Course of Fecal Calprotectin after mRNA SARS-CoV-2 Vaccination in Patients with Inflammatory Bowel Diseases
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Study Population
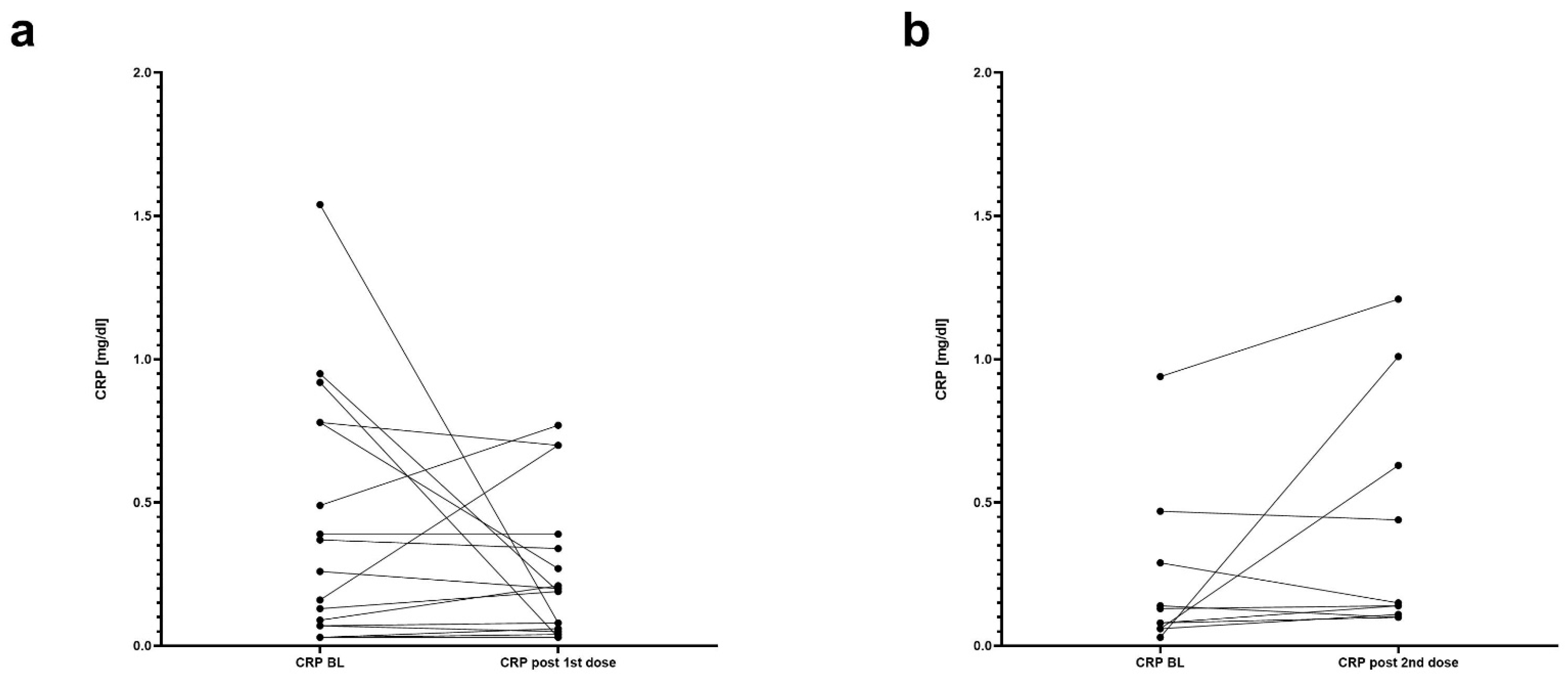
3.2. The Course of Fecal Calprotectin and Association with IBD-Related Adverse Events
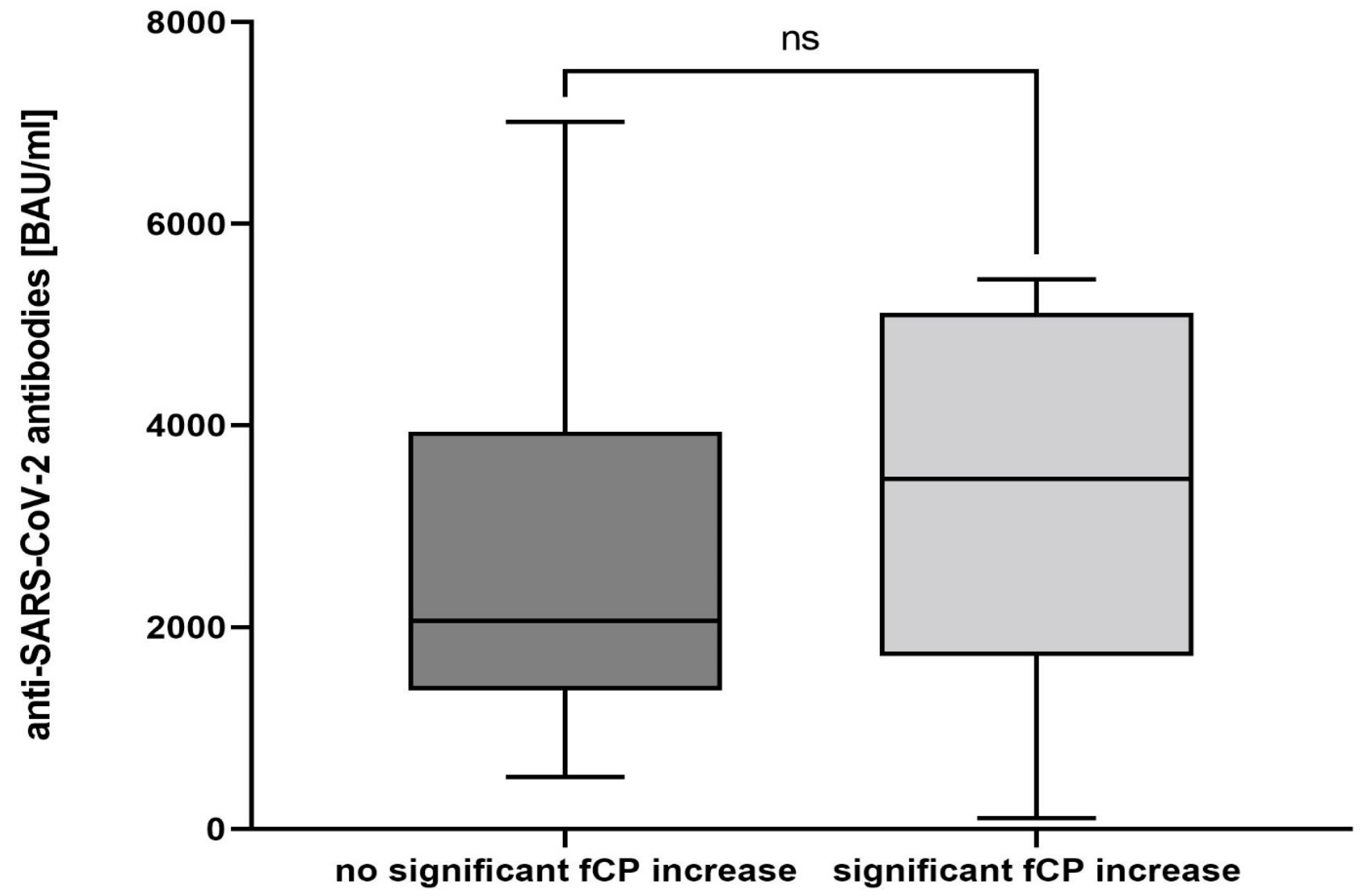
3.3. Exploratory Outcomes
3.4. SARS-CoV-2 Antibody Testing
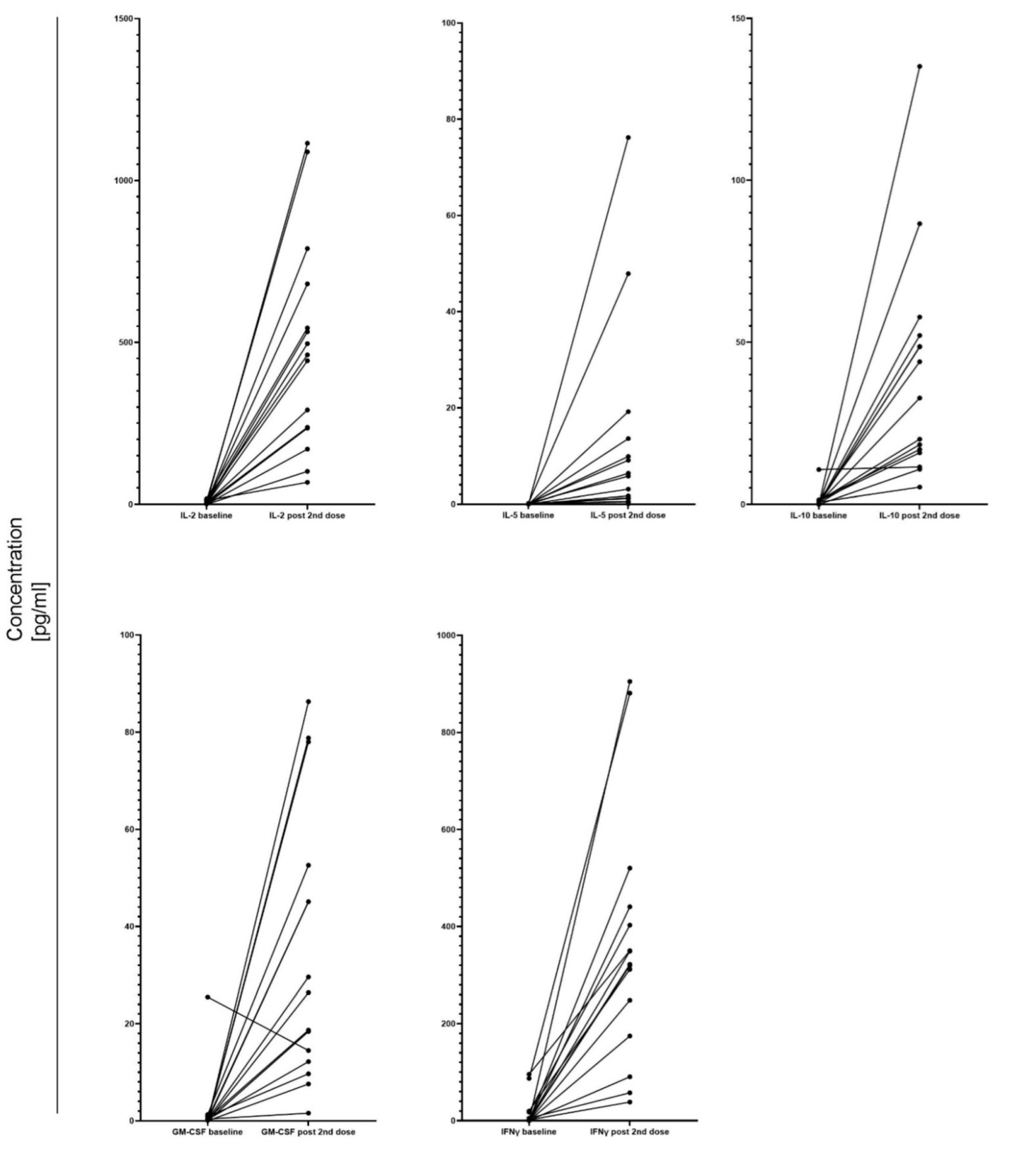
3.5. Cellular Response after COVID-19 Vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burke, K.E.; Kochar, B.; Allegretti, J.R.; Winter, R.W.; Lochhead, P.; Khalili, H.; Colizzo, F.P.; Hamilton, M.J.; Chan, W.W.; Ananthakrishnan, A.N. Immunosuppressive Therapy and Risk of COVID-19 Infection in Patients With Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2021, 27, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Brenner, E.J.; Ungaro, R.C.; Gearry, R.B.; Kaplan, G.G.; Kissous-Hunt, M.; Lewis, J.D.; Ng, S.C.; Rahier, J.F.; Reinisch, W.; Ruemmele, F.M.; et al. Corticosteroids, But Not TNF Antagonists, Are Associated With Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: Results From an International Registry. Gastroenterology 2020, 159, 481–491.e483. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Jena, A.; Kumar, M.P.; Sharma, V.; Sebastian, S. Risk and outcomes of coronavirus disease in patients with inflammatory bowel disease: A systematic review and meta-analysis. Un. Eur. Gastroenterol. J. 2021, 9, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Facciorusso, A.; Dulai, P.S.; Jairath, V.; Sandborn, W.J. Comparative Risk of Serious Infections With Biologic and/or Immunosuppressive Therapy in Patients With Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2020, 