Mosquirix™ RTS, S/AS01 Vaccine Development, Immunogenicity, and Efficacy
Abstract
:1. Introduction
2. World Health Organization Statistical Analysis of Malarial Infection
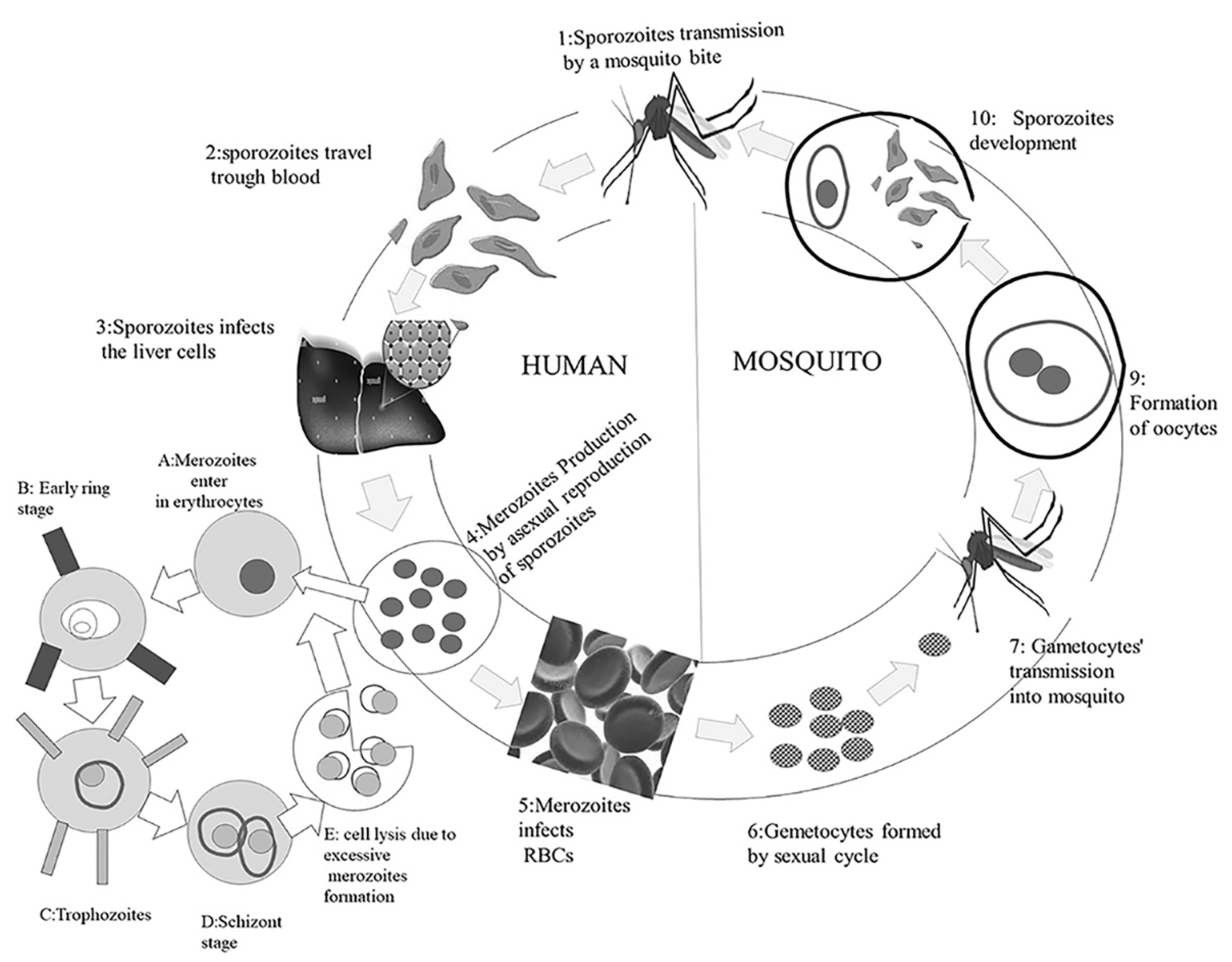
3. Transmission of Malarial Infection
4. Methods to Prevent and Control Malarial Infections and Their Limitations
5. Vaccines—The Most Efficient Tool for Combating Malarial Infection
6. Development of RTS, S/AS01 Vaccine
7. Phase III Clinical Trial of RTS, S/AS01 Vaccine
8. Production of RTS, S/AS01 Vaccine
9. Economic and Community Health Issues
10. Vaccine Administration via Intramuscular Method
11. Immuno-Stimulation
12. Cross Defensive Behavior of Vaccine
13. Public Health Awareness Campaigns about the Side Effects and Advantages of Vaccines
14. Recent Inventions in Malarial Vaccination
15. Future Perspectives
16. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Towards a Global Action Plan for Healthy Lives and Well-Being for All: Uniting to Accelerate Progress towards the Health-Related SDGs; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Ayanful-Torgby, R.; Quashie, N.B.; Boampong, J.N.; Williamson, K.C.; Amoah, L.E. Seasonal variations in Plasmodium falciparum parasite prevalence assessed by varying diagnostic tests in asymptomatic children in southern Ghana. PLoS ONE 2018, 13, e0199172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonam, S.R.; Rénia, L.; Tadepalli, G.; Bayry, J.; Kumar, H.M.S. Plasmodium falciparum Malaria Vaccines and Vaccine Adjuvants. Vaccines 2021, 9, 1072. [Google Scholar] [CrossRef]
- Dondorp, A.M.; Lee, S.J.; Faiz, M.A.; Mishra, S.; Price, R.; Tjitra, E.; Than, M.; Htut, Y.; Mohanty, S.; Yunus, E.B.; et al. The relationship between age and the manifestations of and mortality associated with severe malaria. Clin. Infect. Dis. 2008, 47, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Maitland, K. Management of severe paediatric malaria in resource-limited settings. BMC Med. 2015, 13, 42. [Google Scholar] [CrossRef] [Green Version]
- Aqeel, S.; Naheda, A.; Raza, A.; Khan, W. A micro-epidemiological report on the unstable transmission of malaria in Aligarh, India. Parasite Epidemiol. Control. 2020, 11, e00161. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.; Maire, N.; Molineaux, L.; Smith, T. An epidemiologic model of severe morbidity and mortality caused by Plasmodium falciparum. Am. J. Trop. Med. Hyg. 2006, 75 (Suppl. S2), 63–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anonymous. Severe malaria. Severe malaria. Trop. Med. Int. Health 2014, 19 (Suppl. S1), 7–131. [Google Scholar]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [Green Version]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Lee, J.; Jin, K.; Ahn, S.K.; Lee, S.K.; Kwon, H.W.; Na, B.K.; Kim, T.S. Seroprevalence of Plasmodium vivax Circumsporozoite Protein Antibody in High-Risk Malaria Areas in Korea. Korean J. Parasitol. 2021, 59, 415–419. [Google Scholar] [CrossRef]
