Abstract
A prime–boost strategy of COVID-19 vaccines brings hope to limit the spread of SARS-CoV-2, while the immunogenicity of the vaccines is waning over time. Whether a booster dose of vaccine is needed has become a widely controversial issue. However, no published meta-analysis has focused on the issue. Therefore, this study assessed the immunogenicity and safety of the different combinations of prime–boost vaccinations. Electronic databases including PubMed, the Cochrane Library, Embase, medRxiv, Wanfang and CNKI were used to retrieve the original studies. A total of 28 studies, 9 combinations of prime–boost vaccinations and 5870 subjects were included in the meta-analysis, and random effect models were used to estimate pooled immunogenicity and safety. The immunity against COVID-19 after the prime vaccination waned over time, especially in the populations primed with inactivated vaccines, in which the seropositive rate of antibodies was only 28% (95% CI: 17–40%). Booster vaccination could significantly increase the antibody responses, and heterologous immunization was more effective than homologous immunization (neutralization titers: 1.65 vs. 1.27; anti-RBD IgG: 1.85 vs. 1.15); in particular, the combination of inactivated–mRNA vaccines had the highest antibody responses (neutralization titers: MRAW = 3.64, 95% CI: 3.54–3.74; anti-RBD IgG: 3.73, 95% CI: 3.59–3.87). Moreover, compared with the initial two doses of vaccines, a booster dose did not induce additional or severe adverse events. The administration of the booster dose effectively recalled specific immune responses to SARS-CoV-2 and increased antibody levels, especially in heterologous immunization. Considering the long-term immunogenicity and vaccine equity, we suggest that now, only individuals primed with inactivated vaccines require a booster dose.
1. Introduction
The COVID-19 pandemic has led to a dramatic loss of human life and impacted the world in terms of health, society, and economy [1]. Experts pointed out that immunity through vaccination is critical to reducing the burden of disease to relieve the pressure on governments and the subsequent economic recovery [2]. Multiple effective vaccines are being deployed globally, such as CoronaVac, BNT162b2, ChAdOx1 nCoV-19 and so on. As of 1 April 2022, there were more than ten billion vaccine doses administered worldwide (Johns Hopkins University Coronavirus Resource Center, https://coronavirus.jhu.edu/map.html accessed on 30 March 2022). Nevertheless, it cannot be ignored that serum antibody levels decrease within a few months after the completion of the prime vaccination, thereby reducing its protective effect against the COVID-19 infection [3,4,5]. A meta-analysis found that vaccine efficacy or effectiveness against SARS-CoV-2 infection decreased from one month to six months after full vaccination (two weeks after the second shot of a two-dose vaccine or after a single-dose vaccine) by 21% (95% CI: 13.9–29.8) among people of all ages [6]. Moreover, despite the high coverage of the primary vaccination in some countries, the spread of COVID-19 in these countries is still not well-controlled.
Therefore, more and more countries are recommending a booster dose of a COVID-19 vaccine to the general public [7]. Since August 2021, Israel has already promoted a third dose of the BNT162b2 vaccine to all individuals aged 12 years and older [8]. Moreover, WHO also reported that about 20% of COVID-19 vaccine doses, daily, were used for booster or additional vaccination in the world [9]. However, although the calls to offer booster doses to the public are becoming frequent, some researchers remain conservative about booster vaccinations [10]. In addition, several governments are waiting for more data before making a final decision on whether to recommend a booster dose [11].
To our knowledge, although studies on COVID-19 booster vaccinations have been carried out in many countries, no comprehensive systematic review and meta-analysis has been published focusing on the immunogenicity and safety of the booster dose. Our study will be the first one summarizing the clinical trials of the COVID-19 booster dose to compare their immunogenicity and safety, providing a useful reference for the recommendation of booster vaccinations.
2. Materials and Methods
2.1. Search Strategy and Protocol
The meta-analysis was conducted in accordance with the Cochrane Handbook for Systematic Review of Interventions [12] and reported according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020 statement) [13]. The search was performed in PubMed, the Cochrane Library, Embase, medRxiv, Wanfang and CNKI to identify all published and pre-publication studies, using the key terms “COVID-19”, “vaccin*” and “booster”. Detailed search strategies for all four databases are provided in the supplementary material (Tables S1–S4).
2.2. Eligibility Criteria
The PICOS (population, intervention, comparison, outcome and study design) approach was used to define study eligibility criteria [14]:
- Population—Subjects received primary COVID-19 vaccination and had no history of laboratory-confirmed COVID-19;
- Intervention—Booster dose of the COVID-19 vaccines;
- Comparison—Before and after the booster vaccination, without a control group;
- Outcomes—Antibody responses were assessed on the basis of the increase of antibody concentrations and the levels of antibodies at 14/28 days after booster vaccination. The secondary outcome was long-term immunogenicity after prime vaccination and booster dose safety, including adverse events at the injection site and systemic adverse events.
- Study designs—The articles with a before–after study design were eligible for inclusion. Animal studies, case reports, reviews, editorials and conference abstracts were excluded. Additionally, studies were excluded if there was an overlap in subjects with another study within the same analysis.
We excluded studies that did not specify the type of COVID-19 vaccines or were not published in English or Chinese. Moreover, if the interval between the first immunogenicity blood sampling (used to evaluate the baseline antibodies) and the booster vaccination was more than seven days, studies were also excluded.
2.3. Data Extraction and Quality Assessment
Two authors (Haoyue Cheng and Zhicheng Peng) were independently responsible for data extraction and quality assessment, and disagreements were determined by the third author (Yunxian Yu). For each study, we extracted data on study characteristics (e.g., date of publication, author names, study design, sample size, country), population demographics (e.g., sex ratio, mean age, inclusion criteria, prime–boost vaccination regimen) and outcomes (including immunogenicity of booster vaccinations and incidence of adverse events).
The quality of the included studies was independently evaluated using the Newcastle–Ottawa Scale (NOS), designed for observational and non-randomized studies [15]. The NOS contains three categories (eight subcategories), with a maximum of ten stars awardable. Scores of 0–3, 4–6 and 7–10 stars were considered a low-quality study, moderate-quality study, and high-quality study, respectively.
2.4. Outcomes
Outcome measures of the meta-analysis consist of three parts: antibody responses from booster vaccination, long-term immunogenicity after prime vaccination and booster dose safety. Indicators of immunogenicity included pseudotype virus neutralization titers, anti-RBD IgG concentration and the seropositive rate of antibodies. All of the indicators were measured on day 0 and day 14/28 after the booster dose. The safety outcomes were evaluated through the incidence of adverse events, including local adverse events and systemic adverse events, extracted from the original studies. Local adverse events, including injection site pain, redness, swelling and so on, occurred at the injection site. In addition, systemic adverse events were defined as those events occurring in tissues distant from the injection site, including fever, headache, body aches, fatigue and so on [16].
2.5. Data Synthesis and Statistical Analysis
Data analysis was conducted as recommended in the Cochrane Handbook for Systematic Reviews of Interventions [12]. Before the analysis, antibody data were log-transformed (Log10) and converted to the arithmetic mean. Due to the concentrations of anti-RBD IgG being converted to the binding antibody units/mL (BAU/mL) in the original studies, we used mean difference (MD) rather than standardized mean difference to evaluate the change in antibody responses.
Forest plots were constructed showing the summary and 95% CI estimated in the meta-analysis. The magnitude of between-study heterogeneity was estimated using the I2 statistical parameter. We used random effect models with inverse variance weighting, as we expected variation in effects due to differences in study populations and the methods of antibody tests. All pooled outcomes were stratified across groups of vaccination regimens. Moreover, subgroup analyses were performed to compare the differences in antibody responses across age groups. To identify a potential publication bias, Begg’s tests were conducted with different outcomes (Table S5).
The two-independent-sample t test was used to compare the differences of immunogenicity between different groups. p < 0.05 was considered statistically significant. All the statistical analyses were conducted using R statistical software VERSION 4.0.0 (The R Project for Statistical Computing; https://www.r-project.org).
3. Results
3.1. Characteristics of the Studies
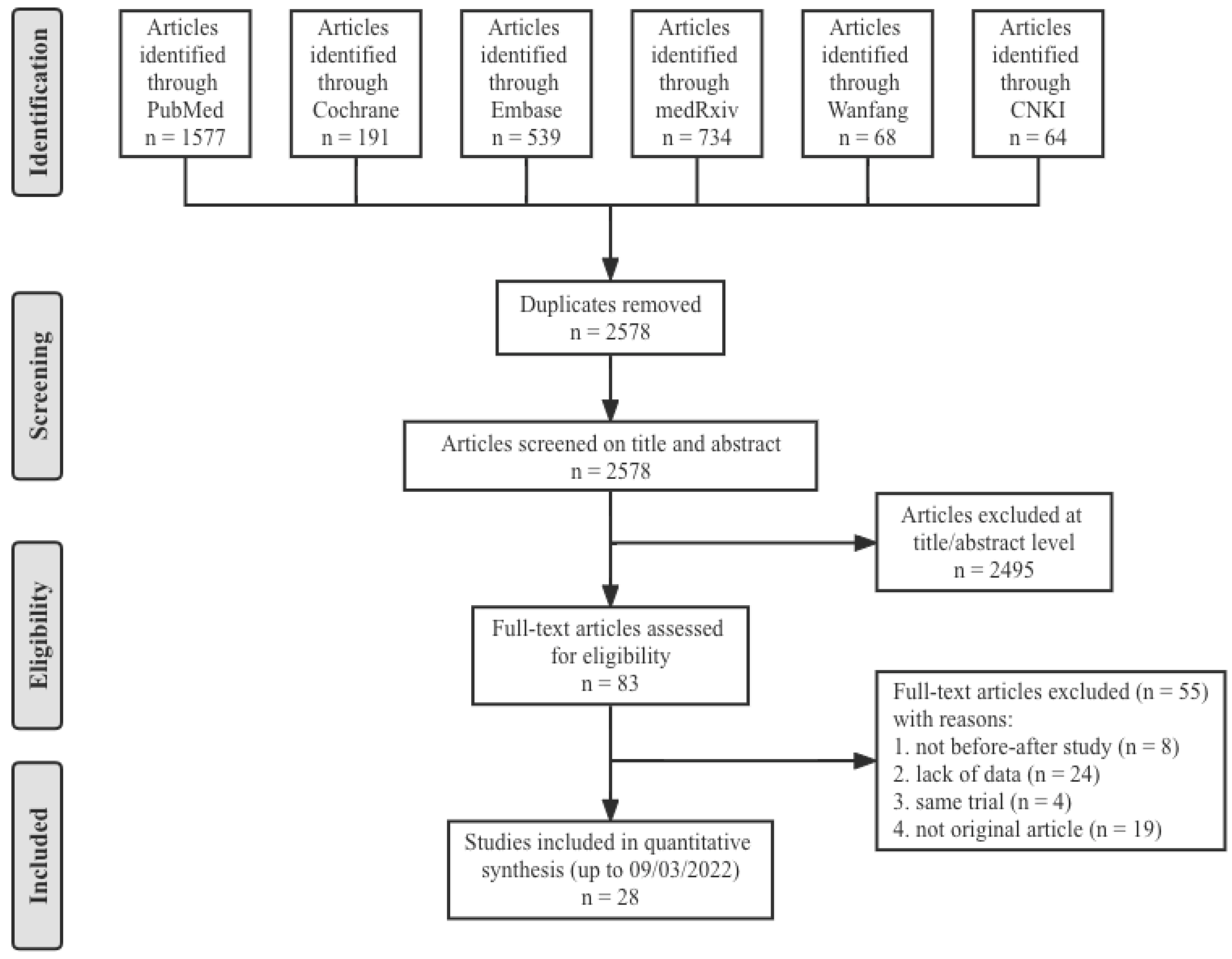
A total of 3173 articles from PubMed (1577), the Cochrane Library (191), Embase (539), medRxiv (734), Wanfang (68) and CNKI (64) were initially included. After screening 2578 titles and abstracts and 83 full-text articles, 28 studies [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44] provided data on nine combinations of COVID-19 booster vaccinations (including homologous immunization and heterologous immunization), and 5870 subjects met the eligibility criteria (Figure 1). The study populations mainly included the general population, health care workers and residents of a care home, which were all without history of laboratory-confirmed COVID-19. Therefore, the antibody responses were totally induced by the vaccines. All twenty-eight studies were before–after studies, and eight COVID-19 vaccines were involved: two inactivated vaccines (CoronaVac and BBIBP-CorV), two mRNA vaccines (mRNA-1273 and BNT162b2), three viral vector vaccines (ChAdOx1 nCoV-19, Ad26.COV2.S and Ad5-nCoV) and one recombinant protein vaccine (ZF2001). The nine specific groups of COVID-19 vaccination regimens were as follows (prime–boost): inactivated–inactivated, mRNA–mRNA, viral vector–viral vector, inactivated–mRNA, inactivated–viral vector, inactivated–recombinant protein, mRNA–viral vector, viral vector–inactivated and viral vector–mRNA. The main characteristics and NOS scores of all included studies are summarized in Table 1. The scores for study quality ranged from six to nine. Twenty-five studies were determined to be high-quality, three studies moderate-quality, and no study was judged as low-quality.
