Percentages of Vaccination Coverage Required to Establish Herd Immunity against SARS-CoV-2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Herd Immunity against SARS-CoV-2
2.2. Vaccination Coverage Required to Establish Herd Immunity against SARS-CoV-2
2.3. Vaccination Coverage Required to Establish Herd Immunity against SARS-CoV-2 with Infections among Vaccinated Individuals
2.4. Assessment of Herd Immunity Levels Achieved with Percentages of Vaccination Coverage of 70%, 80%, and 90%
2.5. Vaccination Coverage Required to Establish Herd Immunity in Populations with Part of the Population Already Protected against SARS-CoV-2
2.6. Statistical Analysis
3. Results
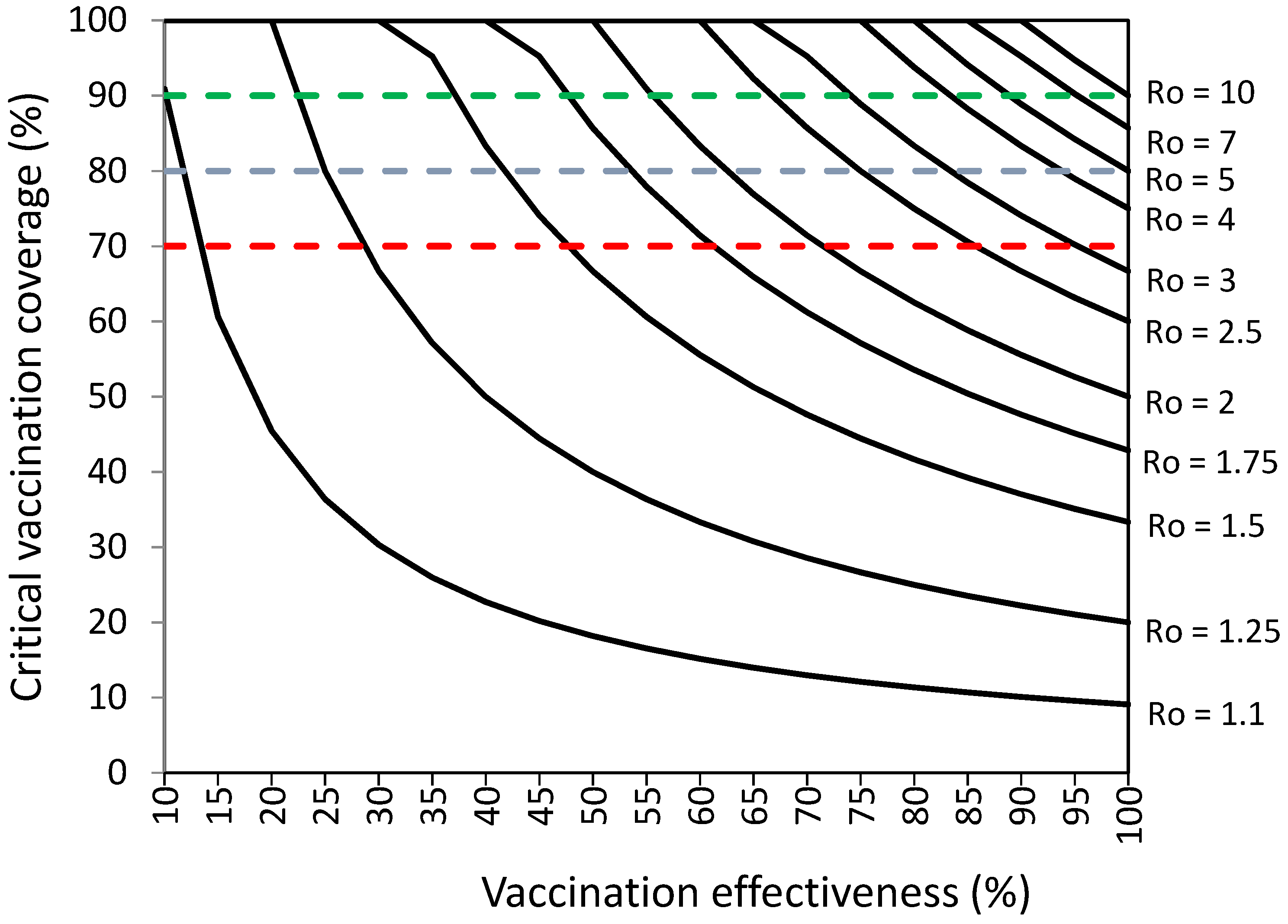
3.1. Vaccination Coverage Required to Establish Herd Immunity against SARS-CoV-2
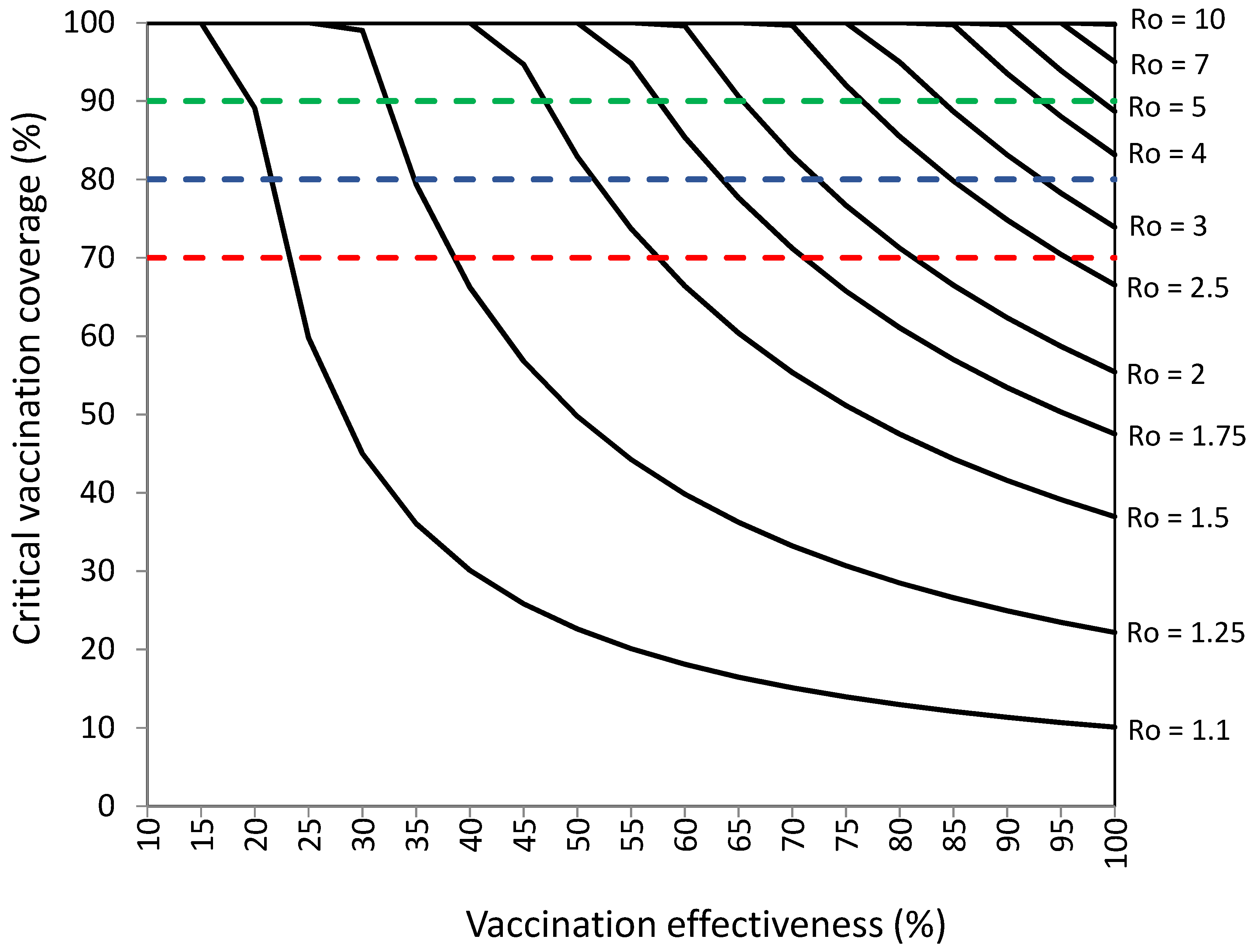
3.2. Vaccination Coverage Required to Establish Herd Immunity against SARS-CoV-2 with Infections among Vaccinated Individuals
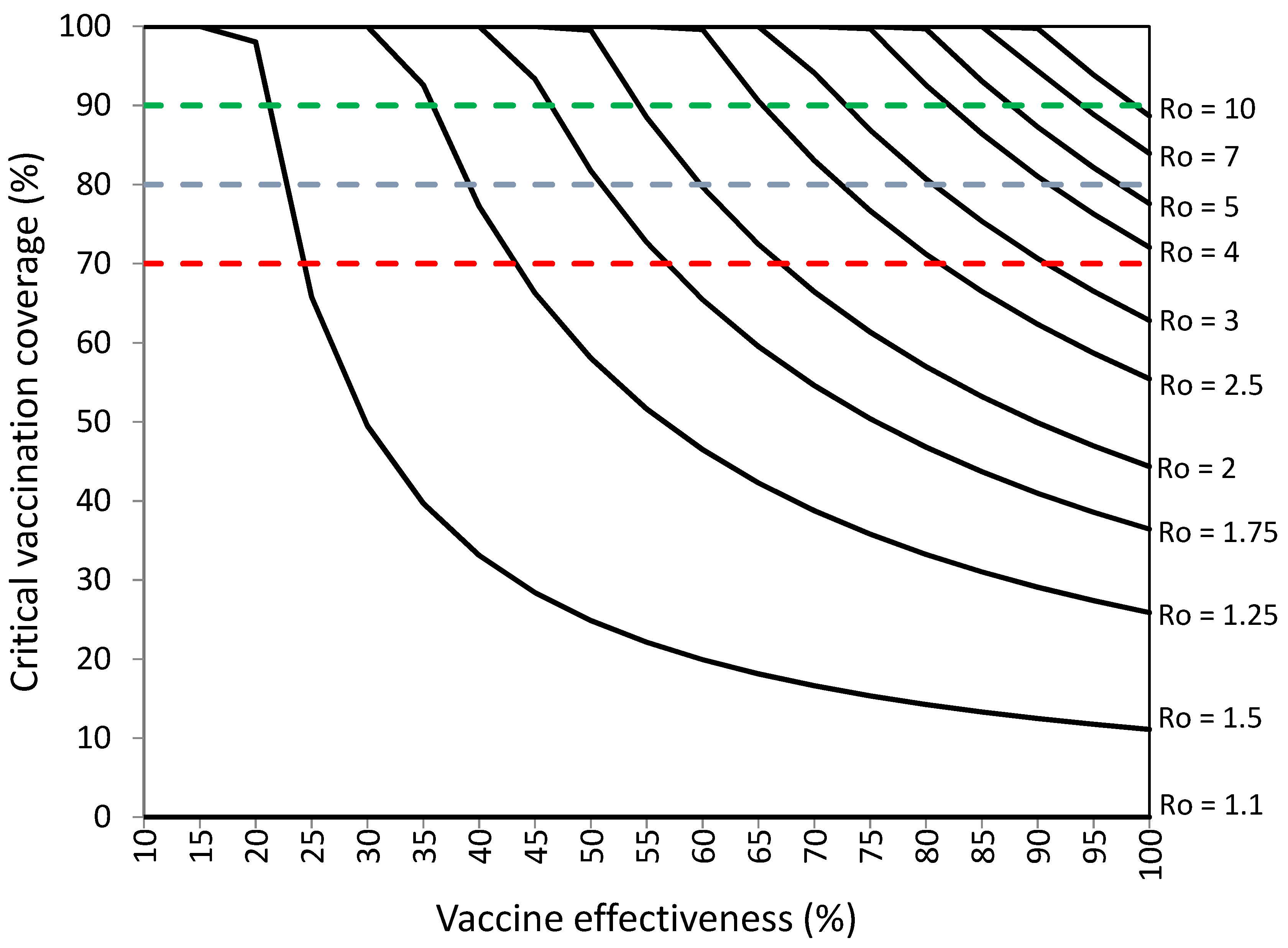
3.3. Assessment of Herd Immunity Levels Achieved with Percentages of Vaccination Coverage of 70%, 80%, and 90%
