Long-Term Immunogenicity upon Pertussis Booster Vaccination in Young Adults and Children in Relation to Priming Vaccinations in Infancy
Abstract
1. Introduction
2. Materials and Methods
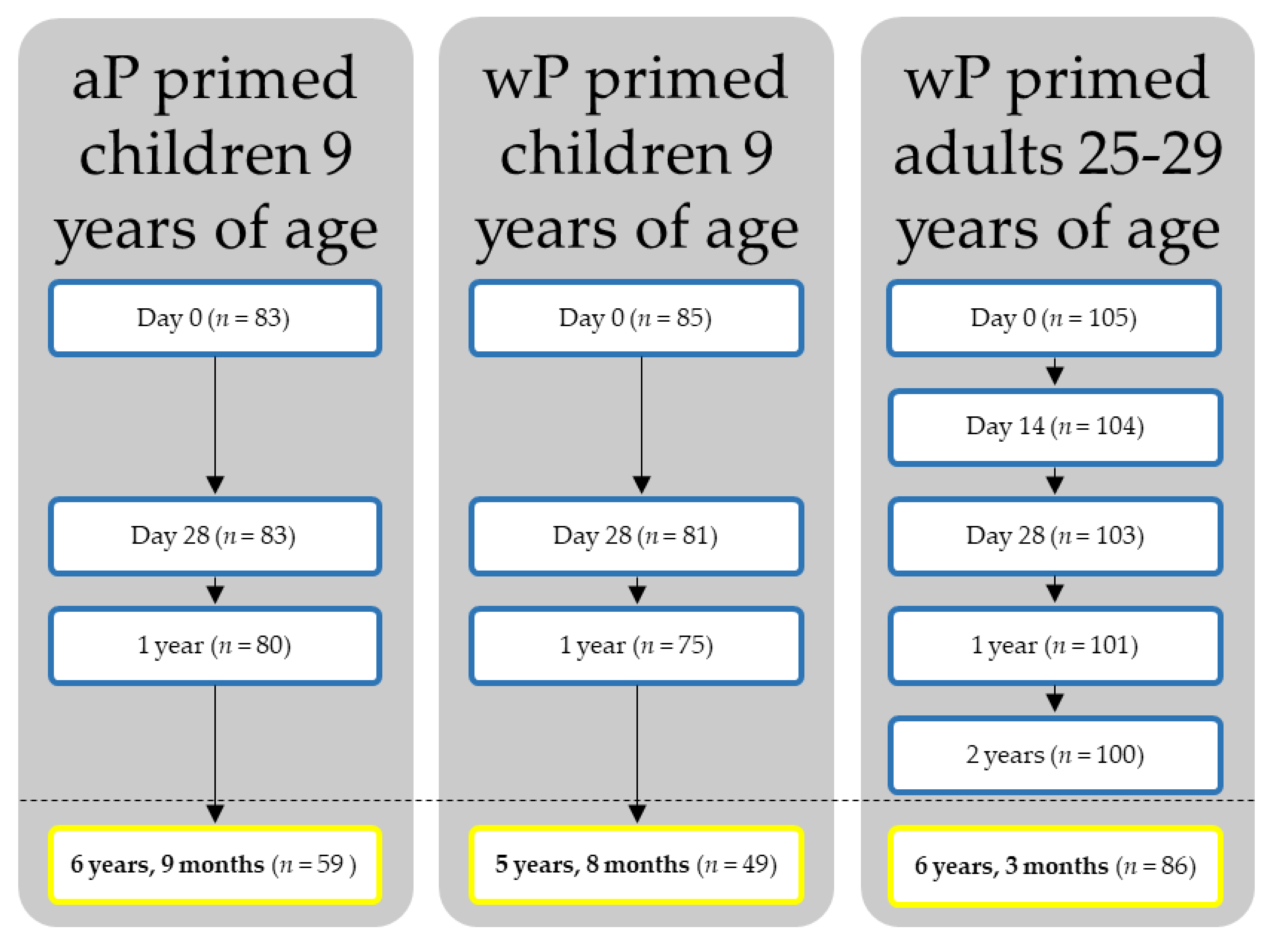
2.1. Study Design and Participants
2.2. Serological Analysis
2.3. Stastical Analyses
3. Results
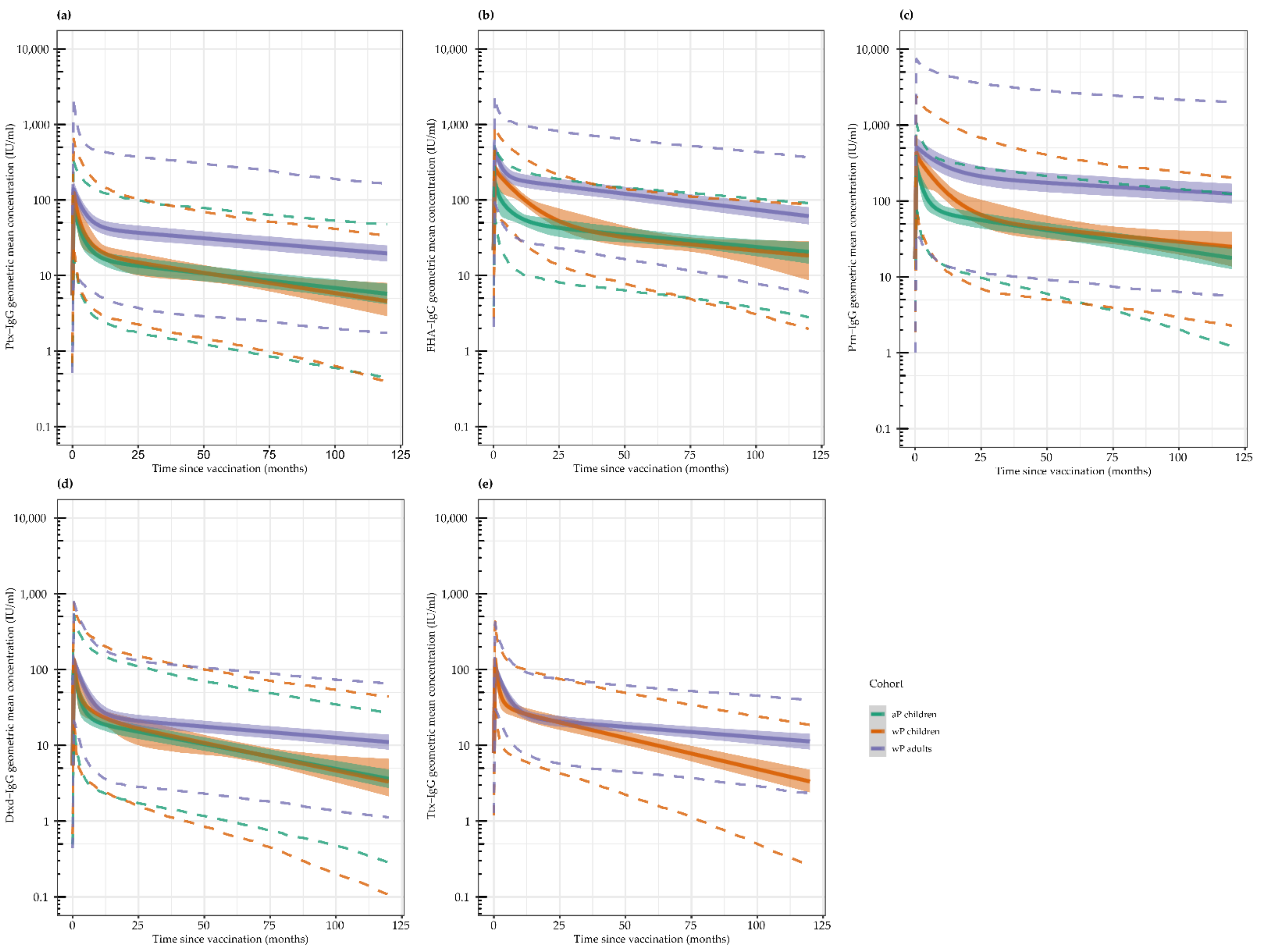
3.1. Geometric Mean Antibody Concentrations (GMCs) per Timepoint
3.2. Antibody Kinetics
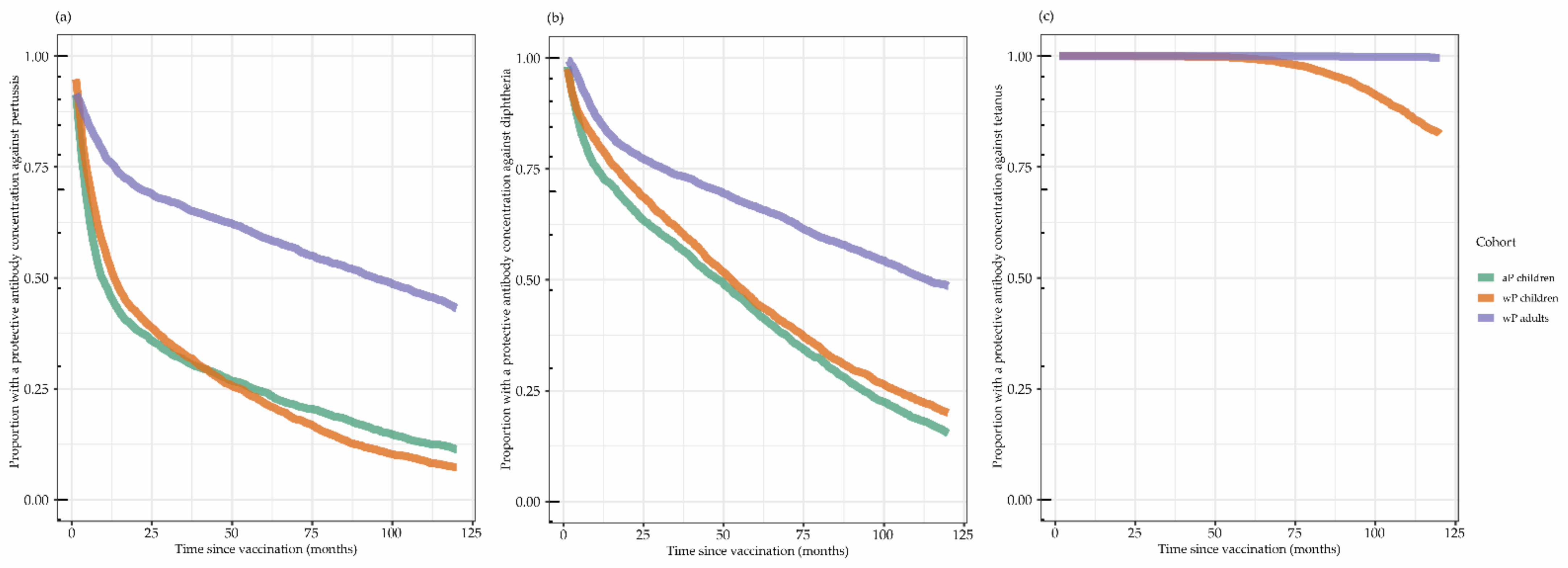
3.3. Proportion with Protective Antibody Concentrations
3.4. Risk Factors to Contract Pertussis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cherry, J.D. Pertussis in Young Infants Throughout the World. Clin. Infect. Dis. 2016, 63, S119–S122. [Google Scholar] [CrossRef] [PubMed]
- Kandeil, W.; Atanasov, P.; Avramioti, D.; Fu, J.; Demarteau, N.; Li, X. The burden of pertussis in older adults: What is the role of vaccination? A systematic literature review. Expert Rev. Vaccines 2019, 18, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D. Historical review of pertussis and the classical vaccine. J. Infect. Dis. 1996, 174 (Suppl. S3), S259–S263. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.W.; Rohani, P. Perplexities of pertussis: Recent global epidemiological trends and their potential causes. Epidemiol. Infect. 2014, 142, 672–684. [Google Scholar] [CrossRef]
- Health Council of the Netherlands. Vaccination against Pertussis; Health Council of the Netherlands: The Hague, The Netherlands, 2004; Volume 2004. [Google Scholar]
- Schurink-van ‘t Klooster, T.; de Melker, H. The National Immunisation Programme in the Netherlands: Surveillance and Developments in 2019–2020; Rijksinstituut voor Volksgezondheid en Milieu (RIVM): Bilthoven, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Lambert, L.C. Pertussis vaccine trials in the 1990s. J. Infect. Dis. 2014, 209 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef]
- David, S.; Vermeer-de Bondt, P.E.; van der Maas, N.A.T. Reactogenicity of infant whole cell pertussis combination vaccine compared with acellular pertussis vaccines with or without simultaneous pneumococcal vaccine in the Netherlands. Vaccine 2008, 26, 5883–5887. [Google Scholar] [CrossRef]
