IgG Antibody Responses and Immune Persistence of Two Doses of BBIBP-CorV Vaccine or CoronaVac Vaccine in People Living with HIV (PLWH) in Shenzhen, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Object Recruitment
2.2. Questionnaire Survey
2.3. Blood Collection and Testing
2.4. Statistical Analysis
3. Results
3.1. Demographics, HIV Characteristics and Types of Vaccinations of PLWH
3.2. The S-RBD-IgG Antibody Data of First Blood Collection at 28 ± 7 Days after PLWH and HC Received Two Doses of BBIBP-CorV Vaccine or CoronaVac Vaccine
3.3. Influence of CD4+T Cell Counts before First Dose of Vaccine on S-RBD-IgG Antibody Responses at 28 ± 7 Days after PLWH Vaccination with Two Doses of BBIBP-CorV Vaccine or CoronaVac Vaccine
3.4. The S-RBD-IgG Antibody Data Induced by Two Doses of BBIBP-CorV Vaccine or CoronaVac Vaccine in PLWH Having CD4+T Cell Counts ≥350 Cells/µL before First Dose of Vaccine and Healthy Controls
3.5. Effects of Vaccination with BBIBP-CorV Vaccine or CoronaVac Vaccine on VLs and CD4+T Cell Counts of PLWH
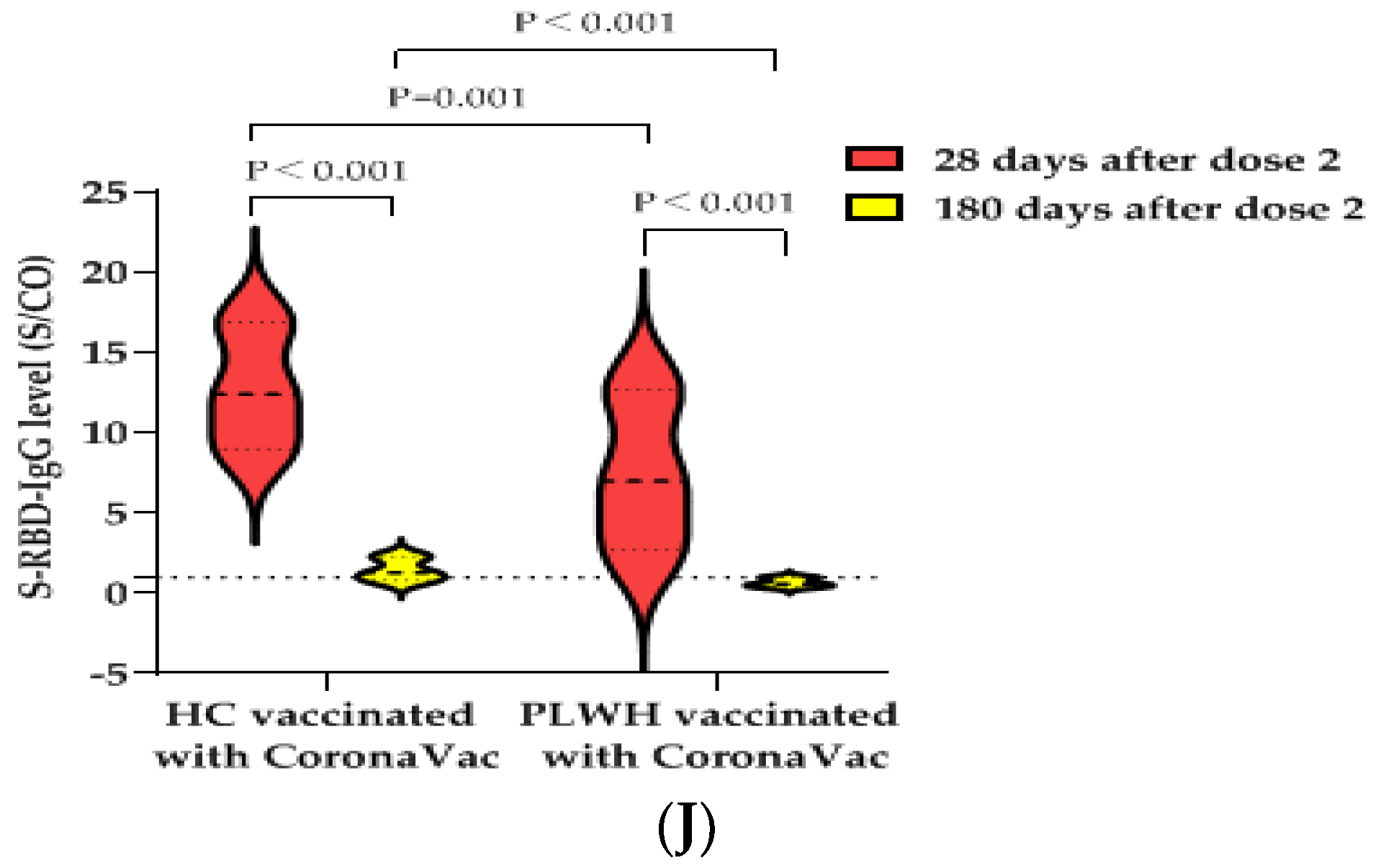
3.6. S-RBD-IgG Antibody Data of Second Blood Collection at 180 ± 20 Days after PLWH and HCs Received Two Doses of BBIBP-CorV Vaccine or CoronaVac Vaccine
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Plummer, M.M.; Pavia, C.S. COVID-19 Vaccines for HIV-Infected Patients. Viruses 2021, 13, 1890. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Guidance for COVID-19 and People with HIV. Available online: https://clinicalinfo.hiv.gov/en/guidelines/guidance-covid-19-and-people-hiv/guidance-covid-19-and-people-hiv?view=fula (accessed on 25 March 2022).
- AIDS and Hepatitis C Professional Group. Expert recommendation for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in patients with HIV infection. Chin. J. Int. Med. 2021, 7, 615–618. [Google Scholar]
- Centers for Disease Control and Prevention. COVID-19 Vaccines for Moderately to Severely Immunocompromised People. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html (accessed on 25 March 2022).
- Huang, X.; Yan, Y.; Su, B.; Xiao, D.; Yu, M.; Jin, X.; Duan, J.; Zhang, X.; Zheng, S.; Fang, Y.; et al. Comparing Immune Responses to Inactivated Vaccines against SARS-CoV-2 between People Living with HIV and HIV-Negative Individuals: A Cross-Sectional Study in China. Viruses 2022, 14, 277. [Google Scholar] [CrossRef]