3.4. Vaccination Coverage Required to Establish Herd Immunity against SARS-CoV-2 When Part of the Population Is Already Protected
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- WHO. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. 11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-COVID-19---11-March-2020. (accessed on 1 January 2022).
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 10 April 2022).
- Our World in Data. Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations. (accessed on 10 April 2022).
- WHO. Status of COVID-19 Vaccines within WHO EUL/PQ Evaluation Process. Available online: https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVID_VAX_18February2022.pdf (accessed on 10 April 2022).
- WHO. COVID-19 Vaccine Tracker and Landscape. Geneva: World Health Organization. 19 October 2020. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 10 April 2022).
- Rotshild, V.; Hirsh-Raccah, B.; Miskin, I.; Muszkat, M.; Matok, I. Comparing the clinical efficacy of COVID-19 vaccines: A systematic review and network meta-analysis. Sci. Rep. 2021, 11, 22777. [Google Scholar] [CrossRef] [PubMed]
- The Institute for Health Metrics and Evaluation (IHME). COVID-19 Vaccine Efficacy Summary. 10 January 2022. Available online: https://www.healthdata.org/covid/COVID-19-vaccine-efficacy-summary (accessed on 10 April 2022).
- WHO. Achieving 70% COVID-19 Immunization Coverage by Mid-2022. Statement of the Independent Allocation of Vaccines Group (IAVG) of COVAX. 23 December 2021. Available online: https://www.who.int/news/item/23-12-2021-achieving-70-COVID-19-immunization-coverage-by-mid-2022 (accessed on 4 January 2022).
- Anderson, R.M.; Vegvari, C.; Truscott, J.; Collyer, B.S. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet 2020, 396, 1614–1616. [Google Scholar] [CrossRef]
- United Kingdom Security Agency. SARS-CoV-2 Variants of Concern and Variants under Investigation in England. UK Security Agency: Technical Briefing 33. 23 December 2021. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1043807/technical-briefing-33.pdf (accessed on 5 April 2022).