- Siciliano, G.; Alano, P. Enlightening the malaria parasite life cycle: Bioluminescent Plasmodium in fundamental and applied research. Front. Microbiol. 2015, 6, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flemming, A. Hitting all stages of the parasite life cycle. Nat. Rev. Microbiol. 2015, 13, 458–459. [Google Scholar] [CrossRef] [PubMed]
- Soulard, V.; Soulard, V.; Bosson-Vanga, H.; Lorthiois, A.; Roucher, C.; Franetich, J.F.; Zanghi, G.; Bordessoulles, M.; Tefit, M.; Thellier, M.; et al. Plasmodium falciparum full life cycle and Plasmodium ovale liver stages in humanized mice. Nat. Commun. 2015, 6, 7690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashley, E.A.; Dhorda, M.; Fairhurst, R.M.; Amaratunga, C.; Lim, P.; Suon, S.; Sreng, S.; Anderson, J.M.; Mao, S.; Sam, B.; et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2014, 371, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Sypniewska, P.; Duda, J.F.; Locatelli, I.; Althaus, C.R.; Althaus, F.; Genton, B. Clinical and laboratory predictors of death in African children with features of severe malaria: A systematic review and meta-analysis. BMC Med. 2017, 15, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Luzolo, A.L.; Ngoyi, D.M. Cerebral malaria. Brain Res. Bull. 2019, 145, 53–58. [Google Scholar] [CrossRef]
- Su, X.-z.; Zhang, C.; Joy, D.A. Host-Malaria Parasite Interactions, and Impacts on Mutual Evolution. Front. Cell. Infect. Microbiol. 2020, 10, 587933. [Google Scholar] [CrossRef]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011, 10, 144. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.V. Vaccines against malaria. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2806–2814. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.U.; Islam, S.U.; Shehzad, A.; Sonn, J.K.; Lee, Y.S. PRPF overexpression induces drug resistance through actin cytoskeleton rearrangement and epithelial-mesenchymal transition. Oncotarget 2017, 8, 56659–56671. [Google Scholar] [CrossRef] [Green Version]
- Shehzad, A.; Ravinayagam, V.; AlRumaih, H.; Aljafary, M.; Almohazey, D.; Almofty, S.; Al-Rashid, N.A.; Al-Suhaimi, E.A. Application of Three-dimensional (3D) Tumor Cell Culture Systems and Mechanism of Drug Resistance. Curr. Pharm. Des. 2019, 25, 3599–3607. [Google Scholar] [CrossRef] [PubMed]
- Miura, K. Progress and prospects for blood-stage malaria vaccines. Expert Rev. Vaccines 2016, 15, 765–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patarroyo, M.E.; Alba, M.P.; Rojas-Luna, R.; Bermudez, A.; Aza-Conde, J. Functionally relevant proteins in Plasmodium falciparum host cell invasion. Immunotherapy 2017, 9, 131–155. [Google Scholar] [CrossRef] [PubMed]
- Draper, S.J.; Sack, B.K.; King, C.R.; Nielsen, C.M.; Rayner, J.C.; Higgins, M.K.; Long, C.A.; Seder, R.A. Malaria Vaccines: Recent Advances and New Horizons. Cell Host Microbe 2018, 24, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Datoo, M.S.; Natama, M.H.; Somé, A.; Traoré, O.; Rouamba, T.; Bellamy, D.; Yameogo, P.; Valia, D.; Tegneri, M.; Ouedraogo, F.; et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: A randomised controlled trial. Lancet 2021, 397, 1809–1818. [Google Scholar] [CrossRef]