- Clark, T.A. Changing pertussis epidemiology: Everything old is new again. J. Infect. Dis. 2014, 209, 978–981. [Google Scholar] [CrossRef]
- Wiley, K.E.; Zuo, Y.; Macartney, K.K.; McIntyre, P.B. Sources of pertussis infection in young infants: A review of key evidence informing targeting of the cocoon strategy. Vaccine 2013, 31, 618–625. [Google Scholar] [CrossRef]
- Zoldi, V.; Sane, J.; Nohynek, H.; Virkki, M.; Hannila-Handelberg, T.; Mertsola, J. Decreased incidence of pertussis in young adults after the introduction of booster vaccine in military conscripts: Epidemiological analyses of pertussis in Finland, 1995–2015. Vaccine 2017, 35, 5249–5255. [Google Scholar] [CrossRef]
- Cassimos, D.C.; Effraimidou, E.; Medic, S.; Konstantinidis, T.; Theodoridou, M.; Maltezou, H.C. Vaccination Programs for Adults in Europe, 2019. Vaccines 2020, 8, 34. [Google Scholar] [CrossRef]
- Haviari, S.; Bénet, T.; Saadatian-Elahi, M.; André, P.; Loulergue, P.; Vanhems, P. Vaccination of healthcare workers: A review. Hum. Vaccin. Immunother. 2015, 11, 2522–2537. [Google Scholar] [CrossRef]
- Voysey, M.; Kelly, D.F.; Fanshawe, T.R.; Sadarangani, M.; O’Brien, K.L.; Perera, R.; Pollard, A.J. The Influence of Maternally Derived Antibody and Infant Age at Vaccination on Infant Vaccine Responses: An Individual Participant Meta-analysis. JAMA Pediatr. 2017, 171, 637–646. [Google Scholar] [CrossRef]
- Pool, V.; Tomovici, A.; Johnson, D.R.; Greenberg, D.P.; Decker, M.D. Humoral immunity 10years after booster immunization with an adolescent and adult formulation combined tetanus, diphtheria, and 5-component acellular pertussis vaccine in the USA. Vaccine 2018, 36, 2282–2287. [Google Scholar] [CrossRef]
- Squeri, R.; Genovese, C. Immunogenicity and antibody persistence of diphteria-tetanus-acellular pertussis vaccination in adolescents and adults: A systematic review of the literature showed different responses to the available vaccines. J. Prev. Med. Hyg. 2020, 61, E530–E541. [Google Scholar] [CrossRef]