- Collie, S.; Moultrie, H.; Bekker, L.-G.; Gray, G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N. Engl. J. Med. 2022, 386, 494–496. [Google Scholar] [CrossRef]
- Goldberg, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. COVID vaccine immunity is wanning—How much does it matter? Nature 2021, 597, 606–607. [Google Scholar] [CrossRef]
- Velavan, T.P.; Pollard, A.J.; Kremsner, P.C. Herd immunity and vaccination of children for COVID-19. Int. J. Infect. Dis. 2020, 98, 14–15. [Google Scholar] [CrossRef]
- Committee on Infectious Diseases. COVID-19 Vaccines in Children and Adolescents. Pediatrics 2022, 149, e2021054332. [Google Scholar] [CrossRef]
- Anderson, R.M.; May, R.M. Infectious Diseases in Humans. Dynamics and Control; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Fine, P.E.M. Herd Immunity: History, Theory, Practice. Epidemiol. Rev. 1993, 15, 265–302. [Google Scholar] [CrossRef]
- Gay, N.J. The Theory of Measles Elimination: Implications for the Design of Elimination Strategies. J. Infect. Dis. 2003, 189 (Suppl. 1), S27–S35. [Google Scholar] [CrossRef]
- Plans-Rubió, P. Evaluation of the Establishment of Herd Immunity in the Population by Means of Serological Surveys and Vaccination Coverage. Hum. Vaccines Immunother. 2012, 8, 184–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plans-Rubió, P. Vaccination Coverage for Routine Vaccines and Herd Immunity Levels against Measles and Pertussis in the World in 2019. Vaccines 2021, 9, 256. [Google Scholar] [CrossRef] [PubMed]
- Rahman, B.; Sadraddin, E.; Porreca, A. The basic reproduction number of SARS-CoV-2 in Wuhan is about to die out, how about the rest of the World? Rev. Med. Virol. 2020, 30, e2111. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gayle, A.A.; Wilder-Smith, A.; Rocklöv, J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020, 27, taaa021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiura, H.; Ito, K.; Anzai, A.; Kobayashi, T.; Piantham, C.; Rodríguez-Morales, A.J. Relative Reproduction Number of SARS-CoV-2 Omicron (B.1.1.529) Compared with Delta Variant in South Africa. J. Clin. Med. 2022, 11, 30. [Google Scholar] [CrossRef]
- Lin, Y.; Ocklöv, J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J. Travel Med. 2021, 28, 1–3. [Google Scholar] [CrossRef]
- Ito, K.; Piantham, C.; Nishiura, H. Relative instantaneous reproduction number of Omicron SARS-CoV-2 variant with respect to the Delta variant in Denmark. J. Med. Virol. 2021, 94, 2265–2268. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Ferguson, F.; Ghani, A.; Cori, A.; Hogan, A.; Hinsley, W.; Volz, E. Growth, Population Distribution and Immune Escape of the Omicron in England. Imperial College London. 16 December 2021. Available online: https://www.imperial.ac.uk/media/imperial-college/medicine/mrc-gida/2021-12-16-COVID19-Report-49.pdf (accessed on 4 January 2022).
- Cheng, H.; Peng, Z.; Luo, W.; Si, S.; Mo, M.; Zhou, H.; Xin, X.; Liu, H.; Yu, Y. Efficacy and Safety of COVID-19 Vaccines in Phase III Trials: A Meta-Analysis. Vaccines 2021, 9, 582. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Transmission dynamics and impact of pandemic influenza A (H1N1) 2009 virus. Wkly. Epidemiol. Rec. 2009, 84, 481–484. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Prevention and Control of Influenza with Vaccines. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 2010, 59, 1–62. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5908a1.htm (accessed on 7 March 2022).
- Embi, P.J.; Levy, M.E.; Naleway, A.L.; Patel, P.; Gaglani, M.; Natarajan, K.; Dascomb, K.; Ong, T.C.; Klein, N.P.; Liao, I.-C.; et al. Effectiveness of 2-Dose Vaccination with mRNA COVID-19 Vaccines Against COVID-19–Associated Hospitalizations Among Immunocompromised Adults—Nine States, January–September 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Murchu, E.O.; Byrne, P.; Carty, P.G.; De Gascun, C.; Keogan, M.; O’Neill, M.; Harrington, P.; Ryan, M. Quantifying the risk of SARS-CoV-2 reinfection over time. Rev. Med. Virol. 2022, 32, e2260. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.J.; Foulkes, S.; Charlett, A.; Atti, A.; Monk, E.J.M.; Simmons, R.; Wellington, E.; Cole, M.J.; Saei, A.; Oguti, B.; et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: A large, multicentre, prospective cohort study (SIREN). Lancet 2021, 397, 1459–1469. [Google Scholar] [CrossRef]
- Pulliam, J.R.C.; Schalkwyk, C.; Govender, N.; Gottberg, A.; Cohen, C.; Groome, M.J. Increased Risk of SARS-CoV-2 Reinfection Associated with Emergence of the Omicron Variant in South Africa. medRxiv 2021. Available online: https://www.medrxiv.org/content/10.1101/2021.11.11.21266068v2 (accessed on 7 March 2022).
