Trends in Varicella Burden of Disease Following Introduction of Routine Childhood Varicella Vaccination in Argentina: A 12-Year Time Series Analysis
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design and Data Sources
2.2. Study Population
2.3. Outcomes and Analytical Methods
2.4. Analysis
2.5. UVV Impact Estimation
3. Results
3.1. Vaccination Coverage
3.2. Descriptive Statistics for Varicella Cases, Incidence, and Mortality
3.3. Time Series Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ávila-Agüero, M.L.; Beltrán, S.; Castillo, J.B.D.; Castillo Diaz, M.E.; Chaparro, L.E.; Deseda, C.; Debbag, R.; Espinal, C.; Falleiros-Arlant, L.H.; Gonzalez Mata, A.J. Varicella epidemiology in Latin America and the Caribbean. Expert Rev. Vaccines 2018, 17, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Warren-Gash, C.; Forbes, H.; Breuer, J. Varicella and herpes zoster vaccine development: Lessons learned. Expert Rev. Vaccines 2017, 16, 1191–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meszner, Z.; Molnar, Z.; Rampakakis, E.; Yang, H.K.; Kuter, B.J.; Wolfson, L.J. Economic burden of varicella in children 1–12 Years of age in Hungary, 2011–2015. BMC Infect. Dis. 2017, 17, 495. [Google Scholar] [CrossRef] [Green Version]
- Fornaro, P.; Gandini, F.; Marin, M.; Pedrazzi, C.; Piccoli, P.; Tognetti, D.; Assael, B.M.; Lucioni, C.; Mazzi, S. Epidemiology and cost analysis of varicella in Italy: Results of a sentinel study in the pediatric practice. Pediatric Infect. Dis. J. 1999, 18, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Iseli, A.; Aebi, C.; Banz, K.; Brunner, M.; Schmutz, A.M.; Heininger, U. Prospective surveillance of varicella-zoster virus infections in an out-patient setting in Switzerland. Hum. Vaccines 2009, 5, 843–846. [Google Scholar] [CrossRef]
- Wagenpfeil, S.; Neiss, A.; Banz, K.; Wutzler, P. Empirical data on the varicella situation in Germany for vaccination decisions. Clin. Microbiol. Infect. 2004, 10, 425–430. [Google Scholar] [CrossRef] [Green Version]
- Blumental, S.; Sabbe, M.; Lepage, P. Varicella paediatric hospitalisations in Belgium: A 1-year national survey. Arch. Dis. Child. 2016, 101, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Liese, J.G.; Grote, V.; Rosenfeld, E.; Fischer, R.; Belohradsky, B.H.; Kries, R.v.; Group, E.V.S. The burden of varicella complications before the introduction of routine varicella vaccination in Germany. Pediatric Infect. Dis. J. 2008, 27, 119–124. [Google Scholar] [CrossRef]
- Neyro, S.E.; Ferolla, F.M.; Molise, C.; Stach, P.; Romano, P.; Marone, S.; Arturo de Mena, M.; Plat, F.; Voto, C.; Soto, P. Clinical and epidemiological impact of varicella infection in children prior to the introduction of the varicella vaccine in the national immunization schedule of Argentina. Arch. Argent. Pediatr 2019, 117, 12–18. [Google Scholar] [CrossRef]
- World Health Organization. Varicella and herpes zoster vaccines: WHO position paper, June 2014–Recommendations. Vaccine 2016, 34, 198–199. [Google Scholar] [CrossRef]
- Barrenechea, G.G.; Chahla, R.E.; Peral, M.; Sánchez, R.; Bastos, L.S. Asociación entre incidencia de varicela y variabilidad climática en la provincia de Tucumán, Argentina, 2005–2019. Rev. Argent. Salud Pública 2021, 13, e62. [Google Scholar]
- Dayan, G.H.; Panero, M.S.; Debbag, R.; Urquiza, A.; Molina, M.; Prieto, S.; del Carmen Perego, M.a.; Scagliotti, G.; Galimberti, D.; Carroli, G. Varicella seroprevalence and molecular epidemiology of varicella-zoster virus in Argentina, 2002. J. Clin. Microbiol. 2004, 42, 5698–5704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirez, M.C. Vacunología en Latinoaméroca: La Varicela y las Vacunas Contra la Varicela. SABIN Vaccine Institute. Available online: https://www.sabin.org/sites/sabin.org/files/pirezvarispa_rev8.1.18.pdf (accessed on 18 January 2022).