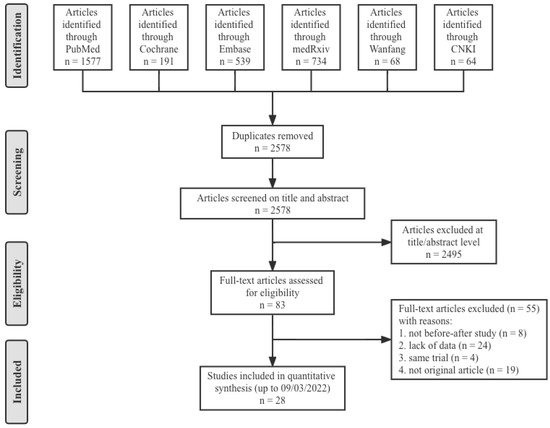
Figure 1.
Flow diagram showing the progress through the stages of meta-analysis.

Table 1.
Characteristics of the original studies included in the meta-analysis.
3.2. Long-Term Immunogenicity after Prime Vaccination
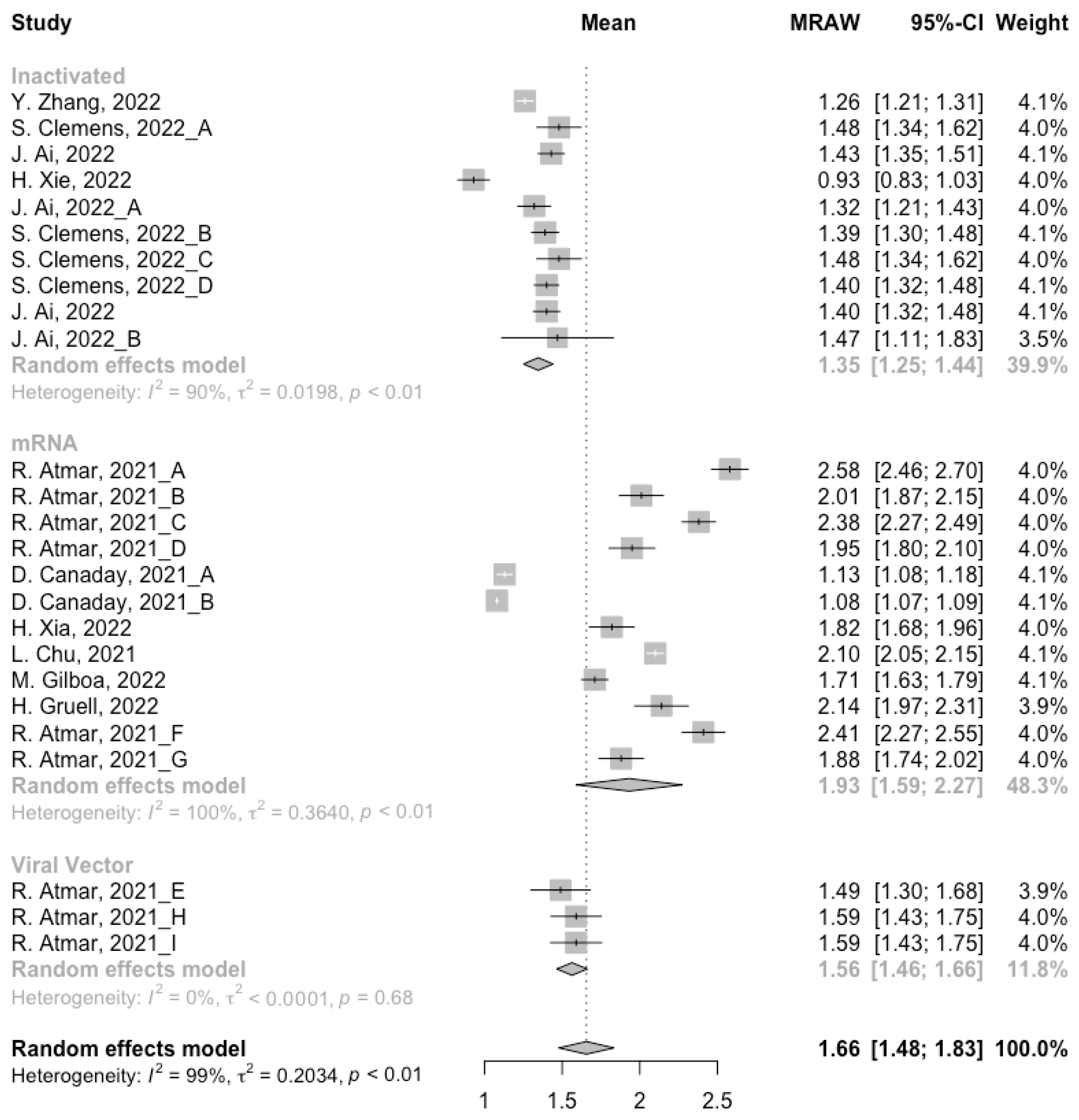
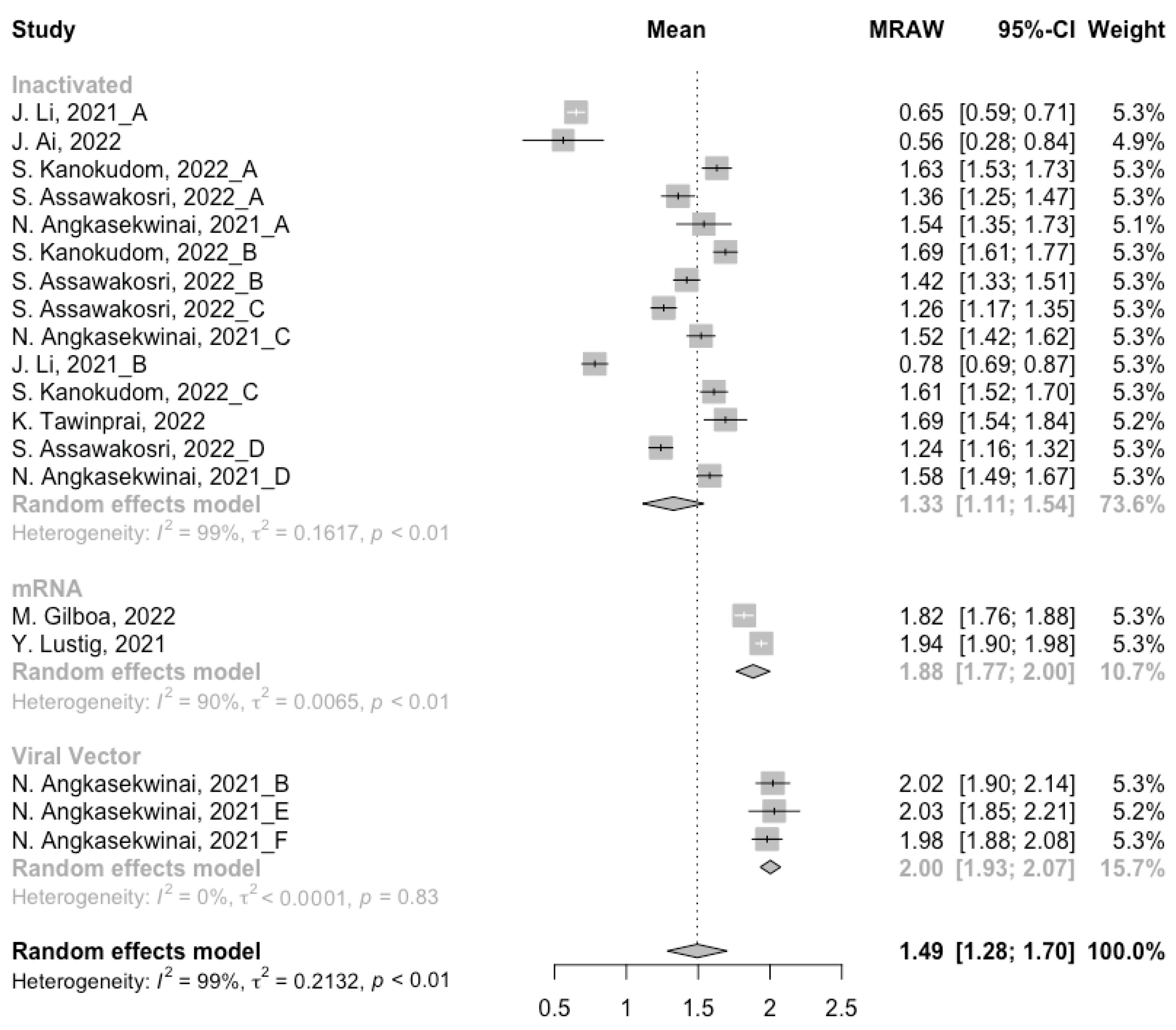
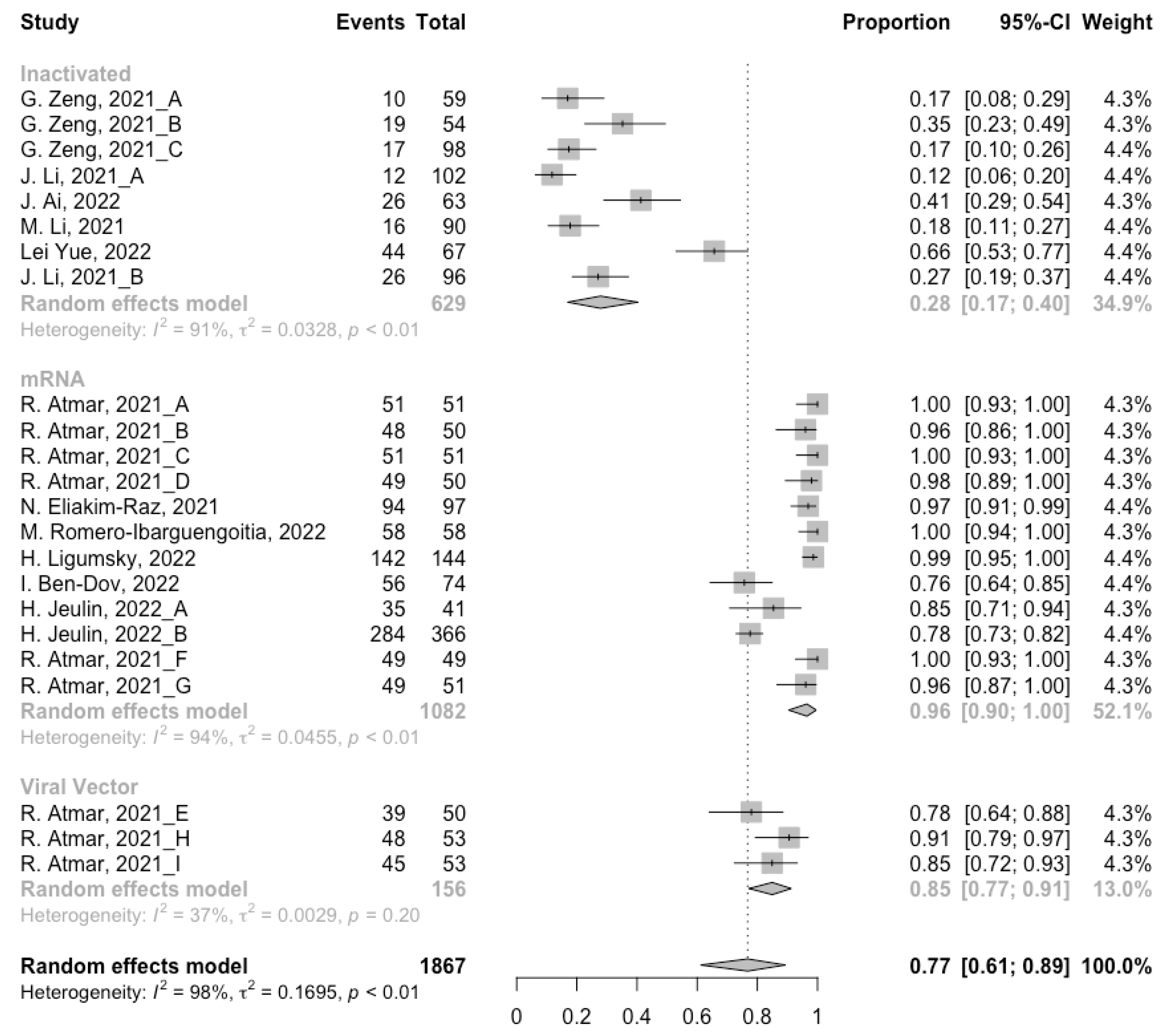
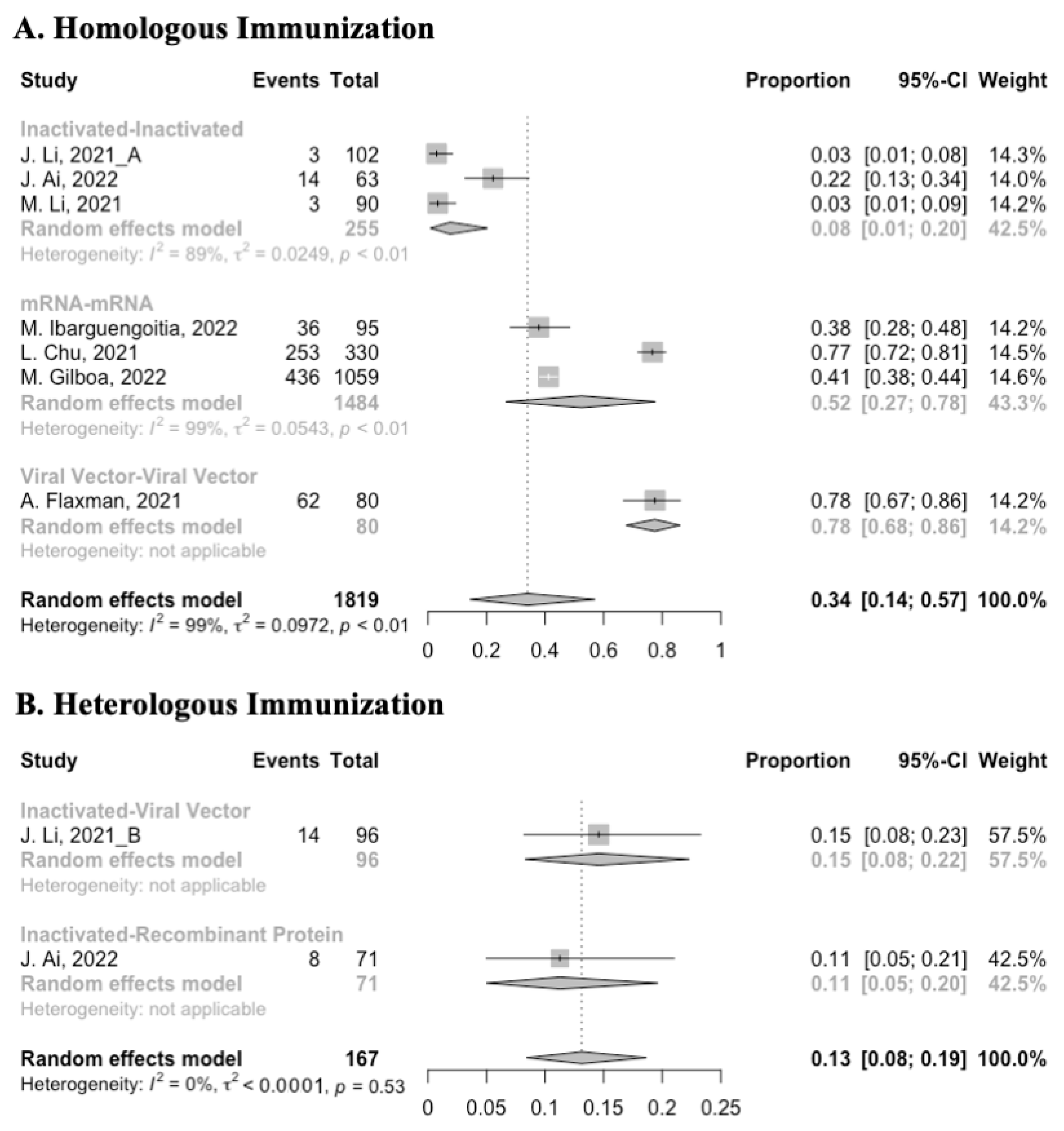
Before analyzing the antibody responses after the third dose of COVID-19 vaccines, the baseline level of the populations primed with different types of vaccines was also of concern. In general, all the populations still had immunity against COVID-19 at least 3 months after the prime vaccination (Figure 2, Figure 3 and Figure 4). However, compared with the published data on the immunogenicity of the vaccines, the results also confirmed that the antibody levels and clinical protective effect against COVID-19 waned over time after vaccinations without a booster dose [45,46]. Furthermore, when the vaccines were divided into three categories according to their types, the long-term immunogenicity of mRNA vaccines and viral vector