BCG-Based Vaccines Elicit Antigen-Specific Adaptive and Trained Immunity against SARS-CoV-2 and Andes orthohantavirus
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of Recombinant Mycobacterium bovis BCG Strains Expressing the Nucleoprotein of SARS-CoV-2 or ANDV
2.2. Expansion and Characterization of rBCG Strains
2.3. Protein Expression Evaluation through Western Blot
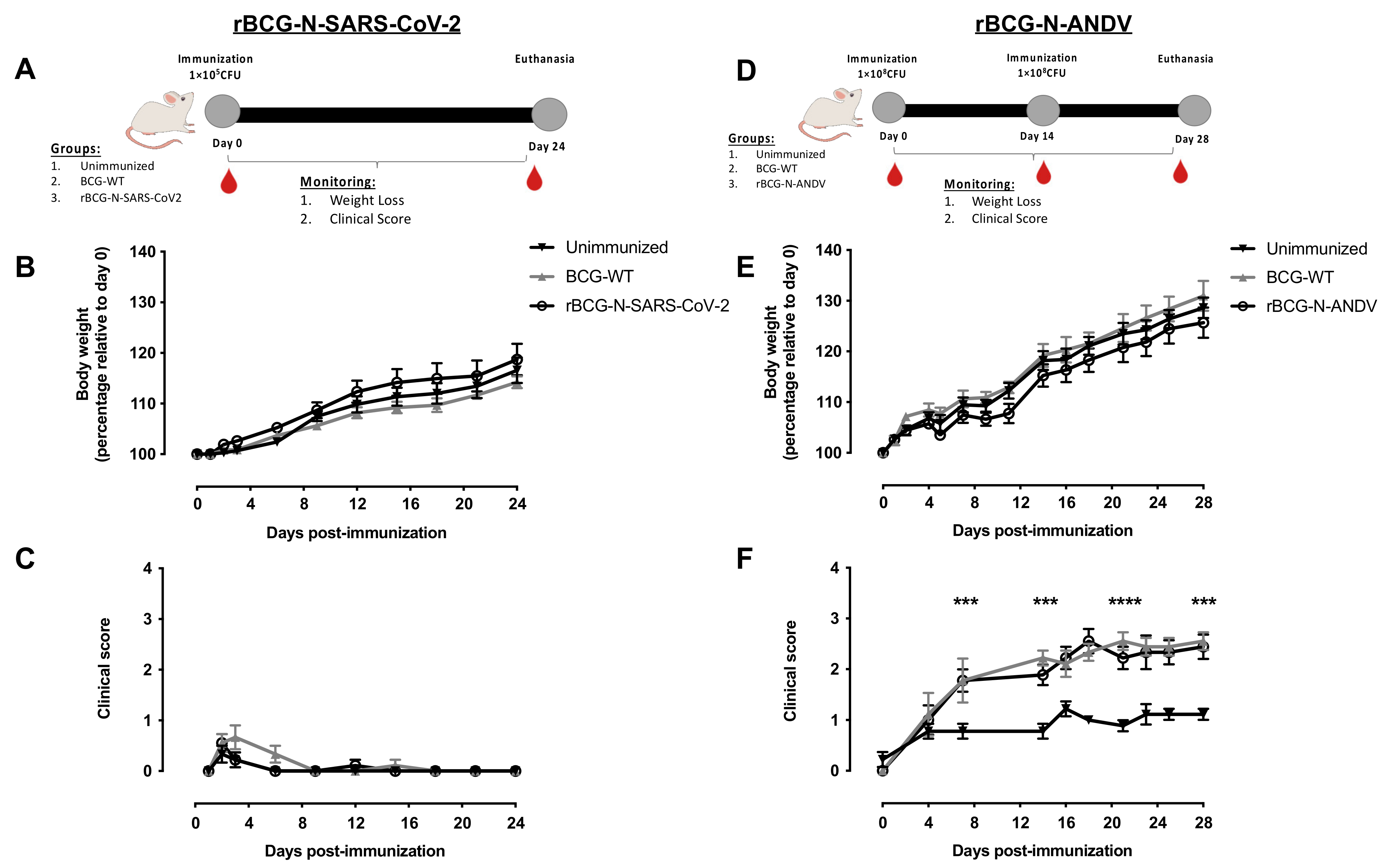
2.4. Mice, Immunization, and Safety Evaluation
2.5. Bone Marrow-Derived Dendritic Cell (BMDCs) Cultures
2.6. T Cell Purification
2.7. Co-Culture Stimulation Assay
2.8. Ex Vivo T Cell Stimulation
2.9. Flow Cytometry Evaluation
2.10. Quantification of Cytokines Secreted during Co-Cultures by ELISA
2.11. Quantification of Specific IgG against SARS-CoV-2 and ANDV Antigens by ELISA
2.12. Statistical Analyses
3. Results
3.1. rBCG Strains Express Either the N-SARS-CoV-2 or N-ANDV Proteins
3.2. The Administration of rBCG-N-SARS-CoV-2 or rBCG-N-ANDV Is Safe and Well-Tolerated in Mice
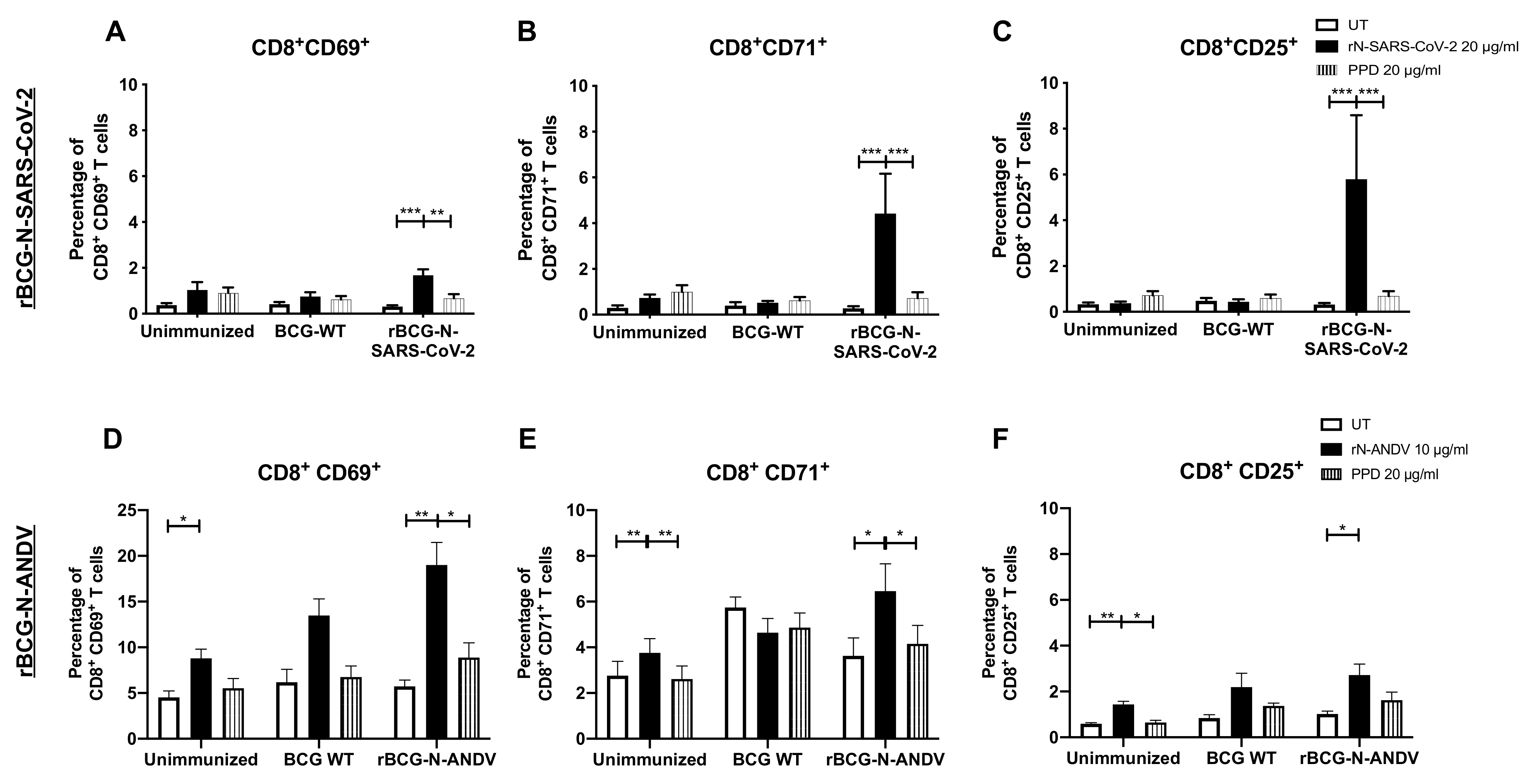
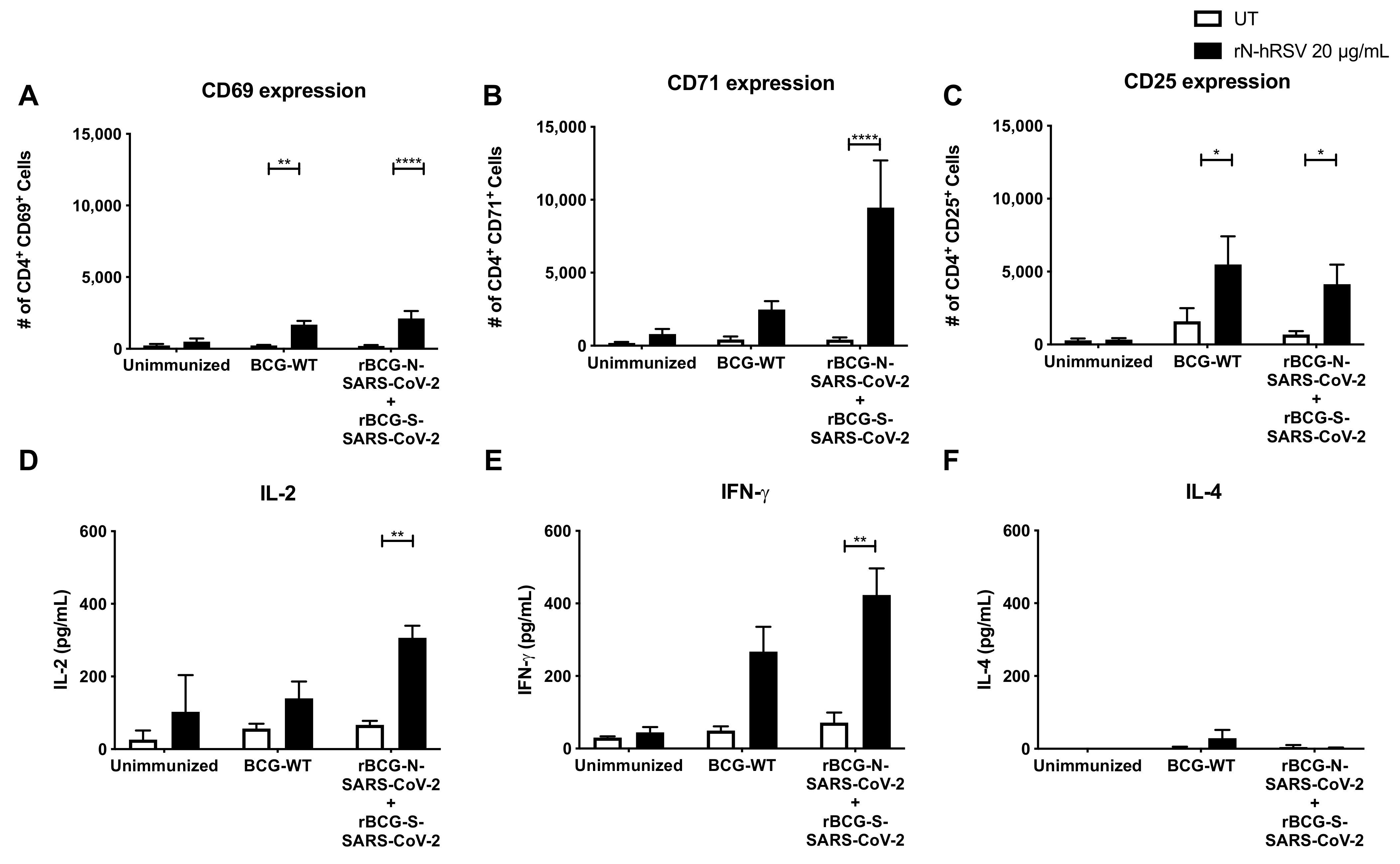
3.3. Administration of One or Two Doses of rBCG Promotes the Activation of T Cells
3.4. Immunization with the Recombinant BCGs Promotes Antiviral Cytokine Secretion in T Cells Stimulated Ex Vivo
3.5. Immunization with Recombinant BCG Vaccine Promotes the Induction of Trained Immunity Parameters
3.6. The Secretion of Specific Antibodies Depends on the Dose and the Number of Immunizations Administrated
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romano, M.; Huygen, K. An update on vaccines for tuberculosis – there is more to it than just waning of BCG efficacy with time. Expert Opin. Biol. Ther. 2012, 12, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Corbel, M.; Fruth, U.; Griffiths, E.; Knezevic, I. Report on a WHO Consultation on the characterisation of BCG strains, Imperial College, London 15–16 December 2003. Vaccine 2004, 22, 2675–2680. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.A.; Gálvez, N.M.S.; Andrade, C.A.; Ramírez, M.A.; Riedel, C.A.; Kalergis, A.M.; Bueno, S.M. BCG vaccination induces cross-protective immunity against pathogenic microorganisms. Trends Immunol. 2022, 43, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Leentjens, J.; Kox, M.; Stokman, R.; Gerretsen, J.; Diavatopoulos, D.A.; Van Crevel, R.; Rimmelzwaan, G.F.; Pickkers, P.; Netea, M.G. BCG vaccination enhances the immunogenicity of subsequent influenza vaccination in healthy volunteers: A randomized, placebo-controlled pilot study. J. Infect. Dis. 2015, 212, 1930–1938. [Google Scholar] [CrossRef] [PubMed]
- Rezende, C.A.F.; De Moraes, M.T.B.; Matos, D.C.D.S.; Mcintoch, D.; Armoa, G.R.G. Humoral response and genetic stability of recombinant BCG expressing hepatitis B surface antigens. J. Virol. Methods 2005, 125, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ritz, N.; Hanekom, W.A.; Robins-Browne, R.; Britton, W.J.; Curtis, N. Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol. Rev. 2008, 32, 821–841. [Google Scholar] [CrossRef]