- van der Lee, S.; van Rooijen, D.M.; de Zeeuw-Brouwer, M.L.; Bogaard, M.J.M.; van Gageldonk, P.G.M.; Marinovic, A.B.; Sanders, E.A.M.; Berbers, G.A.M.; Buisman, A.M. Robust Humoral and Cellular Immune Responses to Pertussis in Adults after a First Acellular Booster Vaccination. Front. Immunol. 2018, 9, 681. [Google Scholar] [CrossRef]
- van der Lee, S.; Hendrikx, L.H.; Sanders, E.A.M.; Berbers, G.A.M.; Buisman, A.M. Whole-Cell or Acellular Pertussis Primary Immunizations in Infancy Determines Adolescent Cellular Immune Profiles. Front. Immunol. 2018, 9, 51. [Google Scholar] [CrossRef]
- Schure, R.M.; de Rond, L.; Oztürk, K.; Hendrikx, L.; Sanders, E.; Berbers, G.; Buisman, A.M. Pertussis circulation has increased T-cell immunity during childhood more than a second acellular booster vaccination in Dutch children 9 years of age. PLoS ONE 2012, 7, e41928. [Google Scholar] [CrossRef][Green Version]
- Hijnen, M.; van Gageldonk, P.G.M.; Berbers, G.A.M.; van Woerkom, T.; Mooi, F.R. The Bordetella pertussis virulence factor P.69 pertactin retains its immunological properties after overproduction in Escherichia coli. Protein Expr. Purif. 2005, 41, 106–112. [Google Scholar] [CrossRef]
- van Gageldonk, P.G.M.; van Schaijk, F.G.; van der Klis, F.R.; Berbers, G.A.M. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J. Immunol. Methods 2008, 335, 79–89. [Google Scholar] [CrossRef]
- van der Lee, S.; Stoof, S.P.; van Ravenhorst, M.B.; van Gageldonk, P.G.M.; van der Maas, N.A.T.; Sanders, E.A.M.; Buisman, A.M.; Berbers, G.A.M. Enhanced Bordetella pertussis acquisition rate in adolescents during the 2012 epidemic in the Netherlands and evidence for prolonged antibody persistence after infection. Euro Surveill. 2017, 22, 17-00011. [Google Scholar] [CrossRef]
- World Health Organization. Module 3: Tetanus. In The Immunological Basis for Immunization Series; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization. Module 2: Diphtheria. In The Immunological Basis for Immunization Series; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Long, S.S.; Welkon, C.J.; Clark, J.L. Widespread