- Townsend, J.P.; Hassler, H.B.; Wang, Z.; Miura, S.; Singh, J.; Kumar, S.; Ruddle, N.H.; Galvani, A.P.; Dornburg, A. The durability of immunity against reinfection by SARS-CoV-2: A comparative evolutionary study. Lancet Microbe 2021, 2, e666–e675. [Google Scholar] [CrossRef]
- Sui, Y.; Bekele, Y.; Berzofsky, J.A. Potential SARS-CoV-2 Immune Correlates of Protection in Infection and Vaccine Immunization. Pathogens 2021, 10, 138. [Google Scholar] [CrossRef]
- WHO. Strategy to Achieve Global COVID-19 Vaccination by Mid 2022. Available online: https://reliefweb.int/report/world/strategy-achieve-global-covid-19-vaccination-mid-2022 (accessed on 5 April 2022).
- Hodgson, D.; Flasche, S.; Jit, M.; Kucharski, A.J.; CMMID COVID-19 Working Group. The potential for vaccination-induced herd immunity against the SARS-CoV-2 B.1.1.7 variant. Euro Surveill. 2021, 26, 2100428. [Google Scholar] [CrossRef]
- Plans-Rubió, P. The Vaccination Coverage Required to Establish Herd Immunity against Influenza Viruses. Prev. Med. 2012, 55, 72–77. Available online: http://www.sciencedirect.com/science/article/pii/S0091743512000588 (accessed on 5 February 2022). [CrossRef]
- Plans, P. New preventive strategy to eliminate measles, mumps and rubella from Europe based on the serological assessment of herd immunity levels is the population. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 961–996. [Google Scholar] [CrossRef]
- Chenga, V.C.; Wong, S.; Chuangc, V.W.; Soa, S.Y.; Chena, J.H.; Sridhar, S.; To, K.K.; Chand, J.F.; Hunge, V.F.; Ho, P.; et al. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J. Infect. 2020, 81, 107–114. [Google Scholar] [CrossRef]
- WHO. COVID-19 Strategic Preparedness and Response Plan (SPRP). Geneva: WHO. 2021. Available online: https://www.who.int/publications/i/item/WHO-WHE-2021.02 (accessed on 10 January 2022).
- WHO. COVID-19 Vaccination—Strategic Vision for 2022. Available online: https://cdn.who.int/media/docs/default-source/immunization/sage/covid/global-COVID-19-vaccination-strategic-vision-for-2022_sage-yellow-book.pdf?sfvrsn=4827ec0d_5 (accessed on 10 March 2022).
- Anastasiou, O.E.; Heger, D. Understanding the Influence of Individual and Systemic Factors on Vaccination Take-Up in European Citizens Aged 55 or Older. Vaccines 2021, 9, 169. [Google Scholar] [CrossRef]
- Rhoda, D.A.; Prier, M.L.; Clary, C.B.; Trimner, M.K.; Velandia-Gonzalez, M.; Danovaro-Holliday, M.C.; Cutts, F.T. Using Household Surveys to Assess Missed Opportunities for Simultaneous Vaccination: Longitudinal Examples from Colombia and Nigeria. Vaccines 2021, 9, 795. [Google Scholar] [CrossRef] [PubMed]
- Hogan, A.B.; Winskill, P.; Watson, O.J.; Walker, P.G.T.; Whittaker, C.; Baguelin, M.; Haw, D.; Løchen, A.; Gaythorpe, K.A.M.; Imperial College COVID-19 Response Team; et al. Modelling the Allocation and Impact of a COVID-19 Vaccine. Imperial College London. 25 September 2020. Available online: https://www.imperial.ac.uk/media/imperial-college/medicine/mrc-gida/2020-09-25-COVID19-Report-33.pdf (accessed on 11 April 2022).
- Spinewine, A.; Pétein, C.; Evrard, P.; Vastrade, C.; Laurent, C.; Delaere, B.; Henrard, S. Attitudes towards COVID-19 Vaccination among Hospital Staff—Understanding What Matters to Hesitant People. Vaccines 2021, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Okubo, R.; Yoshioka, T.; Ohfuji, S.; Matsuo, T.; Tabuchi, T. COVID-19 Vaccine Hesitancy and Its Associated Factors in Japan. Vaccines 2021, 9, 662. [Google Scholar] [CrossRef] [PubMed]
- Popa, G.L.; Muntean, A.-A.; Muntean, M.-M.; Popa, M.I. Knowledge and Attitudes on Vaccination in Southern Romanians: A Cross-Sectional Questionnaire. Vaccines 2020, 8, 774. [Google Scholar] [CrossRef] [PubMed]
- Kerekes, S.; Ji, M.; Shih, S.-F.; Chang, H.-Y.; Harapan, H.; Rajamoorthy, Y.; Singh, A.; Kanwar, S.; Wagner, A.L. Differential Effect of Vaccine Effectiveness and Safety on COVID-19 Vaccine Acceptance across Socioeconomic Groups in an International Sample. Vaccines 2021, 9, 1010. [Google Scholar] [CrossRef] [PubMed]
- Almalki, M.J.; Alotaibi, A.A.; Alabdali, S.H.; Zaalah, A.A.; Maghfuri, M.W.; Qirati, N.H.; Jandali, Y.M.; Almalki, S.M. Acceptability of the COVID-19 Vaccine and Its Determinants among University Students in Saudi Arabia: A Cross-Sectional Study. Vaccines 2021, 9, 943. [Google Scholar] [CrossRef]
- European Medicines Agency. EMA Considerations on COVID-19 Vaccine Approval. EMA/592928/2020. Available online: https://www.ema.europa.eu/en/documents/other/ema-considerations-covid-19-vaccine-approval_en.pdf (accessed on 28 April 2022).