- Saso, A.; Kampmann, B. Vaccine responses in newborns. Semin. Immunopathol. 2017, 39, 627–642. [Google Scholar] [CrossRef]
- Whittaker, E.; Goldblatt, D.; McIntyre, P.; Levy, O. Neonatal Immunization: Rationale, Current State, and Future Prospects. Front. Immunol. 2018, 9, 532. [Google Scholar] [CrossRef]
- Covián, C.; Ríos, M.; Berríos-Rojas, R.V.; Bueno, S.M.; Kalergis, A.M. Induction of Trained Immunity by Recombinant Vaccines. Front. Immunol. 2020, 11, 611946. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Jacobs, C.; Xavier, R.J.; van der Meer, J.W.M.; van Crevel, R.; Netea, M.G. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin. Immunol. 2014, 155, 213–219. [Google Scholar] [CrossRef]
- Novakovic, B.; Stunnenberg, H.G. I Remember You: Epigenetic Priming in Epithelial Stem Cells. Immunity 2017, 47, 1019–1021. [Google Scholar] [CrossRef] [PubMed]
- Paris, S.; Chapat, L.; Pasin, M.; Lambiel, M.; Sharrock, T.E.; Shukla, R.; Sigoillot-Claude, C.; Bonnet, J.-M.; Poulet, H.; Freyburger, L.; et al. β-Glucan-Induced Trained Immunity in Dogs. Front. Immunol. 2020, 11, 566893. [Google Scholar] [CrossRef] [PubMed]
- Covián, C.; Fernández-Fierro, A.; Retamal-Díaz, A.; Díaz, F.E.; Vasquez, A.E.; Lay, M.K.; Riedel, C.A.; González, P.A.; Bueno, S.M.; Kalergis, A.M. BCG-Induced Cross-Protection and Development of Trained Immunity: Implication for Vaccine Design. Front. Immunol. 2019, 10, 2806. [Google Scholar] [CrossRef] [PubMed]
- Drummer, C.; Saaoud, F.; Shao, Y.; Sun, Y.; Xu, K.; Lu, Y.; Ni, D.; Atar, D.; Jiang, X.; Wang, H.; et al. Trained Immunity and Reactivity of Macrophages and Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef]
- Moorlag, S.J.C.F.M.; Arts, R.J.W.; van Crevel, R.; Netea, M.G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019, 25, 1473–1478. [Google Scholar] [CrossRef]
- Stensballe, L.G.; Nante, E.; Jensen, I.P.; Kofoed, P.-E.; Poulsen, A.; Jensen, H.; Newport, M.; Marchant, A.; Aaby, P. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea-Bissau: A beneficial effect of BCG vaccination for girls community based case-control study. Vaccine 2005, 23, 1251–1257. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.-Y.; Yang, J.-S.; Kim, J.W.; Kim, V.N.; Chang, H. The Architecture of SARS-CoV-2 Transcriptome. Cell 2020, 181, 914–921. [Google Scholar] [CrossRef]
- Lotfi, M.; Rezaei, N. SARS-CoV-2: A comprehensive review from pathogenicity of the virus to clinical consequences. J. Med. Virol. 2020, 92, 1864–1874. [Google Scholar] [CrossRef]
- Khan, S.; Siddique, R.; Shereen, M.A.; Ali, A.; Liu, J.; Bai, Q.; Bashir, N.; Xue, M. Emergence of a Novel Coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2: Biology and Therapeutic Options. J. Clin. Microbiol. 2020, 58, e00187-20. [Google Scholar] [CrossRef]