silent transmission of pertussis in families: Antibody correlates of infection and symptomatology. J. Infect. Dis. 1990, 161, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Sato, H. Anti-pertussis toxin IgG and anti-filamentous hemagglutinin IgG production in children immunized with pertussis acellular vaccine and comparison of these titers with the sera of pertussis convalescent children. Dev. Biol. Stand. 1985, 61, 367–372. [Google Scholar] [PubMed]
- Olin, P.; Hallander, H.O.; Gustafsson, L.; Reizenstein, E.; Storsaeter, J. How to make sense of pertussis immunogenicity data. Clin. Infect. Dis. 2001, 33 (Suppl. S4), S288–S291. [Google Scholar] [CrossRef] [PubMed]
- Mollers, M.; Ruijs, W.L.M. Kinkhoest Richtlijn. Available online: lci.rivm.nl/richtlijnen/kinkhoest (accessed on 19 September 2020).
- Slifka, M.K.; Antia, R.; Whitmire, J.K.; Ahmed, R. Humoral immunity due to long-lived plasma cells. Immunity 1998, 8, 363–372. [Google Scholar] [CrossRef]
- Teunis, P.F.; van Eijkeren, J.C.; de Graaf, W.F.; Marinović, A.B.; Kretzschmar, M.E. Linking the seroresponse to infection to within-host heterogeneity in antibody production. Epidemics 2016, 16, 33–39. [Google Scholar] [CrossRef]
- van Ravenhorst, M.B.; Bonacic Marinovic, A.; van der Klis, F.R.; van Rooijen, D.M.; van Maurik, M.; Stoof, S.P.; Sanders, E.A.; Berbers, G.A. Long-term persistence of protective antibodies in Dutch adolescents following a meningococcal serogroup C tetanus booster vaccination. Vaccine 2016, 34, 6309–6315. [Google Scholar] [CrossRef]
- Plummer, M. JAGS: A Program for Analysis of Bayesian Graphical Models Using Gibbs Sampling. In Proceedings of the 3rd International Workshop on Distributed Statistical Computing, Vienna, Austria, 20–22 March 2003; pp. 1–10. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Versteegen, P.; Berbers, G.A.M.; Smits, G.; Sanders, E.A.M.; van der Klis, F.R.M.; de Melker, H.E.; van der Maas, N.A.T. More than 10 years after introduction of an acellular pertussis vaccine in infancy: A cross-sectional serosurvey of pertussis in The Netherlands. Lancet Reg. Health Eur. 2021, 6, 1016. [Google Scholar] [CrossRef]