- Netto, L.C.; Ibrahim, K.Y.; Picone, C.M.; Alves, A.P.; Aniceto, E.V.; Santiago, M.R.; Parmejani, P.S.; Aikawa, N.E.; Medeiros-Ribeiro, A.C.; Pasoto, S.G.; et al. Safety and immunogenicity of CoronaVac in people living with HIV: A prospective cohort study. Lancet HIV 2022, 9, e323–e331. [Google Scholar] [CrossRef]
- Ao, L.; Lu, T.; Cao, Y.; Chen, Z.; Wang, Y.; Li, Z.; Ren, X.; Xu, P.; Peng, M.; Chen, M.; et al. Safety and immunogenicity of inactivated SARS-CoV-2 vaccines in people living with HIV. Emerg. Microbes Infect. 2022, 11, 1126–1134. [Google Scholar] [CrossRef]
- Jeger-Madiot, R.; Heredia, M.; Graff-Dubois, S. Germinal centers B-cell reaction and T follicular helper cells in response to HIV-1 infection. Curr. Opin. HIV AIDS 2019, 14, 246–252. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Zhang, Y.; He, Z.; Huang, H.; Tian, X.; Wang, G.; Chen, D.; Ren, Y.; Jia, L.; Wang, W.; et al. Immunogenicity of an inactivated SARS-CoV-2 vaccine in people living with HIV-1: A non-randomized cohort study. EClinicalMedicine 2022, 43, 101226. [Google Scholar] [CrossRef]
- Liu, Y.; Han, J.; Li, X.; Chen, D.; Zhao, X.; Qiu, Y.; Zhang, L.; Xiao, J.; Li, B.; Zhao, H. COVID-19 Vaccination in People Living with HIV (PLWH) in China: A Cross Sectional Study of Vaccine Hesitancy, Safety, and Immunogenicity. Vaccines 2021, 9, 1458. [Google Scholar] [CrossRef]
- Peng, X.; Ouyang, J.; Isnard, S.; Lin, J.; Fombuena, B.; Zhu, B.; Routy, J.P. Sharing CD4+ T Cell Loss: When COVID-19 and HIV Collide on Immune System. Front. Immunol. 2020, 11, 596631. [Google Scholar] [CrossRef]
- Frater, J.; Ewer, K.J.; Ogbe, A.; Pace, M.; Adele, S.; Adland, E.; Alagaratnam, J.; Aley, P.K.; Ali, M.; Ansari, M.A.; et al. Safety and immunogenicity of the ChAdOx1nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: A single-arm substudy of a phase 2/3 clinical trial. Lancet HIV 2021, 8, e474–e485. [Google Scholar] [CrossRef]