vaccines was higher than that of inactivated vaccines. The baseline levels of neutralization antibody titers and anti-RBD IgG in the populations primed with mRNA vaccines were 1.93 (95% CI: 1.59–2.27) and 1.88 BAU/mL (95% CI: 1.77–2.00), respectively (Figure 2 and Figure 3). Moreover, the seropositive rate of antibodies in the populations primed with inactivated vaccines was 28% (95% CI: 17–40%), while that in the populations primed with mRNA vaccines was nearly 100% (Figure 4). In addition, the long-term immunogenicity of viral vector vaccines was similar to that of mRNA vaccines. Subgroup analyses by age found that there was no significant difference in antibody concentrations between young and old populations (Figure S1).
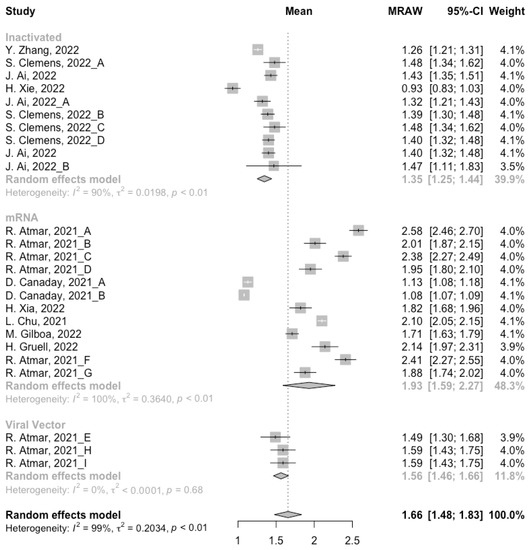
Figure 2.
Forest plot of the pooled log-transformed neutralization antibody titers before booster vaccination.
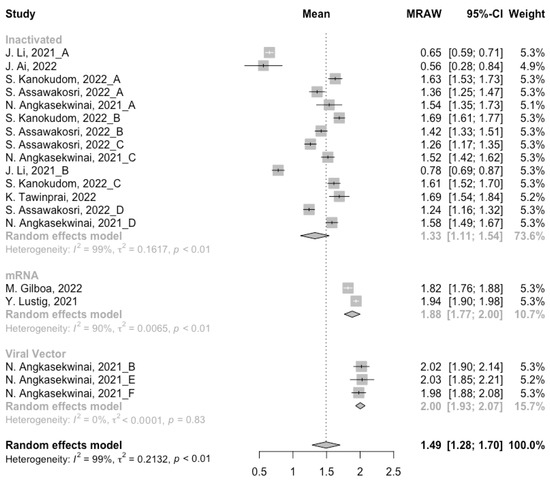
Figure 3.
Forest plot of the pooled log-transformed anti-RBD IgG before booster vaccination.
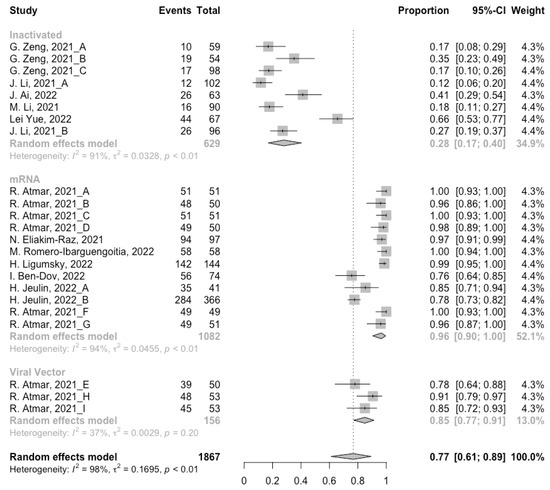
Figure 4.
Forest plot of the pooled seropositive rate of antibodies before booster vaccination.