18, 69–81.e63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.Y.; Dixon, R.; Martinez Pazos, V.; Gnjatic, S.; Colombel, J.F.; Cadwell, K. Serologic Response to Messenger RNA Coronavirus Disease 2019 Vaccines in Inflammatory Bowel Disease Patients Receiving Biologic Therapies. Gastroenterology 2021, 161, 715–718.e4. [Google Scholar] [CrossRef] [PubMed]
- Watad, A.; De Marco, G.; Mahajna, H.; Druyan, A.; Eltity, M.; Hijazi, N.; Haddad, A.; Elias, M.; Zisman, D.; Naffaa, M.E.; et al. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination. Vaccines 2021, 9, 435. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA SARS-CoV-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Botwin, G.J.; Li, D.; Figueiredo, J.; Cheng, S.; Braun, J.; McGovern, D.P.B.; Melmed, G.Y. Adverse Events After SARS-CoV-2 mRNA Vaccination Among Patients With Inflammatory Bowel Disease. Am. J. Gastroenterol. 2021, 116, 1746. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef] [Green Version]
- Walsham, N.E.; Sherwood, R.A. Fecal calprotectin in inflammatory bowel disease. Clin. Exp. Gastroenterol. 2016, 9, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinisch, W.; Panaccione, R.; Bossuyt, P.; Baert, F.; Armuzzi, A.; Hébuterne, X.; Travis, S.; Danese, S.; Sandborn, W.J.; Schreiber, S.; et al. Association of Biomarker Cutoffs and Endoscopic Outcomes in Crohn’s Disease: A Post Hoc Analysis From the CALM Study. Inflamm. Bowel Dis. 2020, 26, 1562–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Leitner, J.; Mahasongkram, K.; Schatzlmaier, P.; Pfisterer, K.; Leksa, V.; Pata, S.; Kasinrerk, W.; Stockinger, H.; Steinberger, P. Differentiation and activation of human CD4 T cells is associated with a gradual loss of myelin and lymphocyte protein. Eur. J. Immunol. 2021, 51, 848–863. [Google Scholar] [CrossRef]