- Bozzi, G.; Lombardi, A.; Ludovisi, S.; Muscatello, A.; Manganaro, L.; Cattaneo, D.; Gori, A.; Bandera, A. Transient increase in plasma HIV RNA after COVID-19 vaccination with mRNA-1272. Int. J. Infect. Dis. 2021, 113, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Badano, M.N.; Sabbione, F.; Keitelman, I.; Pereson, M.; Aloisi, N.; Colado, A.; Ramos, M.V.; Wilczynski, J.M.O.; Pozner, R.G.; Castillo, L.; et al. Humoral Response to the BBIBP-CorV Vaccine over Time in Healthcare Workers with or without Exposure to SARS-CoV-2. Mol. Immunol. 2022, 143, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Jeewandara, C.; Aberathna, I.; Pushpakumara, P.; Kamaladasa, A.; Guruge, D.; Wijesinghe, A.; Gunasekara, B.; Tanussiya, S.; Kuruppu, H.; Ranasinghe, T.; et al. Persistence of Antibody and T Cell Responses to the Sinopharm/BBIBP-CorV Vaccine in Sri Lankan Individuals. MedRxiv 2021. [Google Scholar] [CrossRef]
- Saeed, U.; Uppal, S.R.; Piracha, Z.Z.; Uppal, R. SARS-CoV-2 Spike Antibody Levels Trend among Sinopharm Vaccinated People. Iran. J. Public Health 2021, 50, 1486. [Google Scholar] [CrossRef]
- Assaid, N.; Arich, S.; Charoute, H.; Akarid, K.; Ezzikouri, S.; Maaroufi, A.; Sarih, M. Anti-SARS-CoV-2 Antibody Responses 5 Months Post Complete Vaccination of Moroccan Healthcare Workers. Vaccines 2022, 10, 465. [Google Scholar] [CrossRef]
- Zeng, G.; Wu, Q.; Pan, H.; Li, M.; Yang, J.; Wang, L.; Wu, Z.; Jiang, D.; Deng, X.; Chu, K.; et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: Interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect. Dis. 2022, 22, 483–495. [Google Scholar] [CrossRef]
- Kernéis, S.; Launay, O.; Turbelin, C.; Batteux, F.; Hanslik, T.; Boëlle, P.Y. Long-term immune responses to vaccination in HIV-infected patients: A systematic review and meta-analysis. Clin. Infect. Dis. 2014, 58, 1130–1139. [Google Scholar] [CrossRef]
- Costa, C.; Migliore, E.; Galassi, C.; Scozzari, G.; Ciccone, G.; Coggiola, M.; Pira, E.; Scarmozzino, A.; La Valle, G.; Cassoni, P.; et al. Factors Influencing Level and Persistence of Anti SARS-CoV-2 IgG after BNT162b2 Vaccine: Evidence from a Large Cohort of Healthcare Workers. Vaccines 2022, 10, 474. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Q.; Deng, C.; Liu, M.; Li, L.; Liu, D.; Liu, M.; Ruan, X.; Mei, J.; Mo, R.; et al. Robust induction of B cell and T cell responses by a third dose of inactivated SARS-CoV-2 vaccine. Cell Discov. 2022, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Zhang, Y.; Zhang, H.; Zhang, Q.; Fu, Z.; Lin, K.; Song, J.; Zhao, Y.; Fan, M.; Wang, H.; et al. Safety and immunogenicity of a third-dose homologous BBIBP-CorV boosting vaccination: Interim results from a prospective open-label study. Emerg. Microbes Infect. 