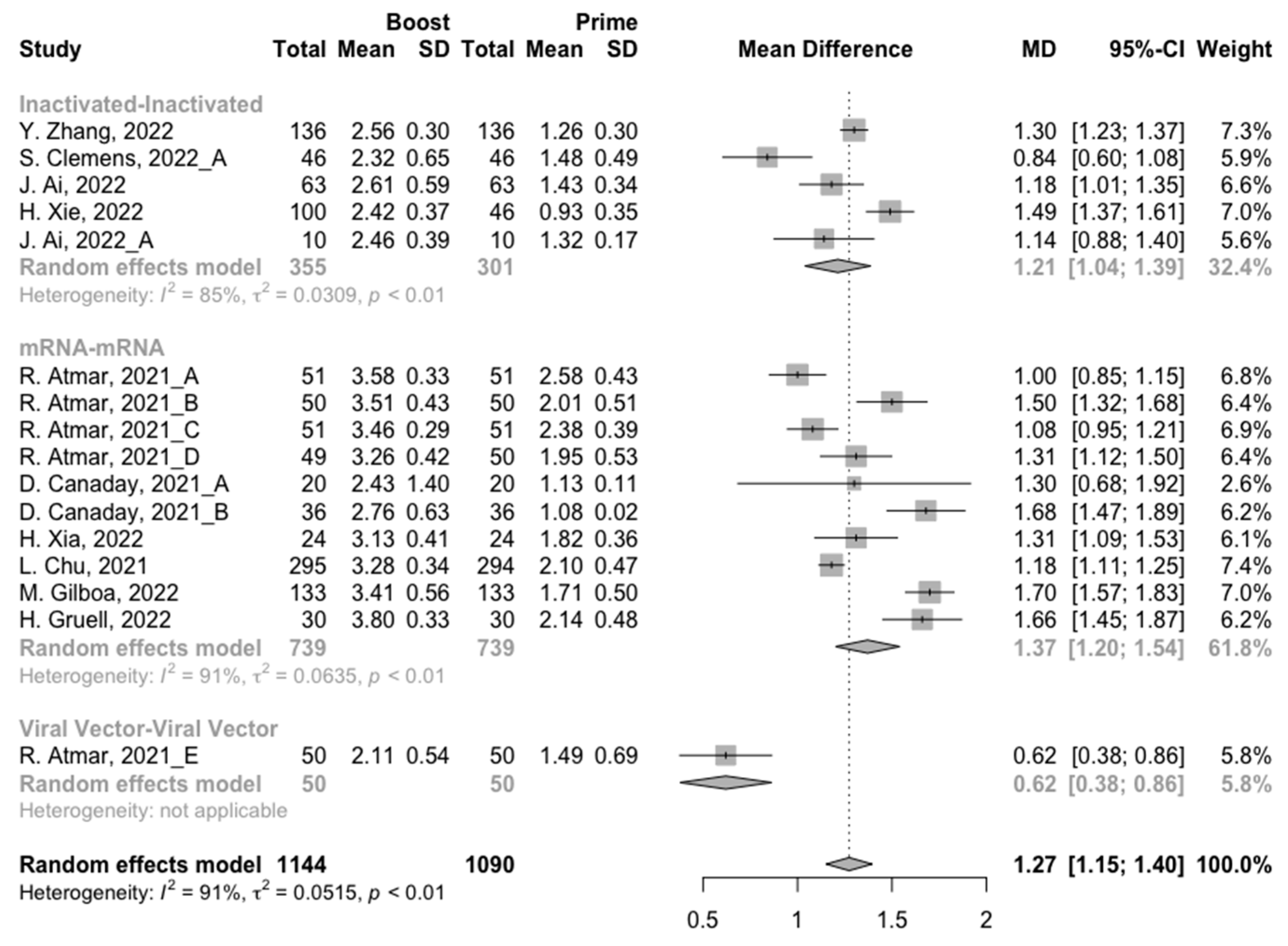
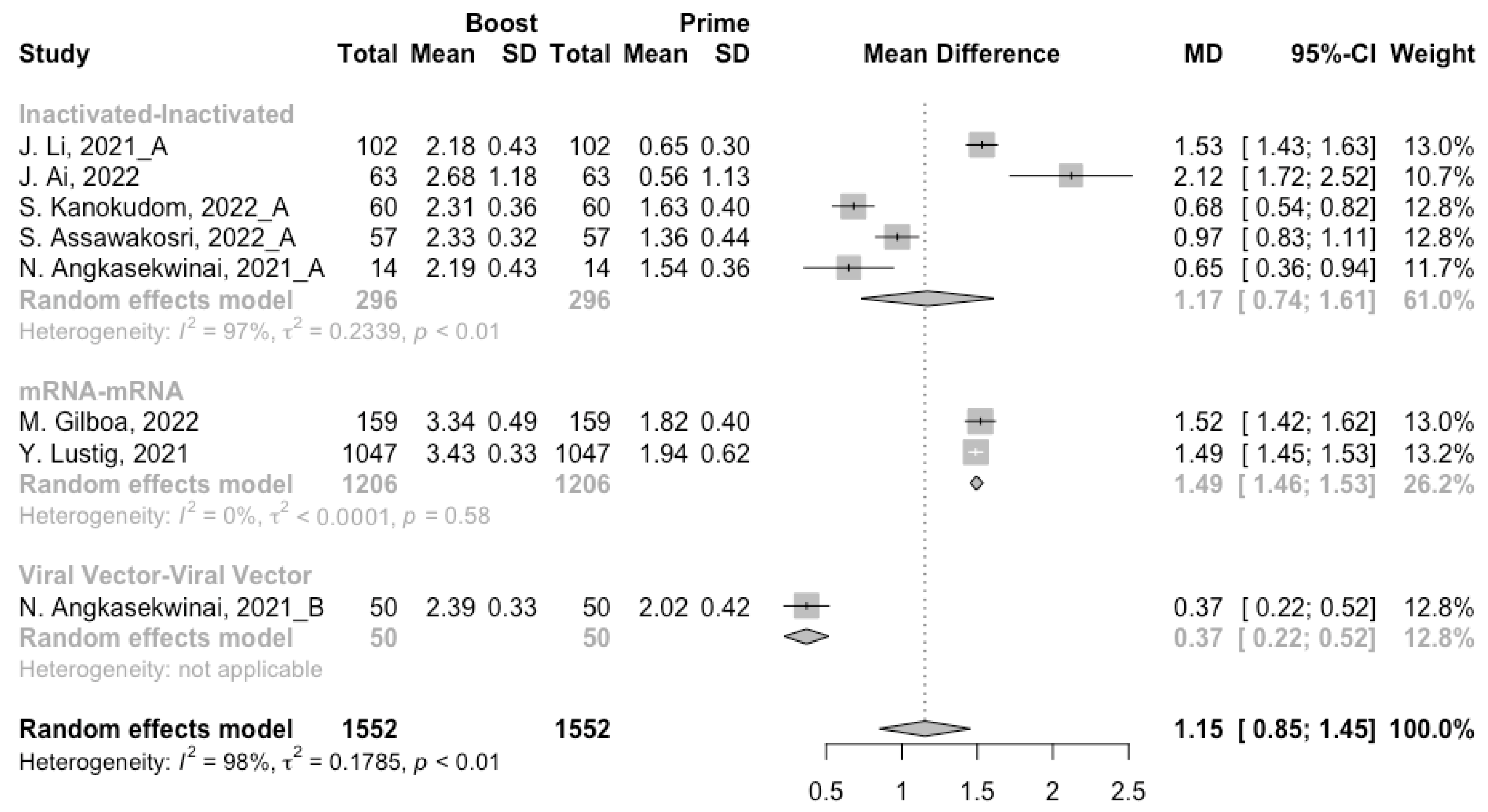
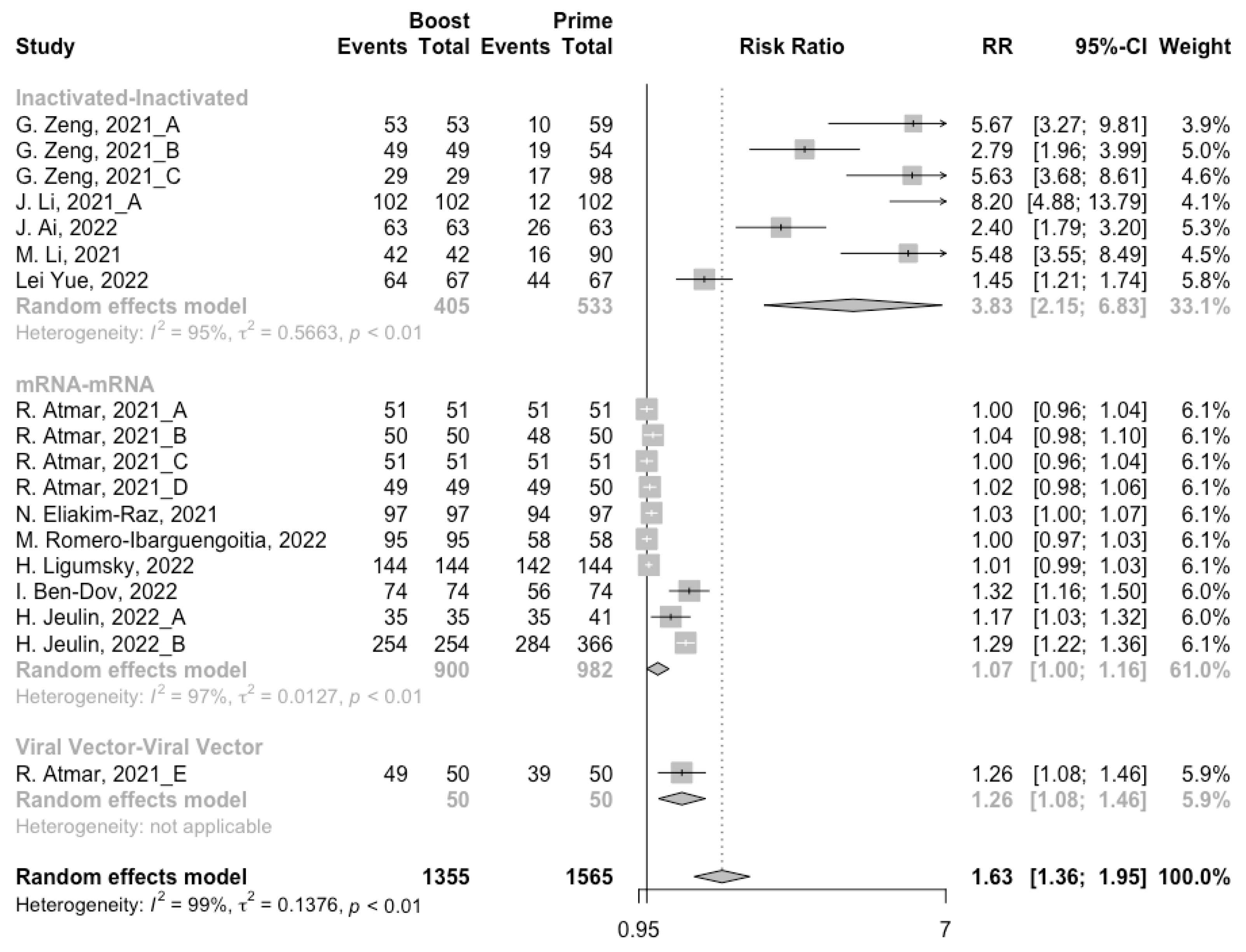
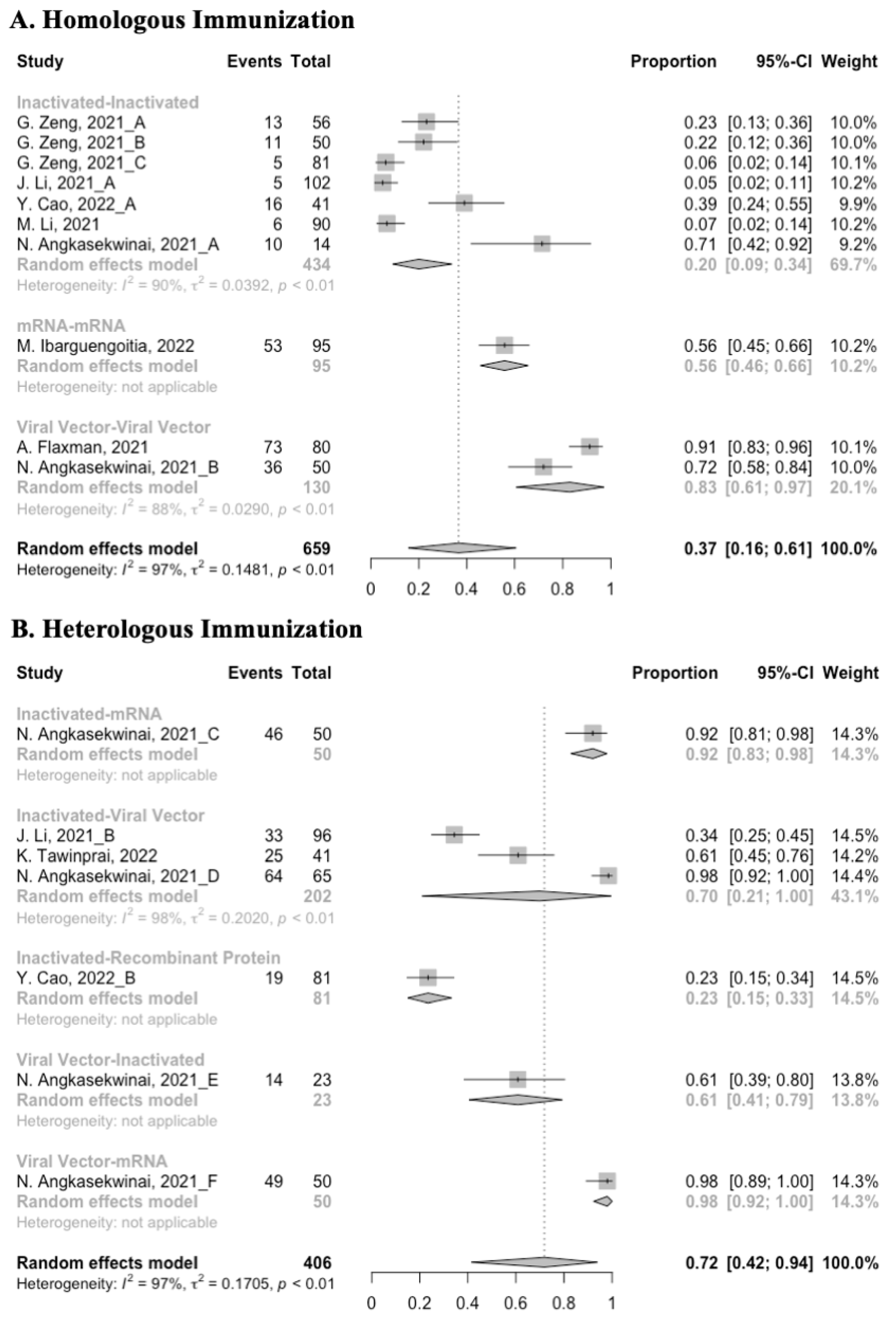
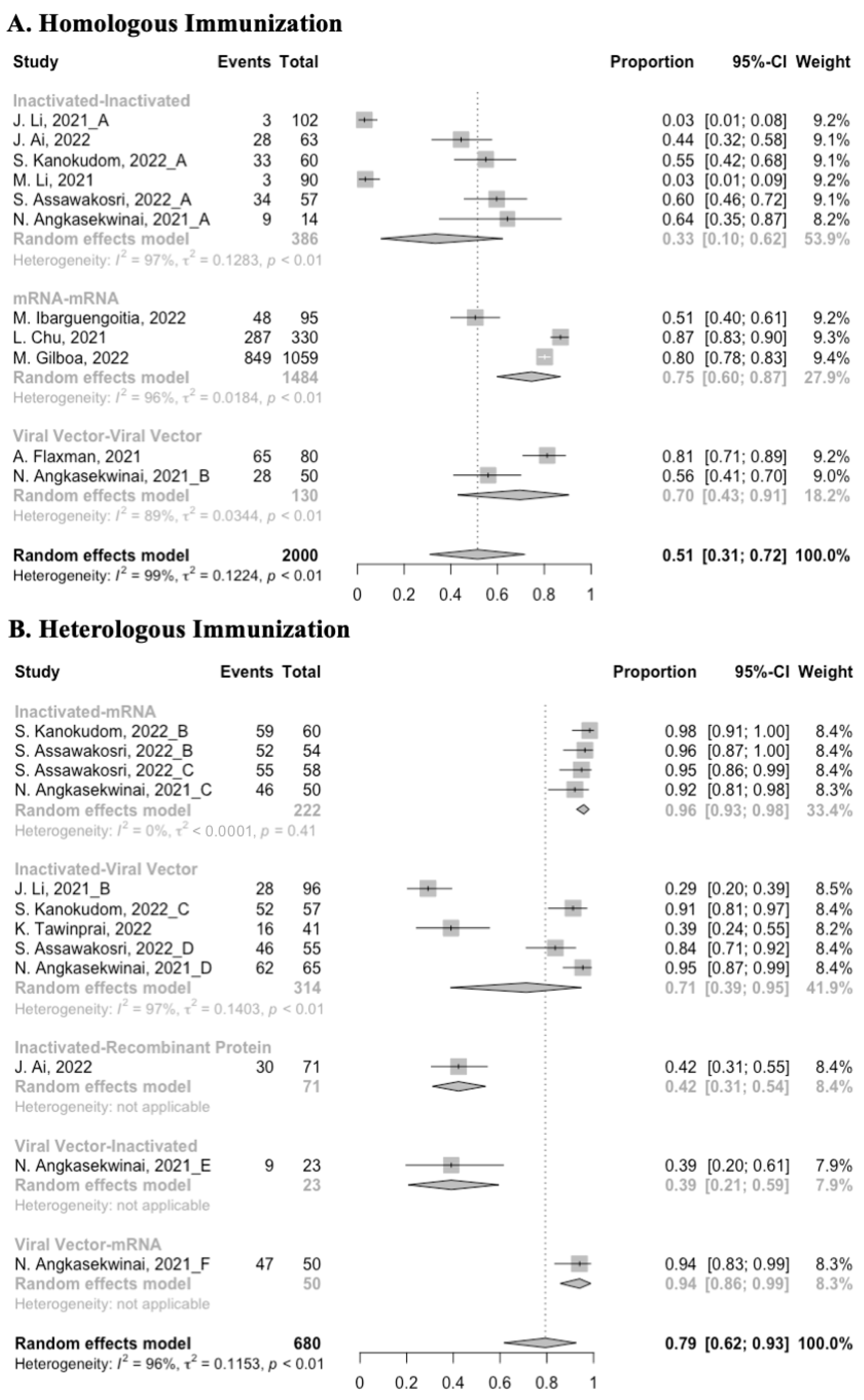
3.3. Antibody Responses after Homologous Boosters
After the homologous booster vaccines, there was a significant rise in antibody concentrations (neutralization titers: MD = 1.27, 95% CI: 1.15–1.40; anti-RBD IgG: MD = 1.15 BAU/mL, 95% CI: 0.85–1.45), and the seropositive rate of antibodies increased to almost 100% (Figure 5, Figure 6 and Figure 7). In this meta-analysis, homologous vaccination was divided into three groups: inactivated–inactivated, mRNA–mRNA and viral vector–viral vector. It is worth noting that mRNA vaccines could induce the most effective antibody responses against SARS-CoV-2 in the populations (neutralization titers: MD = 1.37, 95% CI: 1.20–1.54; anti-RBD IgG: MD = 1.49 BAU/mL, 95% CI: 1.46–1.53), while the immunogenicity of the booster dose with viral vector vaccines may not be as good as expected (neutralization titers: MD = 0.62, 95% CI: 0.38–0.86; anti-RBD IgG: MD = 0.37 BAU/mL, 95% CI: 0.22–0.52) (Figure 5 and Figure 6).
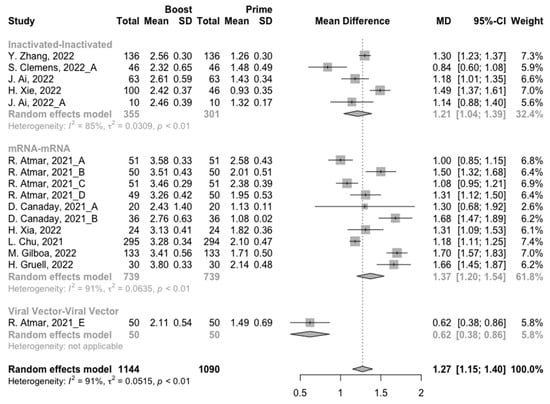
Figure 5.
Forest plot of the pooled log-transformed neutralization antibody titers before and after homologous booster vaccination.
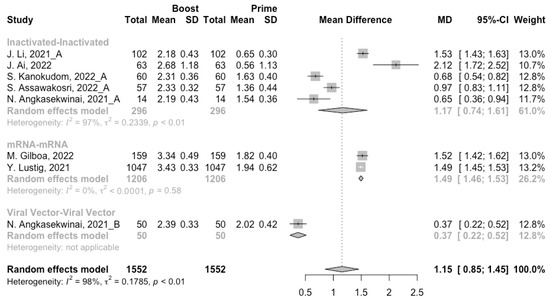
Figure 6.
Forest plot of the pooled log-transformed anti-RBD IgG before and after homologous booster vaccination.
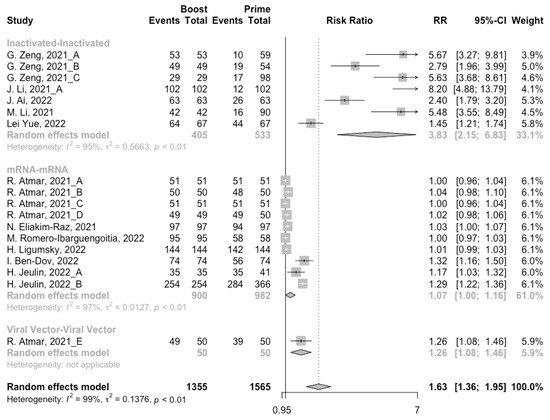
Figure 7.
Forest plot of the pooled seropositive rate of antibodies before and after homologous booster vaccination.