| Vaccine | Effectiveness (%) in Preventing | |||||
|---|---|---|---|---|---|---|
| Ancetral Variant | Delta Variant | Omicron Variant | ||||
| Severe Disease | Infection | Severe Disease | Infection | Severe Disease | Infection | |
| Moderna | 97 | 92 | 97 | 91 | 73 | 48 |
| Pfizer/BioNTech | 95 | 86 | 95 | 84 | 72 | 44 |
| Sputnik | 92 | 86 | 89 | 85 | 67 | 44 |
| Novavax | 89 | 83 | 86 | 82 | 65 | 43 |
| Covaxin | 78 | 73 | 76 | 72 | 57 | 38 |
| Oxford/Astra-Zeneca | 94 | 63 | 94 | 69 | 71 | 36 |
| Sinopharm | 73 | 68 | 71 | 67 | 53 | 35 |
| Janssen | 86 | 72 | 76 | 64 | 57 | 33 |
| CoronaVac | 50 | 47 | 49 | 46 | 37 | 24 |
| Convidecia | 66 | 62 | 64 | 61 | 48 | 22 |
| Ro of SARS-CoV-2 b | Critical Vaccination Coverage (%) a For COVID-19 Vaccination Effectiveness from 10% to 100% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% | |
| 1.1 | − | 89.1 | 45.0 | 30.1 | 22.6 | 18.1 | 15.1 | 13.0 | 11.3 | 10.1 |
| 1.25 | − | − | 99.0 | 66.2 | 49.8 | 39.8 | 33.2 | 28.5 | 24.9 | 22.2 |
| 1.5 | − | − | − | − | 82.9 | 66.4 | 55.4 | 47.5 | 41.6 | 37.0 |
| 1.75 | − | − | − | − | − | 85.4 | 71.2 | 61.1 | 53.4 | 47.5 |
| 2 | − | − | − | − | − | 99.6 | 83.1 | 71.2 | 62.3 | 55.4 |
| 2.25 | − | − | − | − | − | − | 92.3 | 79.1 | 69.3 | 61.6 |
| 2.5 | − | − | − | − | − | − | 99.7 | 85.5 | 74.8 | 66.5 |
| 2.75 | − | − | − | − | − | − | − | 90.7 | 79.3 | 70.6 |
| 3 | − | − | − | − | − | − | − | 95.0 | 83.1 | 73.9 |
| 3.25 | − | − | − | − | − | − | − | 98.6 | 86.3 | 76.8 |
| 3.5 | − | − | − | − | − | − | − | − | 89.1 | 79.2 |
| 3.75 | − | − | − | − | − | − | − | − | 91.4 | 81.3 |
| 4 | − | − | − | − | − | − | − | − | 93.5 | 83.1 |
| 4.25 | − | − | − | − | − | − | − | − | 95.3 | 84.8 |
| 4.5 | − | − | − | − | − | − | − | − | 97.0 | 86.2 |
| 4.75 | − | − | − | − | − | − | − | − | 98.4 | 87.5 |
| 5 | − | − | − | − | − | − | − | − | 99.8 | 88.7 |
| 6 | − | − | − | − | − | − | − | − | − | 92.4 |
| 7 | − | − | − | − | − | − | − | − | − | 93.8 |
| 8 | − | − | − | − | − | − | − | − | − | 98.5 |
| 9 | − | − | − | − | − | − | − | − | − | 97.0 |
| 10 | − | − | − | − | − | − | − | − | − | 99.8 |
| Ro of SARS-CoV-2 b | Critical Vaccination Coverage (%) a for COVID-19 Vaccination Effectiveness from 10% to 100% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% | |
| 1.1 | − | 60.6 | 36.4 | 26.0 | 20.2 | 16.5 | 14.0 | 12.1 | 10.7 | 9.6 |
| 1.25 | − | − | 80.0 | 57.1 | 44.4 | 36.4 | 30.8 | 26.7 | 23.5 | 21.1 |
| 1.5 | − | − | − | 95.2 | 74.1 | 60.6 | 51.3 | 44.4 | 39.2 | 35.1 |
| 1.75 | − | − | − | − | 95.2 | 77.9 | 65.9 | 57.1 | 50.4 | 45.1 |
| 2 | − | − | − | − | − | 90.9 | 76.9 | 66.7 | 58.8 | 52.6 |
| 2.25 | − | − | − | − | − | − | 85.5 | 74.1 | 65.4 | 58.5 |
| 2.5 | − | − | − | − | − | − | 92.3 | 80.0 | 70.6 | 63.2 |