- Na, W.; Moon, H.; Song, D. A comprehensive review of SARS-CoV-2 genetic mutations and lessons from animal coronavirus recombination in one health perspective. J. Microbiol. 2021, 59, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Majid, S.; Khan, M.S.; Rashid, S.; Niyaz, A.; Farooq, R.; Bhat, S.A.; Wani, H.A.; Qureshi, W. COVID-19: Diagnostics, Therapeutic Advances, and Vaccine Development. Curr. Clin. Microbiol. Rep. 2021, 8, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-R.; Cao, Q.-D.; Hong, Z.-S.; Tan, Y.-Y.; Chen, S.-D.; Jin, H.-J.; Tan, K.-S.; Wang, D.-Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med. Res. 2020, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Veleri, S. Neurotropism of SARS-CoV-2 and neurological diseases of the central nervous system in COVID-19 patients. Exp. Brain Res. 2022, 240, 9–25. [Google Scholar] [CrossRef]
- Alonso, D.O.; Iglesias, A.; Coelho, R.; Periolo, N.; Bruno, A.; Córdoba, M.T.; Filomarino, N.; Quipildor, M.; Biondo, E.; Fortunato, E.; et al. Epidemiological description, case-fatality rate, and trends of Hantavirus Pulmonary Syndrome: 9 years of surveillance in Argentina. J. Med. Virol. 2019, 91, 1173–1181. [Google Scholar] [CrossRef]
- Riquelme, R.; Rioseco, M.L.; Bastidas, L.; Trincado, D.; Riquelme, M.; Loyola, H.; Valdivieso, F. Hantavirus Pulmonary Syndrome, Southern Chile, 1995–2012. Emerg. Infect. Dis. 2015, 21. [Google Scholar] [CrossRef]
- Jonsson, C.B.; Figueiredo, L.T.M.; Vapalahti, O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin. Microbiol. Rev. 2010, 23, 412–441. [Google Scholar] [CrossRef]
- MacNeil, A.; Nichol, S.T.; Spiropoulou, C.F. Hantavirus pulmonary syndrome. Virus Res. 2011, 162, 138–147. [Google Scholar] [CrossRef]
- Forthal, D. Adaptive immune responses to SARS-CoV-2. Adv. Drug Deliv. Rev. 2021, 172, 1–8. [Google Scholar] [CrossRef]
- Sattler, A.; Angermair, S.; Stockmann, H.; Heim, K.M.; Khadzhynov, D.; Treskatsch, S.; Halleck, F.; Kreis, M.E.; Kotsch, K. SARS-CoV-2-specific T cell responses and correlations with COVID-19 patient predisposition. J. Clin. Investig. 2020, 130, 6477–6489. [Google Scholar] [CrossRef] [PubMed]
- López, R.; Vial, C.; Graf, J.; Calvo, M.; Ferrés, M.; Mertz, G.; Cuiza, A.; Agüero, B.; Aguilera, D.; Araya, D.; et al. Platelet Count in Patients with Mild Disease at Admission is Associated with Progression to Severe Hantavirus Cardiopulmonary Syndrome. Viruses 2019, 11, 693. [Google Scholar] [CrossRef] [PubMed]
- Llah, S.T.; Mir, S.; Sharif, S.; Khan, S.; Mir, M.A. Hantavirus induced cardiopulmonary syndrome: A public health concern. J. Med. Virol. 2018, 90, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Vaheri, A.; Strandin, T.; Hepojoki, J.; Sironen, T.; Henttonen, H.; Mäkelä, S.; Mustonen, J. Uncovering the mysteries of hantavirus infections. Nat. Rev. Microbiol. 2013, 11, 539–550. [Google Scholar] [CrossRef]