Ultimately, among the populations primed with mRNA vaccines, the levels of neutralizing antibody titers and anti-RBD IgG at 14/28 days after booster vaccination increased to 3.33 (95% CI: 3.20–3.47) and 3.39 BAU/mL (95% CI: 3.31–3.48), respectively (Figures S2 and S3). In addition, the final antibody concentrations of homologous mRNA prime–boost vaccination were significantly higher than that of the other two types of vaccines (p < 0.05).
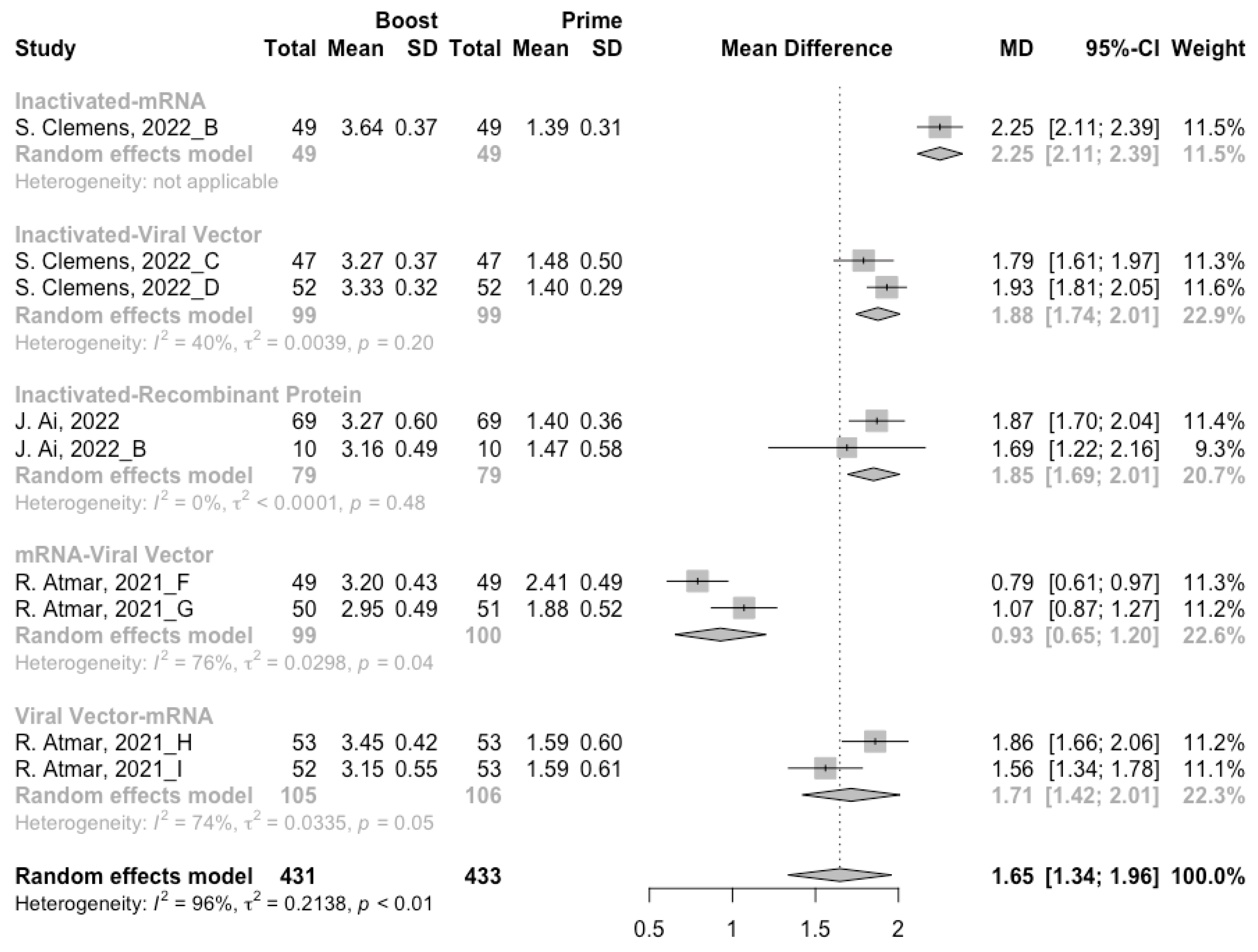
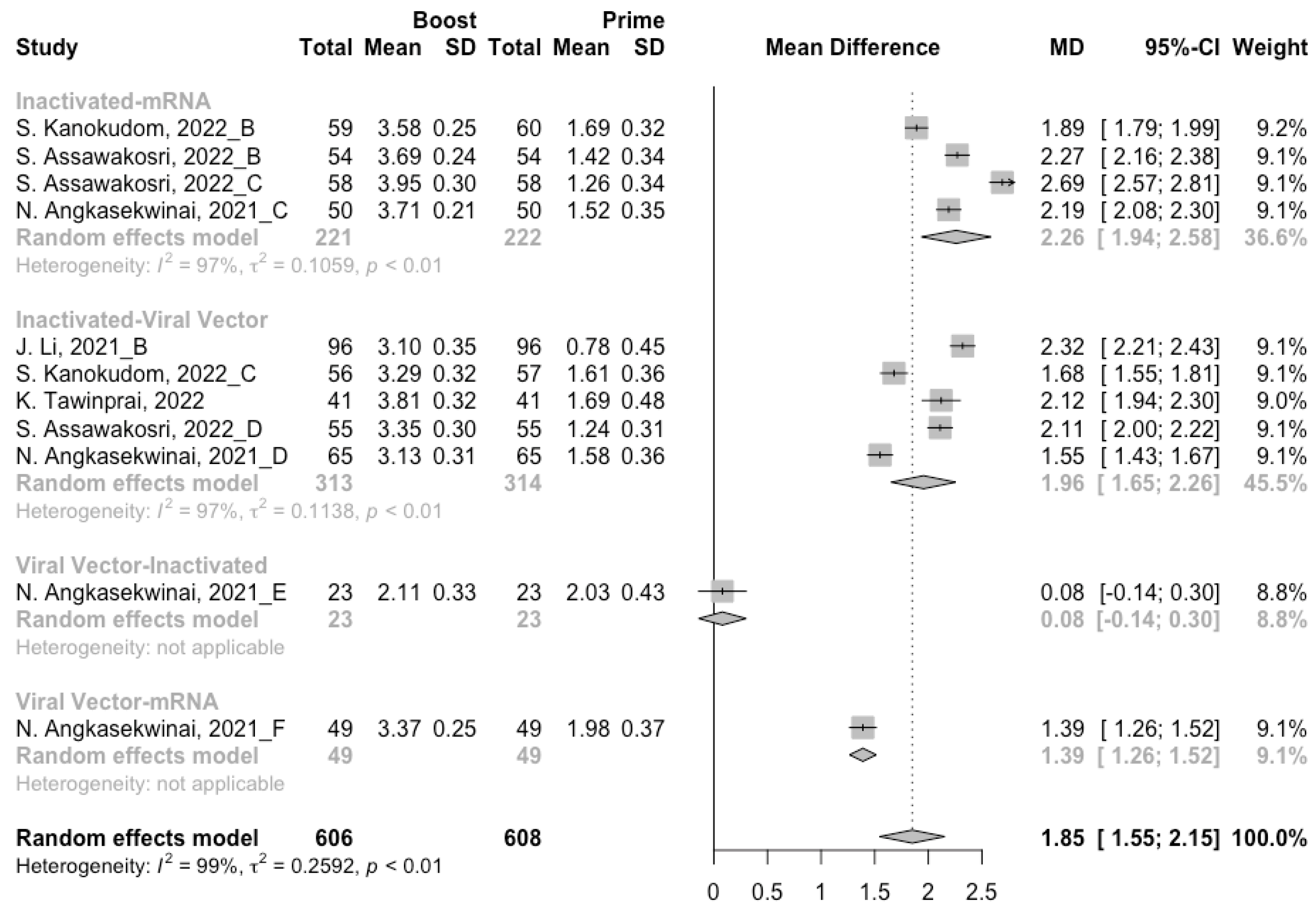
3.4. Antibody Responses after Heterologous Boosters
A total of seven groups of heterologous vaccination regimens were included in this meta-analysis, and most populations were boosted with mRNA or viral vector after inactivated prime. From baseline to day 14/28 after the heterologous booster vaccinations, all groups had a substantial rise in antibody concentrations, and the seropositive rate of antibodies increased to almost 100% (Figure 8, Figure 9 and Figure 10). In general, heterologous immunization induced significantly higher antibody responses at 14/28 days after booster vaccination compared with homologous immunization (p < 0·01): the increase in anti-RBD IgG was 1.85 BAU/mL (95% CI: 1.55–2.15) for heterologous boost versus 1.15 BAU/mL (95% CI: 0.85–1.45) for homologous boost (Figure 6 and Figure 9). We also analyzed the discrepancies between different heterologous vaccination regimens. The populations boosted with heterologous boosters after inactivated vaccines had higher increases in neutralizing antibodies and anti-RBD IgG than those primed with other types of vaccines (Figure 8 and Figure 9). In addition, the results indicated that the mRNA–viral vector group induced the lowest increase in antibody levels compared to the other groups (MD = 0.93, 95% CI: 0.65–1.20).