| 2.75 | − | − | − | − | − | − | 97.9 | 84.8 | 74.9 | 67.0 |
| 3 | − | − | − | − | − | − | − | 88.9 | 78.4 | 70.2 |
| 3.25 | − | − | − | − | − | − | − | 92.3 | 81.4 | 72.9 |
| 3.5 | − | − | − | − | − | − | − | 95.2 | 84.0 | 75.2 |
| 3.75 | − | − | − | − | − | − | − | 97.8 | 86.3 | 77.2 |
| 4 | − | − | − | − | − | − | − | 100 | 88.2 | 78.9 |
| 4.25 | − | − | − | − | − | − | − | − | 90.0 | 80.5 |
| 4.5 | − | − | − | − | − | − | − | − | 91.5 | 81.9 |
| 4.75 | − | − | − | − | − | − | − | − | 92.9 | 83.1 |
| 5 | − | − | − | − | − | − | − | − | 94.1 | 84.2 |
| 6 | − | − | − | − | − | − | − | − | 98,0 | 87.7 |
| 7 | − | − | − | − | − | − | − | − | − | 90.2 |
| 8 | − | − | − | − | − | − | − | − | − | 92.1 |
| 9 | − | − | − | − | − | − | − | − | − | 93.6 |
| 10 | − | − | − | − | − | − | − | − | − | 94.7 |
| Ro of SARS-CoV-2 | COVID-19 Vaccination Effectiveness (%) Required to Establish Herd Immunity with 70%, 80%, and 90% Vaccination Coverage | |||||
|---|---|---|---|---|---|---|
| Vaccination Coverage a | Vaccination Coverage and 9.8% of Infections in Vaccinated Individuals b | |||||
| 70% | 80% | 90% | 70% | 80% | 90% | |
| 1.1 | 13.0 | 11.4 | 10.1 | 22.8 | 21.2 | 19.9 |
| 1.25 | 28.6 | 25.0 | 22.2 | 38.4 | 34.8 | 32.0 |
| 1.5 | 47.6 | 41.7 | 37.0 | 57.4 | 51.5 | 46.8 |
| 1.75 | 61.2 | 53.6 | 47.6 | 71.0 | 63.4 | 57.4 |
| 2 | 71.4 | 62.5 | 55.6 | 81.2 | 72.3 | 65.4 |
| 2.25 | 79.4 | 69.4 | 61.7 | 89.2 | 79.2 | 71.5 |
| 2.5 | 85.7 | 75.0 | 66.7 | 95.5 | 84.8 | 76.5 |
| 2.75 | 90.9 | 79.5 | 70.7 | − | 89.3 | 80.5 |
| 3 | 95.2 | 83.3 | 74.1 | − | 93.1 | 83.9 |
| 3.25 | 98.9 | 86.5 | 76.9 | − | 96.3 | 86.7 |
| 3.5 | − | 89.3 | 79.4 | − | 99.1 | 89.2 |
| 3.75 | − | 91.7 | 81.5 | − | − | 91.3 |
| 4 | − | 93.8 | 83.3 | − | − | 93.1 |
| 4.25 | − | 95.6 | 85.0 | − | − | 94.8 |
| 4.5 | − | 97.2 | 86.4 | − | − | 96.2 |
| 4.75 | − | 98.7 | 87.7 | − | − | 97.5 |
| 5 | − | 100.0 | 88.9 | − | − | 98.7 |
| 6 | − | − | 92.6 | − | − | − |
| 7 | − | − | 95.2 | − | − | − |
| 8 | − | − | 97,2 | − | − | − |
| 9 | − | − | 98,8 | − | − | − |
| 10 | − | − | 100.0 | − | − | − |
| Vaccine | Ro of SARS-CoV-2 Blocked by COVID-19 Vaccination Programs Achieving 70%, 80% and 90% Vaccination Coverage a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vaccination Coverage | Vaccination Coverage | Vaccination Coverage | |||||||
| 70% | 80% | 90% | 70% | 80% | 90% | 70% | 80% | 90% | |
| Ancestral Variant | Delta Variant | Omicron Variant | |||||||
| Ro | Ro | Ro | Ro | Ro | Ro | Ro | Ro | Ro | |
| Moderna | 2.36 | 2.92 | 3.84 | 2.32 | 2.85 | 3.71 | 1.37 | 1.44 | 1.52 |
| Pfizer/BioNTech | 2.14 | 2.56 | 3.18 | 2.08 | 2.46 | 3.01 | 1.31 | 1.38 | 1.44 |
| Sputnik | 2.14 | 2.56 | 3.18 | 2.11 | 2.51 | 3.09 | 1.31 | 1.38 | 1.44 |
| Novavax | 2.05 | 2.41 | 2.93 | 2.02 | 2.37 | 2.86 | 1.30 | 1.36 | 1.43 |