- Giglio, N.; Monsanto, H.; Rampakakis, E.; Yang, H.K.; Kuter, B.J.; Wolfson, L.J. Economic burden of varicella in children 1–12 years of age in Argentina, 2009–2014. J. Med. Econ. 2018, 21, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Barrenechea, G.G.; Bastos, L.S. Evaluation of impact of one dose varicella vaccine on the incidence of chickenpox in Argentina. Vaccine 2020, 38, 330–335. [Google Scholar] [CrossRef]
- Baxter, R.; Ray, P.; Tran, T.N.; Black, S.; Shinefield, H.R.; Coplan, P.M.; Lewis, E.; Fireman, B.; Saddier, P. Long-term effectiveness of varicella vaccine: A 14-year, prospective cohort study. Pediatrics 2013, 131, e1389–e1396. [Google Scholar] [CrossRef] [Green Version]
- Kuter, B.; Matthews, H.; Shinefield, H.; Black, S.; Dennehy, P.; Watson, B.; Reisinger, K.; Kim, L.L.; Lupinacci, L.; Hartzel, J. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatric Infect. Dis. J. 2004, 23, 132–137. [Google Scholar] [CrossRef]
- Gentile, A.; Giglio, N.; Lucion, M.F.; Martínez, A.C.; Pejito, N.; Juarez, M.D.V. Impact of varicella vaccine on nosocomial outbreaks and management of post exposure prophylaxis following in a paediatric hospital. PLoS ONE 2021, 16, e0251496. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention. Varicella Vaccine Effectiveness and Duration of Protection. Available online: https://www.cdc.gov/vaccines/vpd-vac/varicella/hcp-effective-duration.htm (accessed on 18 January 2022).
- Arlant, L.H.F.; Garcia, M.C.P.; Avila Aguero, M.L.; Cashat, M.; Parellada, C.I.; Wolfson, L.J. Burden of varicella in Latin America and the Caribbean: Findings from a systematic literature review. BMC Public Health 2019, 19, 528. [Google Scholar] [CrossRef]
- World Health Organization. WHO Vaccine-Preventable Diseases: Monitoring System. 2020 Global Summary. Available online: https://apps.who.int/immunization_monitoring/globalsummary/schedules (accessed on 2 February 2022).
- Neyro, S.; Rancaño, C.; Iglesias, M.; Katz, N.; Juarez, M.D.V.; Pasinovich, M.; Saralegui, M.; Biscayart, C. 2476. Impact of the vaccination strategy on varicella burden disease in Argentina. Open Forum Infect. Dis. 2018, 5, S742. [Google Scholar] [CrossRef]
- Quian, J.; Rüttimann, R.; Romero, C.; Dall’Orso, P.; Cerisola, A.; Breuer, T.; Greenberg, M.; Verstraeten, T. Impact of universal varicella vaccination on 1-year-olds in Uruguay: 1997–2005. Arch. Dis. Child. 2008, 93, 845–850. [Google Scholar] [CrossRef]
- Scotta, M.C.; Paternina-de la Ossa, R.; Lumertz, M.S.; Jones, M.H.; Mattiello, R.; Pinto, L.A. Early impact of universal varicella vaccination on childhood varicella and herpes zoster hospitalizations in Brazil. Vaccine 2018, 36, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Kupek, E.; Tritany, E.F. Impact of vaccination against varicella on the reduction of the disease incidence in children and adolescents from Florianópolis, Brazil. J. Pediatr. 2009, 85, 365–368. [Google Scholar] [CrossRef]
- Avila-Aguero, M.L.; Ulloa-Gutierrez, R.; Camacho-Badilla, K.; Soriano-Fallas, A.; Arroba-Tijerino, R.; Morice-Trejos, A. Varicella prevention in Costa Rica: Impact of a one-dose schedule universal vaccination. Expert Rev. Vaccines 2017, 16, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Salud Argentina. Lineamientos Técnicos Introducción de la Segunda dosis de Vacuna Contra Varicela al Calendario Nacional de Inmunizaciones. Available online: https://bancos.salud.gob.ar/recurso/lineamientos-tecnicos-introduccion-de-la-segunda-dosis-de-la-vacuna-contra-varicela (accessed on 18 January 2022).
- Ministerio de Salud Argentina. Registros Federales Asociados al Ciudadano: SNVS–Sistema Nacional de Vigilancia en Salud. Available online: https://sisa.msal.gov.ar/sisadoc/docs/050216/snvs_intro.jsp (accessed on 12 August 2020).
- Ministerio de Salud Argentina. Lineamientos Técnicos: Fundamentos de la Introducción de la Vacuna Contra la Varicela al Calendario Nacional de Inmunizaciones. Available online: https://bancos.salud.gob.ar/recurso/fundamentos-de-la-introduccion-de-la-vacuna-contra-varicela (accessed on 12 August 2020).
- Ministerio de Salud Argentina. SISA–Sistema Integrado de Información Sanitaria Argentino. Available online: https://sisa.msal.gov.ar/sisadoc/docs/0101/intro_caracteristicas.jsp (accessed on 12 August 2020).
- Dirección de Estadísticas e Información de Salud–DEIS. Defunciones. Available online: http://www.deis.msal.gov.ar/index.php/base-de-datos/ (accessed on 12 August 2020).