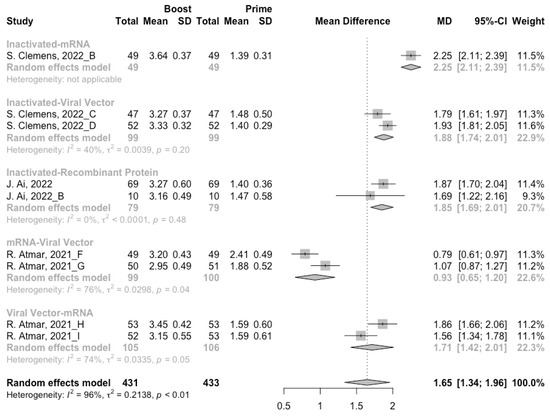
Figure 8.
Forest plot of the pooled log-transformed neutralization antibody titers before and after heterologous booster vaccination.
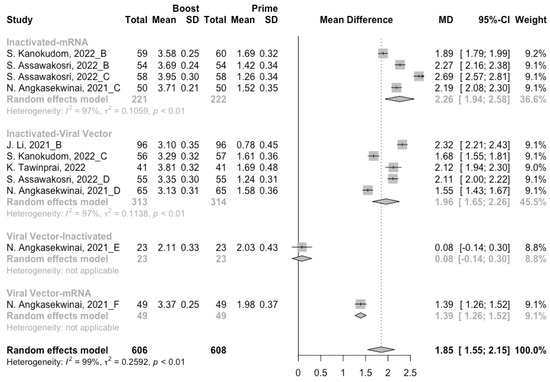
Figure 9.
Forest plot of the pooled log-transformed anti-RBD IgG before and after heterologous booster vaccination.
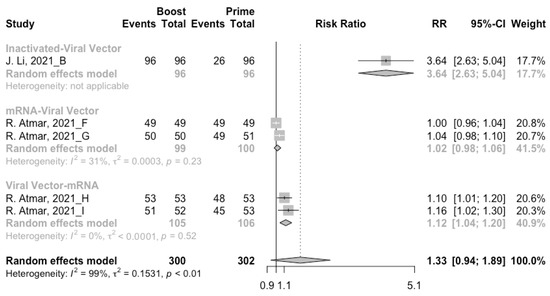
Figure 10.
Forest plot of the pooled seropositive rate of antibodies before and after heterologous booster vaccination.
At 14/28 days post-boost, the populations boosted with mRNA vaccines after an inactivated vaccine prime had the highest neutralization titers (MRAW = 3.64, 95% CI: 3.54–3.74) and anti-RBD IgG (MRAW = 3.73 BAU/mL, 95% CI: 3.59–3.87) (Figures S4 and S5). By contrast, boosting with inactivated vaccines may not be able to improve the antibody responses, but the number of relevant studies was too small to obtain a stable result. Moreover, the result of subgroup analyses implied that age did not affect the immunogenicity of the booster vaccines, regardless of homologous or heterologous immunization (Figures S6 and S7).
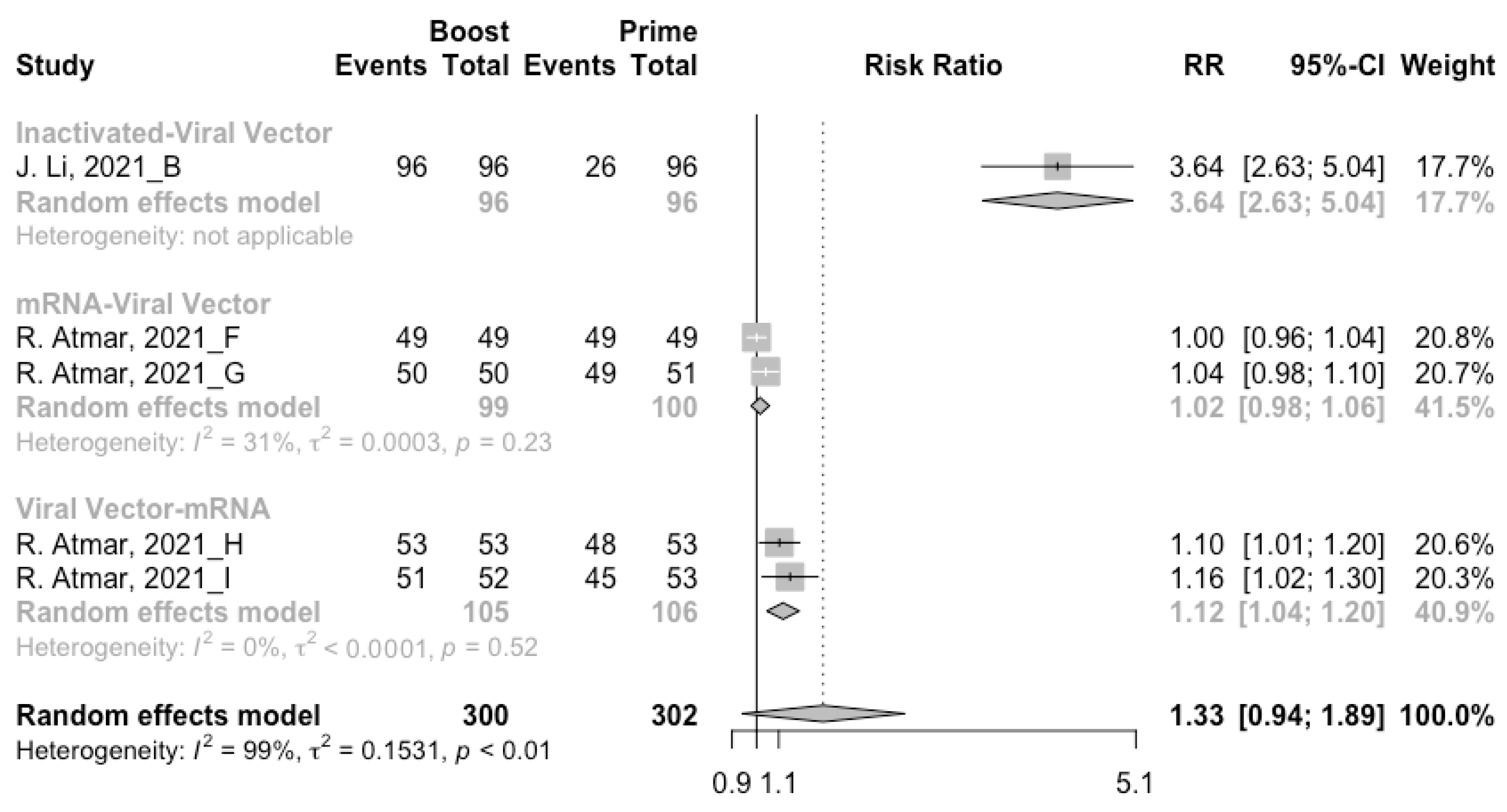
3.5. Booster Dose Safety
Concerning safety, we collected the data on total adverse events and divided them into local adverse events and systemic adverse events. The incidence of total adverse events (72% vs. 37%) and local adverse events (79% vs. 51%) was statistically higher in the homologous vaccination group compared with the heterologous vaccination group after the booster dose (Figure 11, Figure 12 and Figure 13). Moreover, no matter which type of vaccination regimen was applied, as long as the booster dose was mRNA or virus vector vaccines, the incidence of adverse events in the populations was higher. It may indicate that the adverse events were directly related to the mRNA and virus vector vaccines, but not to the prime–boost vaccination regimens.
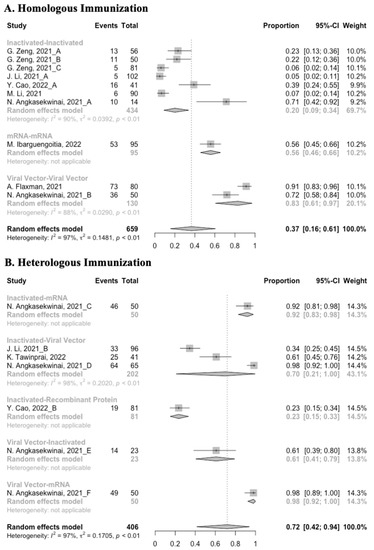
Figure 11.
Forest plot of the overall incidence of total adverse events after booster vaccination.
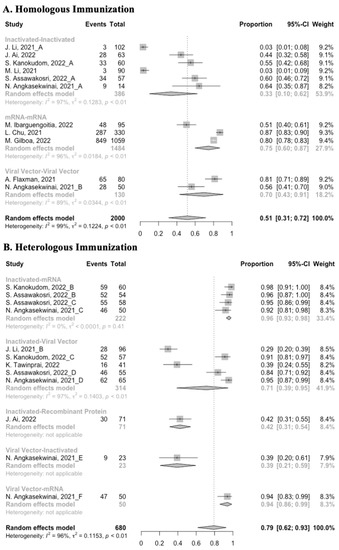
Figure 12.
Forest plot of the overall incidence of local adverse events after booster vaccination.