- Dalby, T.; Petersen, J.W.; Harboe, Z.B.; Krogfelt, K.A. Antibody responses to pertussis toxin display different kinetics after clinical Bordetella pertussis infection than after vaccination with an acellular pertussis vaccine. J. Med. Microbiol. 2010, 59, 1029–1036. [Google Scholar] [CrossRef]
- Warfel, J.M.; Zimmerman, L.I.; Merkel, T.J. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc. Natl. Acad. Sci. USA 2014, 111, 787–792. [Google Scholar] [CrossRef]
- PERISCOPE Consortium. PERISCOPE: Road towards effective control of pertussis. Lancet Infect. Dis. 2019, 19, e179–e186. [Google Scholar] [CrossRef]
- van der Lee, S.; Sanders, E.A.M.; Berbers, G.A.M.; Buisman, A.M. Whole-cell or acellular pertussis vaccination in infancy determines IgG subclass profiles to DTaP booster vaccination. Vaccine 2018, 36, 220–226. [Google Scholar] [CrossRef]
- Aalberse, R.C.; Stapel, S.O.; Schuurman, J.; Rispens, T. Immunoglobulin G4: An odd antibody. Clin. Exp. Allergy 2009, 39, 469–477. [Google Scholar] [CrossRef]
- da Silva Antunes, R.; Babor, M.; Carpenter, C.; Khalil, N.; Cortese, M.; Mentzer, A.J.; Seumois, G.; Petro, C.D.; Purcell, L.A.; Vijayanand, P.; et al. Th1/Th17 polarization persists following whole-cell pertussis vaccination despite repeated acellular boosters. J. Clin. Investig. 2018, 128, 3853–3865. [Google Scholar] [CrossRef]
- McAlister, S.M.; van den Biggelaar, A.H.J.; Woodman, T.L.; Hutton, H.; Thornton, R.B.; Richmond, P.C. An observational study of antibody responses to a primary or subsequent pertussis booster vaccination in Australian healthcare workers. Vaccine 2021, 39, 1642–1651. [Google Scholar] [CrossRef]
- Macina, D.; Evans, K.E. Bordetella pertussis in School-Age Children, Adolescents and Adults: A Systematic Review of Epidemiology and Mortality in Europe. Infect. Dis. Ther. 2021, 10, 2071–2118. [Google Scholar] [CrossRef]
- Wilkinson, K.; Righolt, C.H.; Elliott, L.J.; Fanella, S.; Mahmud, S.M. Pertussis vaccine effectiveness and duration of protection-A systematic review and meta-analysis. Vaccine 2021, 39, 3120–3130. [Google Scholar] [CrossRef]