- Noack, D.; Goeijenbier, M.; Reusken, C.B.E.M.; Koopmans, M.P.G.; Rockx, B.H.G. Orthohantavirus Pathogenesis and Cell Tropism. Front. Cell. Infect. Microbiol. 2020, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, S.M.; Nofchissey, R.A.; Ye, C.; Dunnum, J.L.; Cook, J.A.; Bradfute, S.B. Use of a Novel Detection Tool to Survey Orthohantaviruses in Wild-Caught Rodent Populations. Viruses 2022, 14, 682. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Valdebenito, C.; Calvo, M.; Vial, C.; Mansilla, R.; Marco, C.; Palma, R.E.; Vial, P.A.; Valdivieso, F.; Mertz, G.; Ferrés, M. Person-to-person household and nosocomial transmission of andes hantavirus, Southern Chile, 2011. Emerg. Infect. Dis. 2014, 20, 1629–1636. [Google Scholar] [CrossRef]
- Martinez, V.P.; Bellomo, C.; San Juan, J.; Pinna, D.; Forlenza, R.; Elder, M.; Padula, P.J. Person-to-person transmission of Andes virus. Emerg. Infect. Dis. 2005, 11, 1848–1853. [Google Scholar] [CrossRef]
- Mori, M.; Rothman, A.L.; Kurane, I.; Montoya, J.M.; Nolte, K.B.; Norman, J.E.; Waite, D.C.; Koster, F.T.; Ennis, F.A. High levels of cytokine-producing cells in the lung tissues of patients with fatal hantavirus pulmonary syndrome. J. Infect. Dis. 1999, 179, 295–302. [Google Scholar] [CrossRef]
- Maleki, K.T.; García, M.; Iglesias, A.; Alonso, D.; Ciancaglini, M.; Hammar, U.; Ljunggren, H.-G.; Schierloh, P.; Martínez, V.P.; Klingström, J. Serum Markers Associated with Severity and Outcome of Hantavirus Pulmonary Syndrome. J. Infect. Dis. 2019, 219, 1832–1840. [Google Scholar] [CrossRef]
- Angulo, J.; Martínez-Valdebenito, C.; Marco, C.; Galeno, H.; Villagra, E.; Vera, L.; Lagos, N.; Becerra, N.; Mora, J.; Bermúdez, A.; et al. Serum levels of interleukin-6 are linked to the severity of the disease caused by Andes Virus. PLoS Negl. Trop. Dis. 2017, 11, e0005757. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.A.; Campos, G.M.; Moreli, M.L.; Moro Souza, R.L.; Saggioro, F.P.; Figueiredo, G.G.; Livonesi, M.C.; Moraes Figueiredo, L.T. Role of mixed Th1 and Th2 serum cytokines on pathogenesis and prognosis of hantavirus pulmonary syndrome. Microbes Infect. 2008, 10, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Safronetz, D.; Zivcec, M.; Lacasse, R.; Feldmann, F.; Rosenke, R.; Long, D.; Haddock, E.; Brining, D.; Gardner, D.; Feldmann, H.; et al. Pathogenesis and host response in Syrian hamsters following intranasal infection with Andes virus. PLoS Pathog. 2011, 7, e1002426. [Google Scholar] [CrossRef] [PubMed]
- McDonough, A.A.; Veiras, L.C.; Minas, J.N.; Ralph, D.L. Considerations when quantitating protein abundance by immunoblot. Am. J. Physiol. Cell Physiol. 2015, 308, C426–C433. [Google Scholar] [CrossRef]