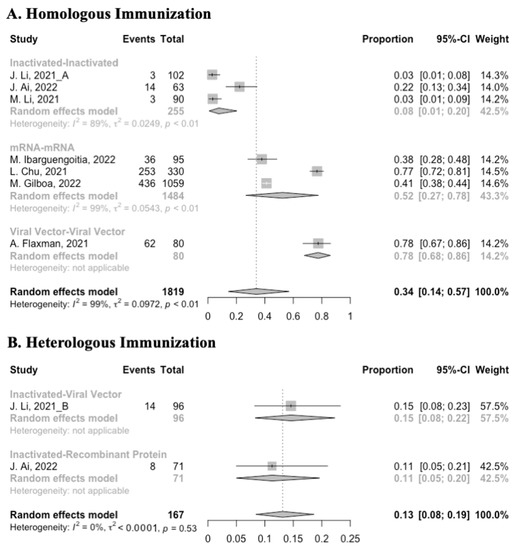
Figure 13.
Forest plot of the overall incidence of systemic adverse events after booster vaccination.
4. Discussion
In this meta-analysis of before–after studies, we found that the immunity against COVID-19 after the prime vaccination waned over time, especially the long-term immunogenicity of the inactivated vaccines. Booster vaccination could significantly ameliorate the antibody responses, and heterologous immunization was more effective than homologous immunization. A heterologous prime–boost regimen with inactivated vaccine followed by an mRNA vaccine boost markedly increased the antibody concentrations, which may be the most effective vaccination strategy. Moreover, compared with the initial two doses of vaccines, a booster dose did not induce additional or severe adverse events.
Lowering the rates of infection help break the cycle of viral transmission, which can eventually reduce cases of severe COVID-19 and death [47]. However, up to now, multiple studies have indicated a decrease in the immunogenicity of COVID-19 vaccines over time [4,5,48]. A meta-analysis evaluating four vaccines further found that, although the vaccine efficacy against severe disease remained high (≥70%) for up to 6 months after vaccination, the decline of vaccine efficacy against SARS-CoV-2 infection could not be ignored [6]. Waning antibody concentrations is a plausible explanation for the decrease in vaccine efficacy against infection and disease [47]. The results of this meta-analysis are consistent with this explanation, especially the alarming decline in the seropositive rate of antibodies of the inactivated vaccines. Moreover, Feikin et al. [6] indicated that the decrease in vaccine efficacy over time was not caused by the SARS-CoV-2 variants. To sum up, from the perspective of maintaining the immunogenicity of the COVID-19 vaccines, a booster dose is critical.
Humoral immunity and cell-mediated immunity are two types of adaptive immune responses that enable the human body to defend itself against SARS-CoV-2. However, neutralizing antibodies that can intercept viruses before they penetrate cells do not have much staying power, while cellular immune responses are longer-lasting [47]. Therefore, a booster dose is a “trigger” that can stimulate B cells to secrete more neutralizing antibodies, so as to prevent the invasion of SARS-CoV-2 [49]. The results of this meta-analysis confirmed the benefit of booster vaccinations, and it also indicated that the immunogenicity of heterologous immunization was much better. The mechanism for this difference is that using dissimilar platforms can induce protection from different pathways [50]. Different types of vaccines have their own advantages. The theoretical advantage of inactivated vaccines is that they contain additional viral proteins, such as nucleoprotein, which can potentially extend the protection beyond anti-spike protein responses [26]. The mRNA vaccines are able to elicit extremely high neutralizing and binding antibody titers (especially the anti-spike IgG), while the vector-based vaccines produce polyclonal antibodies [51,52]. Palgen et al. [53] indicated another plausible mechanism that could explain the better immunogenicity of heterologous boosters. Preexisting trained innate cells and antibodies to the same vaccine tend to impair antigen presentation in individuals boosted with homologous vaccines. However, when an unrelated heterologous vaccine is administered, cells may produce subsequent robust responses of naive cells via epigenetic reprogramming. Unfortunately, since the original studies did not provide enough data on B cell and T cell responses, the mechanism could not be verified in this meta-analysis. In addition to the type of COVID-19 booster vaccines, Chiu et al. [54] found that the order of prime–boost also mattered. In addition, the results of our study showed that the immunogenicity of the viral vector–mRNA vaccination regimen was better than that of the mRNA–viral vector vaccination regimen. Therefore, further studies are required to clarify the underlying mechanisms and the best order of vaccinations.
The meta-analysis summarized the immunogenicity and safety of the COVID-19 booster vaccines in healthy populations without a history of laboratory-confirmed COVID-19. However, it has several limitations. First, most of the original studies were limited in several countries where vaccination was widely promoted, such as China, USA and Thailand. In addition, due to government policies, individuals in a country always accepted a certain type of vaccine. Therefore, the representativeness of the meta-analysis results may be affected by race, vaccination strategy and so on. Second, the interval between prime and boost may influence the efficacy of COVID-19 vaccines [54,55]. However, most original studies did not provide information about the interval, which induced the lack of the relevant subgroup analyses. Third, when the studies were stratified by the type of vaccines, the number of studies in each group was small. Therefore, achieving adequate statistical power may be difficult, and a cautious approach in interpreting the results is warranted. Fourth, although there was some consistency between different measurement methods, specific processes and laboratory equipment varied in different studies. It may be less accurate to directly compare the immunogenicity of different studies [56].
In general, our study is the first meta-analysis confirming the immunogenicity and safety of COVID-19 booster vaccination, especially the superiority of heterologous immunization. However, any discussion around the requirement of boosters cannot be had in a vacuum. Vaccine equity remains an issue that cannot be ignored. At the current rate of vaccination, low-income countries are unable to achieve substantial protection levels until at least 2023 [57]. This situation is not conducive to controlling the worldwide spread of COVID-19 and will drive SARS-CoV-2 evolution [58]. Therefore, it is critical to find a balance between vaccine equity and booster vaccination.
5. Conclusions
There is no doubt that the administration of the booster dose effectively recalls specific immune responses to SARS-CoV-2 and increases antibody levels, and heterologous immunization is more effective than homologous immunization. Considering long-term immunogenicity and vaccine equity, we suggest that a booster dose be required in individuals primed with inactivated vaccines, while individuals primed with other types of vaccines can appropriately hold off on the administration of boosters.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines10050798/s1, Table S1: Literature search terms used for PubMed. The final search strategy applied to conduct this meta-analysis is reported at step #22; Table S2: Literature search terms used for the Cochrane Library. The final search strategy applied to conduct this meta-analysis is reported at step #26; Table S3: Literature search terms used for Embass. The final search strategy applied to conduct this meta-analysis is reported at step #20; Table S4: Literature search terms used for Medrxiv, Wanfang and CNKI; Table S5: Results of Begg’s test in different outcomes; Figure S1: Forest plot of the pooled log-transformed neutralization antibody titers before booster vaccination in different age groups; Figure S2: Forest plot of the pooled log-transformed neutralization antibody titers after homologous booster vaccination; Figure S3: Forest plot of the pooled log-transformed anti-RBD IgG after homologous booster vaccination; Figure S4: Forest plot of the pooled log-transformed neutralization antibody titers after heterologous booster vaccination; Figure S5: Forest plot of the pooled log-transformed anti-RBD IgG after heterologous booster vaccination; Figure S6: Forest plot of the pooled log-transformed neutralization antibody titers before and after homologous booster vaccination in different age groups; Figure S7: Forest plot of the pooled log-transformed neutralization antibody titers before and after heterologous booster vaccination in different age groups.
Author Contributions
Y.Y., H.C., Z.P. and M.M. contributed substantially to the study topic and designed the search strategy. S.S., X.A. and Y.Z. developed the search strategy. H.C., Z.P., P.C. and H.Z. contributed to data extraction, analysis, and interpretation. All authors wrote the first draft of the article and gave final approval of the version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Chinese National Natural Science Foundation, grant number 81973055; the National Key Research and Development Programme of China, grant number 2021YFC2701901; major research and development projects of the Zhejiang Science and Technology Department, grant number 2018C03010; the Key Laboratory of Intelligent Preventive Medicine of Zhejiang Province, grant number 2020E10004; and the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang, grant number 2019R01007.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Impact of COVID-19 on People’s Livelihoods, Their Health and Our Food Systems. Available online: https://www.who.int/news/item/13-10-2020-impact-of-covid-19-on-people%27s-livelihoods-their-health-and-our-food-systems (accessed on 30 March 2022).
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Choi, A.; Koch, M.; Wu, K.; Chu, L.; Ma, L.; Hill, A.; Nunna, N.; Huang, W.; Oestreicher, J.; Colpitts, T.; et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: An interim analysis. Nat. Med. 2021, 27, 2025–2031. [Google Scholar] [CrossRef] [PubMed]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Yasuhara, A.; Ito, M.; Akasaka, O.; Nakamura, M.; Nakachi, I.; Koga, M.; Mitamura, K.; Yagi, K.; Maeda, K.; et al. Antibody titers against SARS-CoV-2 decline, but do not disappear for several months. EClinicalMedicine 2021, 32, 100734. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Rzymski, P.; Camargo, C.A., Jr.; Fal, A.; Flisiak, R.; Gwenzi, W.; Kelishadi, R.; Leemans, A.; Nieto, J.J.; Ozen, A.; Perc, M.; et al. COVID-19 Vaccine Boosters: The Good, the Bad, and the Ugly. Vaccines 2021, 9, 1299. [Google Scholar] [CrossRef]
- Reardon, S. Will Giving COVID Booster Shots Make It Harder to Vaccinate the Rest of the World? Available online: https://www.scientificamerican.com/article/will-giving-COVID-booster-shots-make-it-harder-to-vaccinate-the-rest-of-the-world/ (accessed on 30 March 2022).
- World Health Organization. Interim Statement on Booster Doses for COVID-19 Vaccination. Available online: https://www.who.int/news/item/22-12-2021-interim-statement-on-booster-doses-for-covid-19-vaccination---update-22-december-2021 (accessed on 30 March 2022).
- Mahase, E. COVID-19: Third vaccine dose boosts immune response but may not be needed, say researchers. BMJ 2021, 373, n1659. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19 booster vaccines: What we know and who’s doing what. BMJ 2021, 374, n2082. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. 2021, 74, 790–799. [Google Scholar] [CrossRef]
- Stone, P.W. Popping the (PICO) question in research and evidence-based practice. Appl. Nurs. Res. 2002, 15, 197–198. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar]
- Centers for Disease Control and Prevention. Understanding Adverse Events and Side Effects. Available online: https://www.cdc.gov/vaccinesafety/ensuringsafety/sideeffects/index.html (accessed on 21 April 2022).