| Covaxin | 1.79 | 2.02 | 2.32 | 1.77 | 1.99 | 2.27 | 1.25 | 1.29 | 1.34 |
| Oxford/Astra-Zeneca | 1.59 | 1.74 | 1.92 | 1.71 | 1.90 | 2.14 | 1.22 | 1.27 | 1.31 |
| Sinopharm | 1.69 | 1.87 | 2.10 | 1.67 | 1.84 | 2.06 | 1.21 | 1.25 | 1.29 |
| Janssen | 1.77 | 1.99 | 2.27 | 1.61 | 1.77 | 1.95 | 1.19 | 1.23 | 1.26 |
| CoronaVac | 1.35 | 1.42 | 1.50 | 1.34 | 1.41 | 1.48 | 1.11 | 1.13 | 1.15 |
| Convidecia | 1.58 | 1.72 | 1.89 | 1.56 | 1.69 | 1.85 | 1.09 | 1.11 | 1.12 |
| AstraZeneca (AZ 3D) | 2.43 | 3.05 | 4.10 | 1.79 | 2.02 | 2.32 | |||
| Pfizer (PF 3D) | 2.47 | 3.13 | 4.26 | 1.89 | 2.16 | 2.53 | |||
| Vaccine | Ro of SARS-CoV-2 Blocked by COVID-19 Vaccination Programs Achieving 70%, 80%, and 90% Vaccination Coverage a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vaccination Coverage | Vaccination Coverage | Vaccination Coverage | |||||||
| 70% | 80% | 90% | 70% | 80% | 90% | 70% | 80% | 90% | |
| Ancestral Variant | Delta Variant | Omicron Variant | |||||||
| Ro | Ro | Ro | Ro | Ro | Ro | Ro | Ro | Ro | |
| Moderna | 2.81 | 3.79 | 5.81 | 2.75 | 3.68 | 5.52 | 1.51 | 1.62 | 1.76 |
| Pfizer/BioNTech | 2.51 | 3.21 | 4.42 | 2.43 | 3.05 | 4.10 | 1.45 | 1.54 | 1.66 |
| Sputnik | 2.51 | 3.21 | 4.42 | 2.47 | 3.13 | 4.26 | 1.45 | 1.54 | 1.66 |
| Novavax | 2.39 | 2.98 | 3.95 | 2.35 | 2.91 | 3.82 | 1.43 | 1.52 | 1.63 |
| Covaxin | 2.04 | 2.40 | 2.92 | 2.02 | 2.36 | 2.84 | 1.36 | 1.44 | 1.52 |
| Oxford/Astra-Zeneca | 1.79 | 2.02 | 2.31 | 1.93 | 2.23 | 2.64 | 1.34 | 1.40 | 1.48 |
| Sinopharm | 1.91 | 2.19 | 2.58 | 1.88 | 2.16 | 2.52 | 1.32 | 1.39 | 1.46 |
| Janssen | 2.02 | 2.36 | 2.84 | 1.81 | 2.05 | 2.36 | 1.30 | 1.36 | 1.42 |
| CoronaVac | 1.49 | 1.60 | 1.73 | 1.47 | 1.58 | 1.71 | 1.20 | 1.24 | 1.28 |
| Convidecia | 1.77 | 1.98 | 2.26 | 1.75 | 1.95 | 2.22 | 1.18 | 1.21 | 1.25 |
| AstraZeneca (AZ 3D) | 2.91 | 4.01 | 6.42 | 2.04 | 2.40 | 2.92 | |||
| Pfizer (PF 3D) | 2.97 | 4.14 | 6.81 | 2.17 | 2.60 | 3.26 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plans-Rubió, P. Percentages of Vaccination Coverage Required to Establish Herd Immunity against SARS-CoV-2. Vaccines 2022, 10, 736. https://doi.org/10.3390/vaccines10050736
Plans-Rubió P. Percentages of Vaccination Coverage Required to Establish Herd Immunity against SARS-CoV-2. Vaccines. 2022; 10(5):736. https://doi.org/10.3390/vaccines10050736
Chicago/Turabian StylePlans-Rubió, Pedro. 2022. "Percentages of Vaccination Coverage Required to Establish Herd Immunity against SARS-CoV-2" Vaccines 10, no. 5: 736. https://doi.org/10.3390/vaccines10050736
APA StylePlans-Rubió, P. (2022). Percentages of Vaccination Coverage Required to Establish Herd Immunity against SARS-CoV-2. Vaccines, 10(5), 736. https://doi.org/10.3390/vaccines10050736