- Soto, J.A.; Gálvez, N.M.S.; Pacheco, G.A.; Canedo-Marroquín, G.; Bueno, S.M.; Kalergis, A.M. Induction of Protective Immunity by a Single Low Dose of a Master Cell Bank cGMP-rBCG-P Vaccine Against the Human Metapneumovirus in Mice. Front. Cell. Infect. Microbiol. 2021, 11, 662714. [Google Scholar] [CrossRef]
- Soto, J.A.; Gálvez, N.M.S.; Rivera, C.A.; Palavecino, C.E.; Céspedes, P.F.; Rey-Jurado, E.; Bueno, S.M.; Kalergis, A.M. Recombinant BCG Vaccines Reduce Pneumovirus-Caused Airway Pathology by Inducing Protective Humoral Immunity. Front. Immunol. 2018, 9, 2875. [Google Scholar] [CrossRef]
- Lechuga, G.C.; Souza-Silva, F.; Sacramento, C.Q.; Trugilho, M.R.O.; Valente, R.H.; Napoleão-Pêgo, P.; Dias, S.S.G.; Fintelman-Rodrigues, N.; Temerozo, J.R.; Carels, N.; et al. SARS-CoV-2 Proteins Bind to Hemoglobin and Its Metabolites. Int. J. Mol. Sci. 2021, 22, 9035. [Google Scholar] [CrossRef]
- Céspedes, P.P.F.; Rey-Jurado, E.; Espinoza, J.A.; Rivera, C.A.; Canedo-Marroquín, G.; Bueno, S.M.; Kalergis, A.M. A single, low dose of a cGMP recombinant BCG vaccine elicits protective T cell immunity against the human respiratory syncytial virus infection and prevents lung pathology in mice. Vaccine 2017, 35, 757–766. [Google Scholar] [CrossRef]
- Bueno, S.M.; González, P.A.; Cautivo, K.M.; Mora, J.E.; Leiva, E.D.; Tobar, H.E.; Fennelly, G.J.; Eugenin, E.A.; Jacobs, W.R.; Riedel, C.A.; et al. Protective T cell immunity against respiratory syncytial virus is efficiently induced by recombinant BCG. Proc. Natl. Acad. Sci. USA 2008, 105, 20822–20827. [Google Scholar] [CrossRef]
- Cautivo, K.M.; Bueno, S.M.; Cortes, C.M.; Wozniak, A.; Riedel, C.A.; Kalergis, A.M. Efficient Lung Recruitment of Respiratory Syncytial Virus-Specific Th1 Cells Induced by Recombinant Bacillus Calmette-Guerin Promotes Virus Clearance and Protects from Infection. J. Immunol. 2010, 185, 7633–7645. [Google Scholar] [CrossRef]
- Palavecino, C.E.; Cespedes, P.F.; Gomez, R.S.; Kalergis, A.M.; Bueno, S.M.; Céspedes, P.F.; Gómez, R.S.; Kalergis, A.M.; Bueno, S.M.; Cespedes, P.F.; et al. Immunization with a Recombinant Bacillus Calmette-Guerin Strain Confers Protective Th1 Immunity against the Human Metapneumovirus. J. Immunol. 2014, 192, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Inaba, K.; Inaba, M.; Romani, N.; Aya, H.; Deguchi, M.; Ikehara, S.; Muramatsu, S.; Steinman, R.M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992, 176, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Céspedes, P.F.; Gonzalez, P.A.; Kalergis, A.M. Human metapneumovirus keeps dendritic cells from priming antigen-specific naive T cells. Immunology 2013, 139, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, P.F.; Bueno, S.M.; Ramirez, B.A.; Gomez, R.S.; Riquelme, S.A.; Palavecino, C.E.; Mackern-Oberti, J.P.; Mora, J.E.; Depoil, D.; Sacristan, C.; et al. Surface expression of the hRSV nucleoprotein impairs immunological synapse formation with T cells. Proc. Natl. Acad. Sci. USA 2014, 111, E3214–E3223. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Demeure, C.E.; Rubio, M.; Delespesse, G.; Sarfati, M. Human monocyte-derived dendritic cells induce naive T cell differentiation into T helper cell type 2 (Th2) or Th1/Th2 effectors. Role of stimulator/responder ratio. J. Exp. Med. 2000, 192, 405–412. [Google Scholar] [CrossRef]
- Shete, A.; Mohandas, S.; Jain, R.; Yadav, P.D. A qualitative IgG ELISA for detection of SARS-CoV-2-specific antibodies in Syrian hamster serum samples. STAR Protoc. 2021, 2, 100573. [Google Scholar] [CrossRef]
- Stover, C.K.; de la Cruz, V.F.; Fuerst, T.R.; Burlein, J.E.; Benson, L.A.; Bennett, L.T.; Bansal, G.P.; Young, J.F.; Lee, M.H.; Hatfull, G.F. New use of BCG for recombinant vaccines. Nature 1991, 351, 456–460. [Google Scholar] [CrossRef]
- Abarca, K.; Rey-Jurado, E.; Muñoz-Durango, N.; Vázquez, Y.; Soto, J.A.; Gálvez, N.M.S.; Valdés-Ferrada, J.; Iturriaga, C.; Urzúa, M.; Borzutzky, A.; et al. Safety and immunogenicity evaluation of recombinant BCG vaccine against respiratory syncytial virus in a randomized, double-blind, placebo-controlled phase I clinical trial. EClinicalMedicine 2020, 27, 100517. [Google Scholar] [CrossRef]
- Dutta, N.K.; Mazumdar, K.; Gordy, J.T. The Nucleocapsid Protein of SARS-CoV-2: A Target for Vaccine Development. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Zhuang, Z.; Lai, X.; Sun, J.; Chen, Z.; Zhang, Z.; Dai, J.; Liu, D.; Li, Y.; Li, F.; Wang, Y.; et al. Mapping and role of T cell response in SARS-CoV-2-infected mice. J. Exp. Med. 2021, 218. [Google Scholar] [CrossRef]
- de Carvalho Nicacio, C.; Gonzalez Della Valle, M.; Padula, P.; Björling, E.; Plyusnin, A.; Lundkvist, A. Cross-protection against challenge with Puumala virus after immunization with nucleocapsid proteins from different hantaviruses. J. Virol. 2002, 76, 6669–6677. [Google Scholar] [CrossRef] [PubMed]
- Safronetz, D.; Hegde, N.R.; Ebihara, H.; Denton, M.; Kobinger, G.P.; St Jeor, S.; Feldmann, H.; Johnson, D.C. Adenovirus vectors expressing hantavirus proteins protect hamsters against lethal challenge with andes virus. J. Virol. 2009, 83, 7285–7295. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.W.; Josleyn, M.; Ballantyne, J.; Brocato, R. A novel Sin Nombre virus DNA vaccine and its inclusion in a candidate pan-hantavirus vaccine against hantavirus pulmonary syndrome (HPS) and hemorrhagic fever with renal syndrome (HFRS). Vaccine 2013, 31, 4314–4321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dong, Y.; Ma, T.; Zhang, X.; Ying, Q.; Han, M.; Zhang, M.; Yang, R.; Li, Y.; Wang, F.; Liu, R.; et al. Incorporation of CD40 ligand or granulocyte-macrophage colony stimulating factor into Hantaan virus (HTNV) virus-like particles significantly enhances the long-term immunity potency against HTNV infection. J. Med. Microbiol. 