- Zeng, G.; Wu, Q.; Pan, H.; Li, M.; Yang, J.; Wang, L.; Wu, Z.; Jiang, D.; Deng, X.; Chu, K.; et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: Interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect. Dis. 2022, 22, 483–495. [Google Scholar] [CrossRef]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous COVID-19 Booster Vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hou, L.; Guo, X.; Jin, P.; Wu, S.; Zhu, J.; Pan, H.; Wang, X.; Song, Z.; Wan, J.; et al. Heterologous prime-boost immunization with CoronaVac and Convidecia. medRxiv 2021. [Google Scholar] [CrossRef]
- Flaxman, A.; Marchevsky, N.G.; Jenkin, D.; Aboagye, J.; Aley, P.K.; Angus, B.; Belij-Rammerstorfer, S.; Bibi, S.; Bittaye, M.; Cappuccini, F.; et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: A substudy of two randomised controlled trials (COV001 and COV002). Lancet 2021, 398, 981–990. [Google Scholar] [CrossRef]
- Canaday, D.H.; Oyebanji, O.A.; White, E.; Keresztesy, D.; Payne, M.; Wilk, D.; Carias, L.; Aung, H.; Denis, K.S.; Sheehan, M.L.; et al. Significantly elevated antibody levels and neutralization titers in nursing home residents after SARS-CoV-2 BNT162b2 mRNA booster vaccination. medRxiv 2021. [Google Scholar] [CrossRef]
- Cao, Y.; Hao, X.; Wang, X.; Wu, Q.; Song, R.; Zhao, D.; Song, W.; Wang, Y.; Yisimayi, A.; Wang, W.; et al. Humoral immunogenicity and reactogenicity of CoronaVac or ZF2001 booster after two doses of inactivated vaccine. Cell Res. 2022, 32, 107–109. [Google Scholar] [CrossRef]
- Eliakim-Raz, N.; Leibovici-Weisman, Y.; Stemmer, A.; Ness, A.; Awwad, M.; Ghantous, N.; Stemmer, S.M. Antibody Titers Before and After a Third Dose of the SARS-CoV-2 BNT162b2 Vaccine in Adults Aged ≥60 Years. JAMA 2021, 326, 2203–2204. [Google Scholar] [CrossRef]
- Ai, J.; Zhang, H.; Zhang, Q.; Zhang, Y.; Lin, K.; Fu, Z.; Song, J.; Zhao, Y.; Fan, M.; Wang, H.; et al. Recombinant protein subunit vaccine booster following two-dose inactivated vaccines dramatically enhanced anti-RBD responses and neutralizing titers against SARS-CoV-2 and Variants of Concern. Cell Res. 2022, 32, 103–106. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Qiao, N.; Wang, X.; Ding, L.; Zhu, X.; Liang, Y.; Han, Z.; Liu, F.; Zhang, X.; et al. Early assessment of the safety and immunogenicity of a third dose (booster) of COVID-19 immunization in Chinese adults. Front. Med. 2022, 16, 93–101. [Google Scholar] [CrossRef]
- Costa Clemens, S.A.; Weckx, L.; Clemens, R.; Almeida Mendes, A.V.; Ramos Souza, A.; Silveira, M.B.V.; da Guarda, S.N.F.; de Nobrega, M.M.; de Moraes Pinto, M.I.; Gonzalez, I.G.S.; et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): A phase 4, non-inferiority, single blind, randomised study. Lancet 2022, 399, 521–529. [Google Scholar] [CrossRef]
- Ai, J.; Zhang, Y.; Zhang, H.; Zhang, Q.; Fu, Z.; Lin, K.; Song, J.; Zhao, Y.; Fan, M.; Wang, H.; et al. Safety and immunogenicity of a third-dose homologous BBIBP-CorV boosting vaccination: Interim results from a prospective open-label study. Emerg. Microbes Infect. 2022, 11, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wen, X.; Li, J.; Chen, W.; Chen, M.; Zhang, L.; Lv, M.; Zhou, S.; Bai, S.; Zhao, W.; et al. Evaluation of Immunogenicity by Pseudovirus Neutralization Assays for SARS-CoV-2 Variants after Primary and Booster Immunization. Int. J. Infect. Dis. 2022, 117, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Kanokudom, S.; Assawakosri, S.; Suntronwong, N.; Auphimai, C.; Nilyanimit, P.; Vichaiwattana, P.; Thongmee, T.; Yorsaeng, R.; Srimuan, D.; Thatsanatorn, T.; et al. Safety and Immunogenicity of the Third Booster Dose with Inactivated, Viral Vector, and mRNA COVID-19 Vaccines in Fully Immunized Healthy Adults with Inactivated Vaccine. Vaccines 2022, 10, 86. [Google Scholar] [CrossRef]
- Xia, H.; Zou, J.; Kurhade, C.; Cai, H.; Yang, Q.; Cutler, M.; Cooper, D.; Muik, A.; Jansen, K.U.; Xie, X.; et al. Neutralization and durability of 2 or 3 doses of the BNT162b2 vaccine against Omicron SARS-CoV-2. Cell Host Microbe 2022, 30, 485–488.E3. [Google Scholar] [CrossRef]
- Li, M.; Yang, J.; Wang, L.; Wu, Q.; Wu, Z.; Zheng, W.; Wang, L.; Lu, W.; Deng, X.; Peng, C.; et al. A booster dose is immunogenic and will be needed for older adults who have completed two doses vaccination with CoronaVac: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. medRxiv 2021. [Google Scholar] [CrossRef]
- Romero-Ibarguengoitia, M.E.; Rivera-Salinas, D.; Hernández-Ruíz, Y.G.; Armendariz-Vázquez, A.G.; González-Cantú, A.; Barco-Flores, I.A.; González-Facio, R.; Montelongo-Cruz, L.P.; Del Rio-Parra, G.F.; Garza-Herrera, M.R.; et al. Effect of the third dose of BNT162b2 vaccine on quantitative SARS-CoV-2 spike 1-2 IgG antibody titers in healthcare personnel. PLoS ONE 2022, 17, e0263942. [Google Scholar] [CrossRef]
- Chu, L.; Montefiori, D.; Huang, W.; Nestorova, B.; Chang, Y.; Carfi, A.; Edwards, D.K.; Oestreicher, J.; Legault, H.; Girard, B.; et al. Immune Memory Response After a Booster Injection of mRNA-1273 for Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). medRxiv 2021. [Google Scholar] [CrossRef]
- Gilboa, M.; Mandelboim, M.; Indenbaum, V.; Lustig, Y.; Cohen, C.; Rahav, G.; Asraf, K.; Amit, S.; Jaber, H.; Nemet, I.; et al. Early Immunogenicity and Safety of the Third Dose of BNT162b2 Messenger RNA Coronavirus Disease 2019 Vaccine Among Adults Older Than 60 Years: Real-World Experience. J. Infect. Dis. 2022, 225, 785–792. [Google Scholar] [CrossRef]
- Yue, L.; Xie, T.; Yang, T.; Zhou, J.; Chen, H.; Zhu, H.; Li, H.; Xiang, H.; Wang, J.; Yang, H.; et al. A third booster dose may be necessary to mitigate neutralizing antibody fading after inoculation with two doses of an inactivated SARS-CoV-2 vaccine. J. Med. Virol. 2022, 94, 35–38. [Google Scholar] [CrossRef]
- Tawinprai, K.; Siripongboonsitti, T.; Porntharukchareon, T.; Wittayasak, K.; Thonwirak, N.; Soonklang, K.; Sornsamdang, G.; Auewarakul, C.; Mahanonda, N. Immunogenicity and safety of an intradermal fractional third dose of ChAdOx1 nCoV-19/AZD1222 vaccine compared with those of a standard intramuscular third dose in volunteers who previously received two doses of CoronaVac: A randomized controlled trial. Vaccine 2022, 40, 1761–1767. [Google Scholar] [CrossRef]
- Gruell, H.; Vanshylla, K.; Tober-Lau, P.; Hillus, D.; Schommers, P.; Lehmann, C.; Kurth, F.; Sander, L.E.; Klein, F. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat. Med. 2022, 28, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Ligumsky, H.; Dor, H.; Etan, T.; Golomb, I.; Nikolaevski-Berlin, A.; Greenberg, I.; Halperin, T.; Angel, Y.; Henig, O.; Spitzer, A.; et al. Immunogenicity and safety of BNT162b2 mRNA vaccine booster in actively treated patients with cancer. Lancet Oncol. 2022, 23, 193–195. [Google Scholar] [CrossRef]
- Ben-Dov, I.Z.; Tzukert, K.; Aharon, M.; Pri-Chen, H.; Oster, Y.; Oiknine-Djian, E.; Wolf, D.G.; Dranitzki Elhalel, M. Response to tozinameran (BNT162b2) booster in twice-vaccinated kidney transplant and maintenance dialysis patients. J. Nephrol. 2022, 35, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Zhang, H.; Zhang, Y.; Lin, K.; Zhang, Y.; Wu, J.; Wan, Y.; Huang, Y.; Song, J.; Fu, Z.; et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg. Microbes Infect. 2022, 11, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Lustig, Y.; Gonen, T.; Melzer, L.; Gilboa, M.; Indenbaum, V.; Cohen, C.; Amit, S.; Jaber, H.; Doolman, R.; Asraf, K.; et al. Superior immunogenicity and effectiveness of the 3rd BNT162b2 vaccine dose. medRxiv 2021. [Google Scholar] [CrossRef]
- Jeulin, H.; Labat, C.; Duarte, K.; Toupance, S.; Nadin, G.; Craus, D.; Georgiopoulos, I.; Gantois, I.; Goehringer, F.; Benetos, A. History of SARS-CoV-2 infection, anti-spike IgG antibody kinetics and neutralization capacities following the second and third dose of BNT162b2 vaccine in nursing home residents. medRxiv 2022. [Google Scholar] [CrossRef]
- Assawakosri, S.; Kanokudom, S.; Suntronwong, N.; Auphimai, C.; Nilyanimit, P.; Vichaiwattana, P.; Thongmee, T.; Duangchinda, T.; Chantima, W.; Pakchotanon, P.; et al. Neutralizing Activities against the Omicron Variant after a Heterologous Booster in Healthy Adults Receiving Two Doses of CoronaVac Vaccination. J. Infect. Dis. 2022, jiac092. [Google Scholar] [CrossRef]
- Angkasekwinai, N.; Niyomnaitham, S.; Sewatanon, J.; Phumiamorn, S.; Sukapirom, K.; Senawong, S.; Toh, Z.Q.; Umrod, P.; Somporn, T.; Chumpol, S.; et al. The immunogenicity and reactogenicity of four COVID-19 booster vaccinations against SARS-CoV-2 variants of concerns (Delta, Beta, and Omicron) following CoronaVac or ChAdOx1 nCoV-19 primary series. medRxiv 2022. [Google Scholar] [CrossRef]
- Naranbhai, V.; Garcia-Beltran, W.F.; Chang, C.C.; Berrios Mairena, C.; Thierauf, J.C.; Kirkpatrick, G.; Onozato, M.L.; Cheng, J.; St Denis, K.J.; Lam, E.C.; et al. Comparative Immunogenicity and Effectiveness of mRNA-1273, BNT162b2, and Ad26.COV2.S COVID-19 Vaccines. J. Infect. Dis. 2022, 225, 1141–1150. [Google Scholar] [CrossRef]
- Yuan, P.; Ai, P.; Liu, Y.; Ai, Z.; Wang, Y.; Cao, W.; Xia, X.; Zheng, J.C. Safety, Tolerability, and Immunogenicity of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. medRxiv 2020. [Google Scholar] [CrossRef]
- Dolgin, E. COVID vaccine immunity is waning—How much does that matter? Nature 2021, 597, 606–607. [Google Scholar] [CrossRef]
- Hamady, A.; Lee, J.; Loboda, Z.A. Waning antibody responses in COVID-19: What can we learn from the analysis of other coronaviruses? Infection 2022, 50, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Loubet, P.; Laureillard, D.; Martin, A.; Larcher, R.; Sotto, A. Why promoting a COVID-19 vaccine booster dose? Anaesth. Crit. Care Pain Med. 2021, 40, 100967. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Quach, T.H.T.; Tran, T.M.; Phuoc, H.N.; Nguyen, H.T.; Vo, T.K.; Vo, G.V. Reactogenicity and immunogenicity of heterologous prime-boost immunization with COVID-19 vaccine. Biomed. Pharmacother. 2022, 147, 112650. [Google Scholar] [CrossRef] [PubMed]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef]
- Barrett, J.R.; Belij-Rammerstorfer, S.; Dold, C.; Ewer, K.J.; Folegatti, P.M.; Gilbride, C.; Halkerston, R.; Hill, J.; Jenkin, D.; Stockdale, L.; et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. Med. 2021, 27, 279–288. [Google Scholar] [CrossRef]
- Palgen, J.L.; Feraoun, Y.; Dzangué-Tchoupou, G.; Joly, C.; Martinon, F.; Le Grand, R.; Beignon, A.S. Optimize Prime/Boost Vaccine Strategies: Trained Immunity as a New Player in the Game. Front. Immunol. 2021, 12, 612747. [Google Scholar] [CrossRef]
- Chiu, N.-C.; Chi, H.; Tu, Y.-K.; Huang, Y.-N.; Tai, Y.-L.; Weng, S.-L.; Chang, L.; Huang, D.T.-N.; Huang, F.-Y.; Lin, C.-Y. To mix or not to mix? A rapid systematic review of heterologous prime-boost COVID-19 vaccination. Expert Rev. Vaccines 2021, 20, 1211–1220. [Google Scholar] [CrossRef]
- Vallée, A.; Vasse, M.; Mazaux, L.; Bonan, B.; Amiel, C.; Zia-Chahabi, S.; Chan-Hew-Wai, A.; Farfour, E.; Camps, E.; Touche, P.; et al. An Immunogenicity Report for the Comparison between Heterologous and Homologous Prime-Boost Schedules with ChAdOx1-S and BNT162b2 Vaccines. J. Clin. Med. 2021, 10, 3817. [Google Scholar] [CrossRef]
- McDonald, I.; Murray, S.M.; Reynolds, C.J.; Altmann, D.M.; Boyton, R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines 2021, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Rouw, A.; Wexler, A.; Kates, J.; Michaud, J. Tracking Global COVID-19 Vaccine Equity. Available online: https://www.kff.org/coronavirus-COVID-19/issue-brief/tracking-global-COVID-19-vaccine-equity/ (accessed on 30 March 2022).
- Yeh, T.-Y.; Contreras, G.P. Full vaccination against COVID-19 suppresses SARS-CoV-2 delta variant and spike gene mutation frequencies and generates purifying selection pressure. medRxiv 2021. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).