- World Health Organization, Regional Office for Europe. Malaria: Fact Sheet on Sustainable Development Goals (SDGs): Health Targets; World Health Organization: Geneva, Switzerland; Regional Office for Europe: Copenhagen, Denmark, 2017. [Google Scholar]
- Targett, G.A.T.; Moorthy, V.S.; Brown, G.V. Malaria vaccine research and development: The role of the WHO MALVAC committee. Malar. J. 2013, 12, 362. [Google Scholar] [CrossRef] [Green Version]
- Wahid, F.; Khan, T.; Shehzad, A.; Ui-Islam, M.; Kim, Y.Y. Interaction of nanomaterials with cells and their medical applications. J. Nanosci. Nanotechnol. 2014, 14, 744–754. [Google Scholar] [CrossRef]
- Neafsey, D.E.; Juraska, M.; Bedford, T.; Benkeser, D.; Valim, C.; Griggs, A.; Lievens, M.; Abdulla, S.; Adjei, S.; Agbenyega, T.; et al. Genetic Diversity and Protective Efficacy of the RTS, S/AS01 Malaria Vaccine. N. Engl. J. Med. 2015, 373, 2025–2037. [Google Scholar] [CrossRef]
- Daily, J.P. Malaria vaccine trials--beyond efficacy end points. N. Engl. J. Med. 2012, 367, 2349–2351. [Google Scholar] [CrossRef]
- Ndeketa, L.; Mategula, D.; Terlouw, D.J.; Bar-Zeev, N.; Sauboin, C.J.; Biernaux, S. Cost-effectiveness and public health impact of RTS, S/AS01 (E) malaria vaccine in Malawi, using a Markov static model. Wellcome Open Res. 2020, 5, 260. [Google Scholar] [CrossRef]
- Han, L.; Hudgens, M.G.; Emch, M.E.; Juliano, J.J.; Keeler, C.; Martinson, F.; Kamthunzi, P.; Tegha, G.; Lievens, M.; Hoffman, I.F. RTS, S/AS01 Malaria Vaccine Efficacy is Not Modified by Seasonal Precipitation: Results from a Phase 3 Randomized Controlled Trial in Malawi. Sci. Rep. 2017, 7, 7200. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Nussenzweig, V.; Nussenzweig, R.; Vekemans, J.; Leach, A. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Hum. Vaccines 2010, 6, 90–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaslow, D.C.; Biernaux, S. RTS, S: Toward a first landmark on the Malaria Vaccine Technology Roadmap. Vaccine 2015, 33, 7425–7432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shehzad, A.; Lee, J.; Lee, Y.S. Autocrine prostaglandin E₂ signaling promotes promonocytic leukemia cell survival via COX-2 expression and MAPK pathway. BMB Rep. 2015, 48, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Shehzad, A.; Parveen, S.; Qureshi, M.; Subhan, F.; Lee, Y.S. Decursin and decursinol angelate: Molecular mechanism and therapeutic potential in inflammatory diseases. Inflamm. Res. 2018, 67, 209–218. [Google Scholar] [CrossRef]
- Leroux-Roels, G.; Leroux-Roels, I.; Clement, F.; Ofori-Anyinam, O.; Lievens, M.; Jongert, E.; Moris, P.; Ballou, W.R.; Cohen, J. Evaluation of the immune response to RTS, S/AS01 and RTS, S/AS02 adjuvanted vaccines: Randomized, double-blind study in malaria-naive adults. Hum. Vaccines Immunother. 2014, 10, 2211–2219. [Google Scholar] [CrossRef] [Green Version]
- Bojang, K.A. RTS, S/AS02A for malaria. Expert Rev. Vaccines 2006, 5, 611–615. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Collignon, C.; Bourguignon, P.; Wouters, S.; Fierens, K.; Fochesato, M.; Dendouga, N.; Langlet, C.; Malissen, B.; Lambrecht, B.N.; et al. Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells. J. Immunol. 2014, 193, 1920–1930. [Google Scholar] [CrossRef] [Green Version]