2019, 68, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-S.; Pan, Y.; Chen, H.-Q.; Shen, Y.; Wang, X.-C.; Sun, Y.-J.; Tao, K.-H. Induction of SARS-nucleoprotein-specific immune response by use of DNA vaccine. Immunol. Lett. 2004, 92, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Ahlén, G.; Frelin, L.; Nikouyan, N.; Weber, F.; Höglund, U.; Larsson, O.; Westman, M.; Tuvesson, O.; Gidlund, E.-K.; Cadossi, M.; et al. The SARS-CoV-2 N Protein Is a Good Component in a Vaccine. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, M.; Ribeiro Dos Santos, G.; Wang, L.; Cummings, D.A.T.; Azman, A.S.; Paireau, J.; Fontanet, A.; Cauchemez, S.; Salje, H. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 2021, 590, 140–145. [Google Scholar] [CrossRef]
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu-Barnum, S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020, 383, 1544–1555. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef]
- Kim, Y.-I.; Kim, S.-M.; Park, S.-J.; Kim, E.-H.; Yu, K.-M.; Chang, J.-H.; Kim, E.J.; Casel, M.A.B.; Rollon, R.; Jang, S.-G.; et al. Critical role of neutralizing antibody for SARS-CoV-2 reinfection and transmission. Emerg. Microbes Infect. 2021, 10, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.J.; Moore, J.S.; Blighe, K.; Ng, K.Y.; Quinn, N.; Jennings, F.; Warnock, G.; Sharpe, P.; Clarke, M.; Maguire, K.; et al. Evaluation of the IgG antibody response to SARS CoV-2 infection and performance of a lateral flow immunoassay: Cross-sectional and longitudinal analysis over 11 months. BMJ Open 2021, 11, e048142. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, C.; Lanzillotti, C.; Govoni, M.; Pellielo, G.; Mazzoni, E.; Tognon, M.; Martini, F.; Rotondo, J.C. Decreased IgG Antibody Response to Viral Protein Mimotopes of Oncogenic Merkel Cell Polyomavirus in Sera from Healthy Elderly Subjects. Front. Immunol. 2021, 12, 738486. [Google Scholar] [CrossRef]
- Duehr, J.; McMahon, M.; Williamson, B.; Amanat, F.; Durbin, A.; Hawman, D.W.; Noack, D.; Uhl, S.; Tan, G.S.; Feldmann, H.; et al. Neutralizing Monoclonal Antibodies against the Gn and the Gc of the Andes Virus Glycoprotein Spike Complex Protect from Virus Challenge in a Preclinical Hamster Model. MBio 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, Y.; Greig, M.; Liu, G.; Driedger, M.; Langlois, M.-A. Humoral Responses and Serological Assays in SARS-CoV-2 Infections. Front. Immunol. 2020, 11, 610688. [Google Scholar] [CrossRef] [PubMed]
- Nurieva, R.I.; Chung, Y. Understanding the development and function of T follicular helper cells. Cell. Mol. Immunol. 2010, 7, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.W.; Custer, D.M.; Smith, J.; Wahl-Jensen, V. Hantaan/Andes virus DNA vaccine elicits a broadly cross-reactive neutralizing antibody response in nonhuman primates. Virology 2006, 347, 208–216. [Google Scholar] [CrossRef]