- Wagner, N.; Assmus, F.; Arendt, G.; Baum, E.; Baumann, U.; Bogdan, C.; Burchard, G.; Föll, D.; Garbe, E.; Hecht, J.; et al. Impfen bei Immundefizienz. Bundesgesundheitsblatt Gesundh. Gesundh. 2019, 62, 494–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyrin-Biroulet, L.; Sandborn, W.; Sands, B.E.; Reinisch, W.; Bemelman, W.; Bryant, R.V.; D’Haens, G.; Dotan, I.; Dubinsky, M.; Feagan, B.; et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015, 110, 1324–1338. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Burgueño, J.F.; Reich, A.; Hazime, H.; Quintero, M.A.; Fernandez, I.; Fritsch, J.; Santander, A.M.; Brito, N.; Damas, O.M.; Deshpande, A.; et al. Expression of SARS-CoV-2 Entry Molecules ACE2 and TMPRSS2 in the Gut of Patients With IBD. Inflamm. Bowel Dis. 2020, 26, 797–808. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487, 477–481. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, J.; Schiavon, C.R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, L.; et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ojetti, V.; Saviano, A.; Covino, M.; Acampora, N.; Troiani, E.; Franceschi, F. COVID-19 and intestinal inflammation: Role of fecal calprotectin. Dig. Liver Dis. 2020, 52, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Effenberger, M.; Grabherr, F.; Mayr, L.; Schwaerzler, J.; Nairz, M.; Seifert, M.; Hilbe, R.; Seiwald, S.; Scholl-Buergi, S.; Fritsche, G.; et al. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut 2020, 69, 1543–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Gil-Manso, S.; Carbonell, D.; López-Fernández, L.; Miguens, I.; Alonso, R.; Buño, I.; Muñoz, P.; Ochando, J.; Pion, M.; Correa-Rocha, R. Induction of High Levels of Specific Humoral and Cellular Responses to SARS-CoV-2 After the Administration of Covid-19 mRNA Vaccines Requires Several Days. Front. Immunol. 2021, 12, 726960. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mahmud, N.; Trivedi, C.; Reinisch, W.; Lewis, J.D. Risk factors for SARS-CoV-2 infection and course of COVID-19 disease in patients with IBD in the Veterans Affair Healthcare System. Gut 2021, 70, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.N.; Zhang, X.; Dai, X.; Watkins, R.; Adler, J.; Dubinsky, M.C.; Kastl, A.; Bousvaros, A.; Strople, J.A.; Cross, R.K.; et al. Impact of SARS-CoV-2 Vaccination on Inflammatory Bowel Disease Activity and Development of Vaccine-Related Adverse Events: Results From PREVENT-COVID. Inflamm. Bowel Dis. 2021, izab302. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | All Patients (N = 42) |
|---|---|
| Age [years]—median (IQR) | 41.5 (31.0–52.3) |
| Female sex—no. (%) | 19 (45.0) |
| IBD type—no. (%): | |
| -Crohn’s disease | 24 (57.1) |
| -Ulcerative colitis | 17 (40.5) |
| -IBDU | 1 (2.4) |
| Medication at the BL—no. (%): | |
| -Mesalazine | 26 (61.9) |
| -Adalimumab | 11 (26.2) |
| -Infliximab | 6 (14.3) |
| -Ustekinumab | 6 (14.0) |
| -Vedolizumab | 5 (11.9) |
| -Azathioprine | 4 (9.5) |
| -Corticosteroids | 3 (7.1) |
| -Golimumab | 3 (7.1) |
| -Methotrexate | 1 (2.4) |
| -Probiotic Escherichia coli Strain Nissle 1917 | 1 (2.4) |
| Disease duration [years]—median (IQR): | |
| -Crohn’s disease | 14.2 (6.5–20.3) |
| -Ulcerative colitis | 8.5 (4.0–23.8) |
| Clinical remission at the BL—no. (%): | |
| -Crohn’s disease | 16 (38.1) |
| -Ulcerative colitis | 4 (9.5) |
| -IBDU | 1 (2.4) |