2022, 11, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Cao, Y.; Zhou, Y.; Wu, J.; Jia, Z.; Hu, Y.; Yisimayi, A.; Fu, W.; Wang, L.; Liu, P.; et al. A third dose of inactivated vaccine augments the potency, breadth, and duration of anamnestic responses against SARS-CoV-2. MedRxiv 2021. [Google Scholar] [CrossRef]


| Variables | Statistic Value | |
|---|---|---|
| Sex (n, %) | Male | 119 (90.2%) |
| Female | 13 (9.8%) | |
| Age (years, n, %) | 20–29 | 43 (32.3%) |
| 30–39 | 61 (46.2%) | |
| 40–49 | 21 (15.9%) | |
| 50–59 | 7 (5.3%) | |
| Mode of HIV transmission (n, %) | Homosexual sexual transmission | 89 (67.4%) |
| Heterosexual sexual transmission | 42 (31.8%) | |
| Others | 1 (0.8%) | |
| ART time before first dose of vaccine | Not started | 6 (4.5%) |
| (months, n, %) | ≤6 | 10 (7.6%) |
| >6 | 116 (87.9%) | |
| VLs before the first dose of vaccine | <50 | 120 (90.9%) |
| (copies/mL, n, %) | ≥50 | 6 (4.5%) |
| Unknown | 6 (4.5%) | |
| CD4+T cell counts before first dose of vaccine (cells/µL, n, %) | 0–199 200–349 | 7 (5.3%) 19 (14.4%) |
| 350–499 | 35 (26.5%) | |
| ≥500 | 65 (49.2%) | |
| Unknown | 6 (4.5%) | |
| Types of vaccination (n, %) | BBIBP-CorV vaccine | 65 (49.2%) |
| CoronaVac vaccine | 67 (50.8%) | |
| Characteristics | PLWH a (n = 65) | HCs a (n = 65) | p | PLWH b (n = 67) | HCs b (n = 65) | p |
|---|---|---|---|---|---|---|
| Age (years, mean ± SD) | 34.0 ± 8.6 | 34.3 ± 9.4 | 0.845 | 34.1 ± 8.3 | 34.6 ± 8.5 | 0.726 |
| Male (n, %) | 55 (84.6%) | 55 (84.6%) | 1.000 | 64 (95.5%) | 60 (92.3%) | 0.683 |
| Interval between two doses (days, mean ± SD) | 27.3 ± 6.0 | 28.0 ± 5.2 | 0.463 | 25.1 ± 4.3 | 25.0 ± 4.1 | 0.853 |
| Blood collection after dose 2 (days, mean ± SD) | 29.7 ± 3.4 | 28.9 ± 3.7 | 0.221 | 28.2 ± 3.7 | 28.0 ± 3.9 | 0.784 |
| Groups | N | S-RBD-IgG Antibody Seropositivity | S-RBD-IgG Antibody Levels | |||||
|---|---|---|---|---|---|---|---|---|
| Numbers | Rate (95%CI) (%) | Median S/CO Value (IQR) | <1 | 1–9 | 10–19 | ≥20 | ||
| PLWH a | 65 | 53 | 81.5 (71.8, 91.2) | 2.5 (1.2–7.4) | 12 (18.5%) | 42 (64.6%) | 7 (10.8%) | 4 (6.2%) |
| HCs a | 65 | 61 | 93.8 (87.8, 99.8) | 11.4 (6.6–15.6) | 4 (6.2%) | 21 (32.3%) | 33 (50.8%) | 7 (10.8%) |
| p | 0.033 | < 0.001 | <0.001 | |||||