- Shehzad, A.; Qureshi, M.; Anwar, M.N.; Lee, Y.S. Multifunctional Curcumin Mediate Multitherapeutic Effects. J. Food Sci. 2017, 82, 2006–2015. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, K.; Thera, M.A.; Coulibaly, D.; Traoré, K.; Guindo, A.B.; Ouattara, A.; Takala-Harrison, S.; Berry, A.A.; Doumbo, O.K.; Plowe, C.V. Variation in the circumsporozoite protein of Plasmodium falciparum: Vaccine development implications. PLoS ONE 2014, 9, e101783. [Google Scholar] [CrossRef]
- Vreden, S.G.; Verhave, J.P.; Oettinger, T.; Sauerwein, R.W.; Meuwissen, J.H. Phase I clinical trial of a recombinant malaria vaccine consisting of the circumsporozoite repeat region of Plasmodium falciparum coupled to hepatitis B surface antigen. Am. J. Trop. Med. Hyg. 1991, 45, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Garçon, N.; Vaughn, D.W.; Didierlaurent, A.M. Development and evaluation of AS03, an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion. Expert Rev. Vaccines 2012, 11, 349–366. [Google Scholar] [CrossRef]
- Kester, K.E.; McKinney, D.A.; Tornieporth, N.; Ockenhouse, C.F.; Heppner, D.G.; Hall, T., Jr.; Wellde, B.T.; White, K.; Sun, P.; Schwenk, R.; et al. A phase I/IIa safety, immunogenicity, and efficacy bridging randomized study of a two-dose regimen of liquid and lyophilized formulations of the candidate malaria vaccine RTS, S/AS02A in malaria-naïve adults. Vaccine 2007, 25, 5359–5366. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-B.; Xu, J. Better Adjuvants for Better Vaccines: Progress in Adjuvant Delivery Systems, Modifications, and Adjuvant-Antigen Codelivery. Vaccines 2020, 8, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polhemus, M.E.; Remich, S.A.; Ogutu, B.R.; Waitumbi, J.N.; Otieno, L.; Apollo, S.; Cummings, J.F.; Kester, K.E.; Ockenhouse, C.F.; Stewart, A.; et al. Evaluation of RTS, S/AS02A and RTS, S/AS01B in adults in a high malaria transmission area. PLoS ONE 2009, 4, e6465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnandji, S.T.; Asante, K.P.; Lyimo, J.; Vekemans, J.; Soulanoudjingar, S.S.; Owusu, R.; Shomari, M.; Leach, A.; Fernandes, J.; Dosoo, D.; et al. Evaluation of the safety and immunogenicity of the RTS, S/AS01E malaria candidate vaccine when integrated in the expanded program of immunization. J. Infect. Dis. 2010, 202, 1076–1087. [Google Scholar] [CrossRef]
- Bejon, P.; Lusingu, J.; Olotu, A.; Leach, A.; Lievens, M.; Vekemans, J.; Mshamu, S.; Lang, T.; Gould, J.; Dubois, M.C.; et al. Efficacy of RTS, S/AS01E vaccine against malaria in children 5 to 17 months of age. N. Engl. J. Med. 2008, 359, 2521–2532. [Google Scholar] [CrossRef] [Green Version]
- Lell, B.; Agnandji, S.; von Glasenapp, I.; Haertle, S.; Oyakhiromen, S.; Issifou, S.; Vekemans, J.; Leach, A.; Lievens, M.; Dubois, M.C.; et al. A randomized trial assessing the safety and immunogenicity of AS01 and AS02 adjuvanted RTS, S malaria vaccine candidates in children in Gabon. PLoS ONE 2009, 4, e7611. [Google Scholar] [CrossRef]