- Sheridan, S.L.; Frith, K.; Snelling, T.L.; Grimwood, K.; McIntyre, P.B.; Lambert, S.B. Waning vaccine immunity in teenagers primed with whole cell and acellular pertussis vaccine: Recent epidemiology. Expert Rev. Vaccines 2014, 13, 1081–1106. [Google Scholar] [CrossRef]
- Huygen, K.; Caboré, R.N.; Maertens, K.; Van Damme, P.; Leuridan, E. Humoral and cell mediated immune responses to a pertussis containing vaccine in pregnant and nonpregnant women. Vaccine 2015, 33, 4117–4123. [Google Scholar] [CrossRef]
- National Institute for Public Health and the Environment (RIVM). RVP-Richtlijn Uitvoering Rijksvaccinatieprogramma. 2022. Available online: rijksvaccinatieprogramma.nl/professionals/richtlijnen/rvp-richtlijn-uitvoering (accessed on 6 February 2022).
- Berbers, G.; van Gageldonk, P.; Kassteele, J.V.; Wiedermann, U.; Desombere, I.; Dalby, T.; Toubiana, J.; Tsiodras, S.; Ferencz, I.P.; Mullan, K.; et al. Circulation of pertussis and poor protection against diphtheria among middle-aged adults in 18 European countries. Nat. Commun. 2021, 12, 2871. [Google Scholar] [CrossRef]
- Aboud, S.; Matre, R.; Lyamuya, E.F.; Kristoffersen, E.K. Antibodies to tetanus toxoid in women of childbearing age in Dar es Salaam and Bagamoyo, Tanzania. Trop. Med. Int. Health 2001, 6, 119–125. [Google Scholar] [CrossRef]
- Gentili, G.; D’Amelio, R.; Wirz, M.; Matricardi, P.M.; Nisini, R.; Collotti, C.; Pasquini, P.; Stroffolini, T. Prevalence of hyperimmunization against tetanus in Italians born after the introduction of mandatory vaccination of children with tetanus toxoid in 1968. Infection 1993, 21, 80–82. [Google Scholar] [CrossRef]
- Hammarlund, E.; Thomas, A.; Poore, E.A.; Amanna, I.J.; Rynko, A.E.; Mori, M.; Chen, Z.; Slifka, M.K. Durability of Vaccine-Induced Immunity Against Tetanus and Diphtheria Toxins: A Cross-sectional Analysis. Clin. Infect. Dis 2016, 62, 1111–1118. [Google Scholar] [CrossRef]
- National Coordination Centre for Communicable Disease Control (LCI). Tetanus-Richtlijn. Available online: https://lci.rivm.nl/richtlijnen/tetanus (accessed on 6 February 2022).