| PLWH b | 67 | 58 | 86.6 (78.2, 94.9) | 7.0 (2.8–13.3) | 9 (13.4%) | 33 (49.3%) | 17 (24.4%) | 8 (11.9%) |
| HCs b | 65 | 63 | 96.9 (92.6, 100.0) | 12.7 (8.6–16.2) | 2 (3.1%) | 18 (27.7%) | 36 (55.4%) | 9 (13.8%) |
| p | 0.031 | <0.001 | 0.001 | |||||
| Characteristics | PLWH a (n = 49) | HCs a (n = 47) | p | PLWH b (n = 50) | HCs b (n = 36) | p |
|---|---|---|---|---|---|---|
| Age (years, mean ± SD) | 35.0 ± 9.0 | 33.2 ± 9.2 | 0.474 | 34.2 ± 7.9 | 35.2 ± 9.2 | 0.658 |
| Male (n, %) | 43 (87.8%) | 42 (89.4%) | 0.805 | 50 (100%) | 34 (94.4%) | 0.336 |
| Interval between two doses (days, mean ± SD) | 27.3 ± 6.3 | 27.8 ± 4.7 | 0.213 | 25.2 ± 4.6 | 25.2 ± 4.0 | 0.708 |
| First blood collection after dose 2 (days, mean ± SD) | 29.6 ± 3.7 | 29.2 ± 3.7 | 0.471 | 28.1 ± 4.0 | 27.7 ± 3.7 | 0.417 |
| Second blood collection after dose 2 (days, mean ± SD) | 183.3 ± 10.0 | 181.3 ± 8.8 | 0.281 | 179.6 ± 10.1 | 179.8 ± 7.4 | 0.793 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, G.; Xu, L.; Feng, S.; Tang, J.; Wang, X.; Li, G.; Gan, Y.; Zheng, C.; Zhao, J.; Yang, Z. IgG Antibody Responses and Immune Persistence of Two Doses of BBIBP-CorV Vaccine or CoronaVac Vaccine in People Living with HIV (PLWH) in Shenzhen, China. Vaccines 2022, 10, 880. https://doi.org/10.3390/vaccines10060880
Zeng G, Xu L, Feng S, Tang J, Wang X, Li G, Gan Y, Zheng C, Zhao J, Yang Z. IgG Antibody Responses and Immune Persistence of Two Doses of BBIBP-CorV Vaccine or CoronaVac Vaccine in People Living with HIV (PLWH) in Shenzhen, China. Vaccines. 2022; 10(6):880. https://doi.org/10.3390/vaccines10060880
Chicago/Turabian StyleZeng, Guang, Liumei Xu, Shuidong Feng, Jie Tang, Xiaohui Wang, Guilian Li, Yongxia Gan, Chenli Zheng, Jin Zhao, and Zhengrong Yang. 2022. "IgG Antibody Responses and Immune Persistence of Two Doses of BBIBP-CorV Vaccine or CoronaVac Vaccine in People Living with HIV (PLWH) in Shenzhen, China" Vaccines 10, no. 6: 880. https://doi.org/10.3390/vaccines10060880
APA StyleZeng, G., Xu, L., Feng, S., Tang, J., Wang, X., Li, G., Gan, Y., Zheng, C., Zhao, J., & Yang, Z. (2022). IgG Antibody Responses and Immune Persistence of Two Doses of BBIBP-CorV Vaccine or CoronaVac Vaccine in People Living with HIV (PLWH) in Shenzhen, China. Vaccines, 10(6), 880. https://doi.org/10.3390/vaccines10060880