- Olotu, A.; Moris, P.; Mwacharo, J.; Vekemans, J.; Kimani, D.; Janssens, M.; Kai, O.; Jongert, E.; Lievens, M.; Leach, A.; et al. Circumsporozoite-specific T cell responses in children vaccinated with RTS, S/AS01E and protection against P falciparum clinical malaria. PLoS ONE 2011, 6, e25786. [Google Scholar] [CrossRef]
- Owusu-Agyei, S.; Ansong, D.; Asante, K.; Kwarteng Owusu, S.; Owusu, R.; Wireko Brobby, N.A.; Dosoo, D.; Osei Akoto, A.; Osei-Kwakye, K.; Adjei, E.A.; et al. Randomized controlled trial of RTS, S/AS02D and RTS, S/AS01E malaria candidate vaccines given according to different schedules in Ghanaian children. PLoS ONE 2009, 4, e7302. [Google Scholar] [CrossRef]
- RTS, S. Efficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45. [Google Scholar] [CrossRef] [Green Version]
- RTS, S. Clinical Trials Partnership. Efficacy and safety of the RTS, S/AS01 malaria vaccine during 18 months after vaccination: A phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. 2014, 11, e1001685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurens, M.B. RTS, S/AS01 vaccine (Mosquirix™): An overview. Hum. Vaccines Immunother. 2020, 16, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Olotu, A.; Fegan, G.; Wambua, J.; Nyangweso, G.; Leach, A.; Lievens, M.; Kaslow, D.C.; Njuguna, P.; Marsh, K.; Bejon, P. Seven-Year Efficacy of RTS, S/AS01 Malaria Vaccine among Young African Children. N. Engl. J. Med. 2016, 374, 2519–2529. [Google Scholar] [CrossRef] [Green Version]
- Penny, M.A.; Verity, R.; Bever, C.A.; Sauboin, C.; Galactionova, K.; Flasche, S.; White, M.T.; Wenger, E.A.; Van de Velde, N.; Pemberton-Ross, P.; et al. Public health impact and cost-effectiveness of the RTS, S/AS01 malaria vaccine: A systematic comparison of predictions from four mathematical models. Lancet 2016, 387, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Wagnew, Y.; Hagos, T.; Weldegerima, B.; Debie, A. Willingness to Pay for Childhood Malaria Vaccine Among Caregivers of Under-Five Children in Northwest Ethiopia. Clin. Outcomes Res. CEOR 2021, 13, 165–174. [Google Scholar] [CrossRef]
- Aide, P.; Dobaño, C.; Sacarlal, J.; Aponte, J.J.; Mandomando, I.; Guinovart, C.; Bassat, Q.; Renom, M.; Puyol, L.; Macete, E.; et al. Four year immunogenicity of the RTS, S/AS02(A) malaria vaccine in Mozambican children during a phase IIb trial. Vaccine 2011, 29, 6059–6067. [Google Scholar] [CrossRef]
- Campo, J.J.; Dobaño, C.; Sacarlal, J.; Guinovart, C.; Mayor, A.; Angov, E.; Dutta, S.; Chitnis, C.; Macete, E.; Aponte, J.J.; et al. Impact of the RTS, S malaria vaccine candidate on naturally acquired antibody responses to multiple asexual blood stage antigens. PLoS ONE 2011, 6, e25779. [Google Scholar] [CrossRef] [Green Version]
- Dobaño, C.; Campo, J.J.; Dobaño, C.; Sacarlal, J.; Guinovart, C.; Mayor, A.; Angov, E.; Dutta, S.; Chitnis, C.; Macete, E.; et al. Differential Patterns of IgG Subclass Responses to Plasmodium falciparum Antigens in Relation to Malaria Protection and RTS, S Vaccination. Front. Immunol. 2019, 10, 439. [Google Scholar] [CrossRef] [Green Version]