| n | GMC Ptx (95% CI) | GMC FHA (95% CI) | GMC Prn (95% CI) | GMC Dtxd (95% CI) | GMC Ttx (95% CI) | |
|---|---|---|---|---|---|---|
| Children aP-primed 1 | ||||||
| Baseline | 83 | 7 (5–9) | 27 (23–33) | 36 (29–45) | 0.04 (0.03–0.05) | 0.35 (0.29–0.44) |
| 28 days | 83 | 68 (57–81) | 158 (140–179) | 307 (267–352) | 0.61 (0.49–0.75) | 7.44 (6.31–8.76) |
| 1 year | 80 | 19 (15–24) | 60 (50–71) | 99 (84–117) | 0.15 (0.12–0.19) | 1.94 (1.65–2.30) |
| 6 years 9 months | 59 | 14 (10–19) | 46 (38–57) | 46 (36–60) | 0.05 (0.04–0.07) | 3.69 (2.95–4.61) * |
| 6 years 9 months: infected | 7 (12%) | 57 (37–87) | 128 (80–202) | 84 (22–319) | N/A | N/A |
| 6 years 9 months: non-infected | 52 (88%) | 12 (8–16) | 41 (33–50) | 43 (33–55) | N/A | N/A |
| Children wP-primed 2 | ||||||
| Baseline | 83 | 7 (5–10) | 34 (26–44) | 13 (10–18) | 0.05 (0.04–0.06) | 0.46 (0.38–0.56) |
| 28 days | 81 | 112 (91–139) | 282 (244–325) | 343 (275–429) | 0.88 (0.69–1.12) | 9.10 (7.90–10.5) |
| 1 year | 79 | 24 (18–30) | 105 (92–121) | 93 (70–124) | 0.19 (0.14–0.25) | 2.09 (1.78–2.45) |
| 5 years 8 months | 49 | 13 (9–18) | 34 (27–43) | 34 (24–47) | 0.07 (0.05–0.10) | 0.66 (0.52–0.82) |
| 5 years 8 months infected | 1 (2%) | 41 (–) | 183 (–) | 43 (–) | N/A | N/A |
| 5 years 8 months non-infected | 48 (98%) | 13 (9–18) | 33 (27–41) | 33 (23–47) | N/A | N/A |
| Adults wP-primed 3 | ||||||
| Baseline | 104 | 5 (4–7) | 11 (8–13) | 11 (8–14) | 0.09 (0.07–0.11) | 1.28 (1.07–1.53) |
| 14 days | 103 | 130 (94–179) | 408 (343–485) | 369 (277–492) | 1.56 (1.30–1.89) | 11.7 (10.1–13.5) |
| 28 days | 102 | 122 (93–161) | 341 (289–402) | 364 (275–483) | 1.27 (1.07–1.52) | 9.44 (8.36–10.7) |
| 1 year | 100 | 43 (33–55) | 133 (110–160) | 186 (136–253) | 0.35 (0.29–0.43) | 2.97 (2.63–3.34) |
| 2 years | 99 | 34 (26–44) | 109 (90–132) | 146 (107–199) | 0.24 (0.20–0.29) | 2.15 (1.90–2.43) |
| 6 years 3 months | 85 | 32 (25–43) | 72 (59–89) | 132 (97–180) | 0.23 (0.19–0.29) | 2.47 (2.13–2.86) |
| 6 years 3 months: extra vaccination | 18 (21%) | 84 (48–146) | 131 (92–187) | 214 (114–401) | 0.38 (0.22–0.65) | 3.69 (2.86–4.77) |
| 6 years 3 months: no extra vaccination | 67 (79%) | 25 (19–34) | 62 (49–78) | 116 (81–166) | 0.20 (0.17–0.25) | 2.21 (1.88–2.61) |
| n (%) | N Recent Pertussis Infection (%) | Univariate Crude OR (95% CI) | p-Value | Multivariate Adjusted OR (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| Step 1: multivariable model with all participants combined | ||||||
| Sex | 0.322 | |||||
| Male | 79 (45%) | 5 (6.3%) | Ref. | |||
| Female | 96 (55%) | 3 (3.1%) | 0.477 (0.110–2.063) | |||
| Age at inclusion | 0.997 | |||||
| 9 years | 108 (62%) | 8 (7.4%) | Ref. | |||