| fCP [µg/g]—median (IQR): | |
| -baseline | 102.0 (36.5–383.0) |
| -value after the 1st dose | 54.5 (33.5–577.0) |
| -value after the 2nd dose | 174.0 (37.5–823.5) |
| CRP [mg/dL]—median (IQR): | |
| -baseline | 0.15 (0.03–0.49) |
| -value after the 1st dose | 0.19 (0.03–0.37) |
| -value after the 2nd dose | 0.15 (0.10–0.73) |
| IBD-related adverse events: | |
| -after the 1st dose | 2 (4.8) |
| -after the 2nd dose | 2 (4.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokryszka, J.; Wagner, A.; Wiedermann, U.; Tobudic, S.; Herkner, H.; Winkler, S.; Brehovsky, S.; Reinisch, W.; Novacek, G. Course of Fecal Calprotectin after mRNA SARS-CoV-2 Vaccination in Patients with Inflammatory Bowel Diseases. Vaccines 2022, 10, 759. https://doi.org/10.3390/vaccines10050759
Pokryszka J, Wagner A, Wiedermann U, Tobudic S, Herkner H, Winkler S, Brehovsky S, Reinisch W, Novacek G. Course of Fecal Calprotectin after mRNA SARS-CoV-2 Vaccination in Patients with Inflammatory Bowel Diseases. Vaccines. 2022; 10(5):759. https://doi.org/10.3390/vaccines10050759
Chicago/Turabian StylePokryszka, Jagoda, Angelika Wagner, Ursula Wiedermann, Selma Tobudic, Harald Herkner, Stefan Winkler, Sonja Brehovsky, Walter Reinisch, and Gottfried Novacek. 2022. "Course of Fecal Calprotectin after mRNA SARS-CoV-2 Vaccination in Patients with Inflammatory Bowel Diseases" Vaccines 10, no. 5: 759. https://doi.org/10.3390/vaccines10050759
APA StylePokryszka, J., Wagner, A., Wiedermann, U., Tobudic, S., Herkner, H., Winkler, S., Brehovsky, S., Reinisch, W., & Novacek, G. (2022). Course of Fecal Calprotectin after mRNA SARS-CoV-2 Vaccination in Patients with Inflammatory Bowel Diseases. Vaccines, 10(5), 759. https://doi.org/10.3390/vaccines10050759