- Moncunill, G.; Mpina, M.; Nhabomba, A.J.; Aguilar, R.; Ayestaran, A.; Sanz, H.; Campo, J.J.; Jairoce, C.; Barrios, D.; Dong, Y.; et al. Distinct Helper T Cell Type 1 and 2 Responses Associated With Malaria Protection and Risk in RTS, S/AS01E Vaccinees. Clin. Infect. Dis. 2017, 65, 746–755. [Google Scholar] [CrossRef] [Green Version]
- Moorthy, V.S.; Ballou, W.R. Immunological mechanisms underlying protection mediated by RTS, S: A review of the available data. Malar. J. 2009, 8, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, M.T.; Verity, R.; Griffin, J.T.; Asante, K.P.; Owusu-Agyei, S.; Greenwood, B.; Drakeley, C.; Gesase, S.; Lusingu, J.; Ansong, D.; et al. Immunogenicity of the RTS, S/AS01 malaria vaccine and implications for duration of vaccine efficacy: Secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect. Dis. 2015, 15, 1450–1458. [Google Scholar] [CrossRef] [Green Version]
- Kazmin, D.; Nakaya, H.I.; Lee, E.K.; Johnson, M.J.; van der Most, R.; van den Berg, R.A.; Ballou, W.R.; Jongert, E.; Wille-Reece, U.; Ockenhouse, C.; et al. Systems analysis of protective immune responses to RTS, S malaria vaccination in humans. Proc. Natl. Acad. Sci. USA 2017, 114, 2425–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurens, M.B. The Promise of a Malaria Vaccine-Are We Closer? Annu. Rev. Microbiol. 2018, 72, 273–292. [Google Scholar] [CrossRef]
- Ishizuka, A.S.; Lyke, K.E.; DeZure, A.; Berry, A.A.; Richie, T.L.; Mendoza, F.H.; Enama, M.E.; Gordon, I.J.; Chang, L.J.; Sarwar, U.N.; et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat. Med. 2016, 22, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Seder, R.A.; Chang, L.J.; Enama, M.E.; Zephir, K.L.; Sarwar, U.N.; Gordon, I.J.; Holman, L.A.; James, E.R.; Billingsley, P.F.; Gunasekera, A.; et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 2013, 341, 1359–1365. [Google Scholar] [CrossRef] [Green Version]
- Lyke, K.E.; Ishizuka, A.S.; Berry, A.A.; Chakravarty, S.; DeZure, A.; Enama, M.E.; James, E.R.; Billingsley, P.F.; Gunasekera, A.; Manoj, A.; et al. Attenuated PfSPZ Vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc. Natl. Acad. Sci. USA 2017, 114, 2711–2716. [Google Scholar] [CrossRef] [Green Version]
- Sissoko, M.S.; Healy, S.A.; Katile, A.; Omaswa, F.; Zaidi, I.; Gabriel, E.E.; Kamate, B.; Samake, Y.; Guindo, M.A.; Dolo, A.; et al. Safety and efficacy of PfSPZ Vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: A randomised, double-blind phase 1 trial. Lancet Infect. Dis. 2017, 17, 498–509. [Google Scholar] [CrossRef]
- Epstein, J.E.; Tewari, K.; Lyke, K.E.; Sim, B.K.; Billingsley, P.F.; Laurens, M.B.; Gunasekera, A.; Chakravarty, S.; James, E.R.; Sedegah, M.; et al. Live attenuated malaria vaccine designed to protect through hepatic CD8⁺ T cell immunity. Science 2011, 334, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Collins, K.A.; Snaith, R.; Cottingham, M.G.; Gilbert, S.C.; Hill, A. Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci. Rep. 2017, 7, 46621. [Google Scholar] [CrossRef] [Green Version]