| 25–29 years | 67 (38%) | 0 (0.0%) | <0.001 (N/A) | |||
| Priming vaccinations | 0.011 | 0.028 | ||||
| wP | 116 (66%) | 1 (0.9%) | Ref. | Ref. | ||
| aP | 59 (34%) | 7 (12%) | 15.481 (1.857–129.067) | 11.061 (1.293–94.604) | ||
| Ptx < 20 IU/mL at 1 year | 0.025 | 0.063 | ||||
| no | 102 (59%) | 1 (1.0%) | Ref. | Ref. | ||
| yes | 70 (41%) | 7 (10%) | 11.222 (1.349–93.380) | 7.701 (0.895–66.294) | ||
| Antibody concentrations one moths post-vaccination | ||||||
| Ptx Ab at 1 month * | 173 | 8 (4.6%) | 0.440 (0.123–1.577) | 0.208 | ||
| FHA Ab at 1 month * | 173 | 8 (4.6%) | 0.069 (0.005–0.877) | 0.039 | ||
| Prn Ab at 1 month * | 173 | 8 (4.6%) | 0.618 (0.146–2.627) | 0.515 | ||
| Ptx Ab at 1 year * | 172 | 8 (4.7%) | 0.316 (0.087–1.146) | 0.080 | ||
| FHA Ab at 1year * | 172 | 8 (4.7%) | 0.088 (0.011–0.736) | 0.025 | ||
| Prn Ab at 1 year * | 172 | 8 (4.7%) | 0.582 (0.168–2.019) | 0.148 | ||
| Step 2: Pearson’s chi-squared test on priming vaccinations between aP- and wP-primed children (adults excluded) | ||||||
| wP-primed children | 49 (45%) | 1 (2.0%) | N/A | 0.052 | ||
| aP-primed children | 59 (55%) | 7 (12%) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Versteegen, P.; Bonačić Marinović, A.A.; van Gageldonk, P.G.M.; van der Lee, S.; Hendrikx, L.H.; Sanders, E.A.M.; Berbers, G.A.M.; Buisman, A.-M. Long-Term Immunogenicity upon Pertussis Booster Vaccination in Young Adults and Children in Relation to Priming Vaccinations in Infancy. Vaccines 2022, 10, 693. https://doi.org/10.3390/vaccines10050693
Versteegen P, Bonačić Marinović AA, van Gageldonk PGM, van der Lee S, Hendrikx LH, Sanders EAM, Berbers GAM, Buisman A-M. Long-Term Immunogenicity upon Pertussis Booster Vaccination in Young Adults and Children in Relation to Priming Vaccinations in Infancy. Vaccines. 2022; 10(5):693. https://doi.org/10.3390/vaccines10050693
Chicago/Turabian StyleVersteegen, Pauline, Axel A. Bonačić Marinović, Pieter G. M. van Gageldonk, Saskia van der Lee, Lotte H. Hendrikx, Elisabeth A. M. Sanders, Guy A. M. Berbers, and Anne-Marie Buisman. 2022. "Long-Term Immunogenicity upon Pertussis Booster Vaccination in Young Adults and Children in Relation to Priming Vaccinations in Infancy" Vaccines 10, no. 5: 693. https://doi.org/10.3390/vaccines10050693
APA StyleVersteegen, P., Bonačić Marinović, A. A., van Gageldonk, P. G. M., van der Lee, S., Hendrikx, L. H., Sanders, E. A. M., Berbers, G. A. M., & Buisman, A.-M. (2022). Long-Term Immunogenicity upon Pertussis Booster Vaccination in Young Adults and Children in Relation to Priming Vaccinations in Infancy. Vaccines, 10(5), 693. https://doi.org/10.3390/vaccines10050693