- Brocato, R.L.; Josleyn, M.J.; Wahl-Jensen, V.; Schmaljohn, C.S.; Hooper, J.W. Construction and nonclinical testing of a Puumala virus synthetic M gene-based DNA vaccine. Clin. Vaccine Immunol. 2013, 20, 218–226. [Google Scholar] [CrossRef]
- Warner, B.M.; Stein, D.R.; Jangra, R.K.; Slough, M.M.; Sroga, P.; Sloan, A.; Frost, K.L.; Booth, S.; Chandran, K.; Safronetz, D. Vesicular Stomatitis Virus-Based Vaccines Provide Cross-Protection against Andes and Sin Nombre Viruses. Viruses 2019, 11, 645. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Benn, C.S.; Joosten, L.A.B.; Jacobs, C.; van Loenhout, J.; Xavier, R.J.; Aaby, P.; van der Meer, J.W.M.; et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2014, 6, 152–158. [Google Scholar] [CrossRef]
- de Castro, M.J.J.J.; Pardo-Seco, J.; Martinón-Torres, F. Nonspecific (Heterologous) Protection of Neonatal BCG Vaccination Against Hospitalization Due to Respiratory Infection and Sepsis. Clin. Infect. Dis. 2015, 60, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.S.; Kim, W.; Kalaidina, E.; Goss, C.W.; Rauseo, A.M.; Schmitz, A.J.; Hansen, L.; Haile, A.; Klebert, M.K.; Pusic, I.; et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature 2021, 595, 421–425. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto, J.A.; Díaz, F.E.; Retamal-Díaz, A.; Gálvez, N.M.S.; Melo-González, F.; Piña-Iturbe, A.; Ramírez, M.A.; Bohmwald, K.; González, P.A.; Bueno, S.M.; et al. BCG-Based Vaccines Elicit Antigen-Specific Adaptive and Trained Immunity against SARS-CoV-2 and Andes orthohantavirus. Vaccines 2022, 10, 721. https://doi.org/10.3390/vaccines10050721
Soto JA, Díaz FE, Retamal-Díaz A, Gálvez NMS, Melo-González F, Piña-Iturbe A, Ramírez MA, Bohmwald K, González PA, Bueno SM, et al. BCG-Based Vaccines Elicit Antigen-Specific Adaptive and Trained Immunity against SARS-CoV-2 and Andes orthohantavirus. Vaccines. 2022; 10(5):721. https://doi.org/10.3390/vaccines10050721
Chicago/Turabian StyleSoto, Jorge A., Fabián E. Díaz, Angello Retamal-Díaz, Nicolás M. S. Gálvez, Felipe Melo-González, Alejandro Piña-Iturbe, Mario A. Ramírez, Karen Bohmwald, Pablo A. González, Susan M. Bueno, and et al. 2022. "BCG-Based Vaccines Elicit Antigen-Specific Adaptive and Trained Immunity against SARS-CoV-2 and Andes orthohantavirus" Vaccines 10, no. 5: 721. https://doi.org/10.3390/vaccines10050721
APA StyleSoto, J. A., Díaz, F. E., Retamal-Díaz, A., Gálvez, N. M. S., Melo-González, F., Piña-Iturbe, A., Ramírez, M. A., Bohmwald, K., González, P. A., Bueno, S. M., & Kalergis, A. M. (2022). BCG-Based Vaccines Elicit Antigen-Specific Adaptive and Trained Immunity against SARS-CoV-2 and Andes orthohantavirus. Vaccines, 10(5), 721. https://doi.org/10.3390/vaccines10050721