- Regules, J.A.; Cicatelli, S.B.; Bennett, J.W.; Paolino, K.M.; Twomey, P.S.; Moon, J.E.; Kathcart, A.K.; Hauns, K.D.; Komisar, J.L.; Qabar, A.N.; et al. Fractional Third and Fourth Dose of RTS, S/AS01 Malaria Candidate Vaccine: A Phase 2a Controlled Human Malaria Parasite Infection and Immunogenicity Study. J. Infect. Dis. 2016, 214, 762–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledford, H. Malaria vaccine wows and seeds COVID-19 vaccine effort. Nat. Biotechnol. 2021, 39, 786–787. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.U.; Shehzad, A.; Ahmed, M.B.; Lee, Y. Intranasal Delivery of Nanoformulations: A Potential Way of Treatment for Neurological Disorders. Molecules 2020, 25, 1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallory, K.L.; Taylor, J.A.; Zou, X.; Waghela, I.N.; Schneider, C.G.; Sibilo, M.Q.; Punde, N.M.; Perazzo, L.C.; Savransky, T.; Sedegah, M.; et al. Messenger RNA expressing PfCSP induces functional, protective immune responses against malaria in mice. Npj Vaccines 2021, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Al-Suhaimi, E.A.; Aljafary, M.A.; Alkhulaifi, F.M.; Aldossary, H.A.; Alshammari, T.; Al-Qaaneh, A.; Aldahhan, R.; Alkhalifah, Z.; Gaymalov, Z.Z.; Shehzad, A.; et al. Thymus Gland: A Double Edge Sword for Coronaviruses. Vaccines 2021, 9, 1119. [Google Scholar] [CrossRef]
- Wahid, F.; Shehzad, A.; Khan, T.; Kim, Y.Y. MicroRNAs: Synthesis, mechanism, function, and recent clinical trials. Biochim. Biophys. Acta 2010, 1803, 1231–1243. [Google Scholar] [CrossRef] [Green Version]
| Sr. No | Age of the Patient | Number of Patients | Malarial Type | Number of Dosages | Efficiency %Age |
|---|---|---|---|---|---|
| 1 | 6–12 Weeks Aged Infants | 6537 | Clinical Malaria | 3 Doses | 18.3 |
| 4 Doses | 25.9 | ||||
| Severe Malaria | 3 Doses | 10.3 | |||
| 4 Doses | 17.3 | ||||
| 2 | 5–7 Months Aged Children | 8922 | Clinical Malaria | 3 Doses | 28.3 |
| 4 Doses | 36.3 | ||||
| Severe Malaria | 3 Doses | 1.1 | |||
| 4 Doses | 32.2 |
| Malarial Vaccine Types | Mode of Action | Differences | Advantages | Disadvantages |
|---|---|---|---|---|
| RTS, S |
|
|
|
|
| PfSPZ |
|
|
|
|
| R21 |
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadeem, A.Y.; Shehzad, A.; Islam, S.U.; Al-Suhaimi, E.A.; Lee, Y.S. Mosquirix™ RTS, S/AS01 Vaccine Development, Immunogenicity, and Efficacy. Vaccines 2022, 10, 713. https://doi.org/10.3390/vaccines10050713
Nadeem AY, Shehzad A, Islam SU, Al-Suhaimi EA, Lee YS. Mosquirix™ RTS, S/AS01 Vaccine Development, Immunogenicity, and Efficacy. Vaccines. 2022; 10(5):713. https://doi.org/10.3390/vaccines10050713
Chicago/Turabian StyleNadeem, Aroosa Younis, Adeeb Shehzad, Salman Ul Islam, Ebtesam A. Al-Suhaimi, and Young Sup Lee. 2022. "Mosquirix™ RTS, S/AS01 Vaccine Development, Immunogenicity, and Efficacy" Vaccines 10, no. 5: 713. https://doi.org/10.3390/vaccines10050713
APA StyleNadeem, A. Y., Shehzad, A., Islam, S. U., Al-Suhaimi, E. A., & Lee, Y. S. (2022). Mosquirix™ RTS, S/AS01 Vaccine Development, Immunogenicity, and Efficacy. Vaccines, 10(5), 713. https://doi.org/10.3390/vaccines10050713