- Ministerio de Salud Argentina. Estadísticas Vitales: Información Básica Argentina. Available online: http://www.deis.msal.gov.ar/wp-content/uploads/2020/01/Serie5Nro62.pdf (accessed on 12 August 2020).
- Instituto Nacional de Estadística y Censos de la República Argentina–INDEC. Estadísticas: Población. Available online: https://www.indec.gob.ar/ (accessed on 13 August 2020).
- Ministerio de Salud Argentina. Coberturas de Vacunación por Jurisdicción. Available online: https://bancos.salud.gob.ar/recurso/coberturas-de-vacunacion-por-jurisdiccion-2009-2020 (accessed on 13 August 2020).
- de Martino Mota, A.; Carvalho-Costa, F.A. Varicella zoster virus related deaths and hospitalizations before the introduction of universal vaccination with the tetraviral vaccine. J. Pediatr. 2016, 92, 361–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministerio de Salud Pública de la República Oriental del Uruguay. Actualizacion de Varicela a la se 23 de 2012. Available online: https://www.gub.uy/ministerio-salud-publica/sites/ministerio-salud-publica/files/documentos/publicaciones/Informe_Varicela_julio_2012.pdf (accessed on 18 January 2022).
- Pawaskar, M.; Gil-Rojas, Y.; Avendaño, V.; Parellada, C.I.; Rey-Velasco, A.; Beltrán, C.C.; Prieto, E.; Rosselli, D.; Lasalvia, P. The Impact of Universal Varicella Vaccination on the Clinical Burden of Varicella in Colombia: A National Database Analysis, 2008–2019. In Proceedings of the The 39th Annual Meeting of the European Society of Paediatric Infectious Diseases (ESPID), Geneva, Switzerland, 24–29 May 2021. [Google Scholar]
- Paniz-Mondolfi, A.E.; Tami, A.; Grillet, M.E.; Márquez, M.; Hernández-Villena, J.; Escalona-Rodríguez, M.A.; Blohm, G.M.; Mejías, I.; Urbina-Medina, H.; Rísquez, A. Resurgence of vaccine-preventable diseases in Venezuela as a regional public health threat in the Americas. Emerg. Infect. Dis. 2019, 25, 625. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Chickenpox Vaccine Saves Lives and Prevents Serious Illness Infographic. Available online: https://www.cdc.gov/chickenpox/vaccine-infographic.html (accessed on 12 July 2022).
- Guris, D.; Jumaan, A.O.; Mascola, L.; Watson, B.M.; Zhang, J.X.; Chaves, S.S.; Gargiullo, P.; Perella, D.; Civen, R.; Seward, J.F. Changing varicella epidemiology in active surveillance sites—United States, 1995–2005. J. Infect. Dis. 2008, 197, S71–S75. [Google Scholar] [CrossRef]
- Woodward, M.; Marko, A.; Galea, S.; Eagel, B.; Straus, W. Varicella virus vaccine live: A 22-year review of postmarketing safety data. In Open Forum Infectious Diseases; Oxford University Press: New York, NY, USA, 2019; Volume 6. [Google Scholar]
- Centers for Disease Control and Prevention. Vaccine Safety: Chickenpox (Varicella) Vaccines. Available online: https://www.cdc.gov/vaccinesafety/vaccines/varicella-vaccine.html (accessed on 12 July 2022).
- Dirección de Control de enfermedades Inmunoprevenibles–Ministerio de Salud de la Nación; Buenos Aires, Argentina. Adverse Event Reporting–Merck Oka Strain Varicella Vaccine. Personal Communication, 9 July 2022. [Google Scholar]
- Causey, K.; Fullman, N.; Sorensen, R.J.; Galles, N.C.; Zheng, P.; Aravkin, A.; Danovaro-Holliday, M.C.; Martinez-Piedra, R.; Sodha, S.V.; Velandia-González, M.P. Estimating global and regional disruptions to routine childhood vaccine coverage during the COVID-19 pandemic in 2020: A modelling study. Lancet 2021, 398, 522–534. [Google Scholar] [CrossRef]
- Moreno-Montoya, J.; Ballesteros, S.M.; Sotelo, J.C.R.; Cervera, C.L.B.; Barrera-López, P.; De la Hoz-Valle, J.A. Impact of the COVID-19 pandemic on routine childhood immunisation in Colombia. Arch. Dis. Child. 2021, 107, e4. [Google Scholar] [CrossRef]
- Ministerio de Salud Argentina. Report on the Impact of the SARS-CoV-2 Pandemic on National Vaccination Coverage in Argentina. Available online: https://bancos.salud.gob.ar/recurso/informe-sobre-el-impacto-de-la-pandemia-sars-cov-2-en-las-coberturas-nacionales-de (accessed on 1 February 2022).
- Shapiro, E.D.; Vazquez, M.; Esposito, D.; Holabird, N.; Steinberg, S.P.; Dziura, J.; LaRussa, P.S.; Gershon, A.A. Effectiveness of 2 doses of varicella vaccine in children. J. Infect. Dis. 2011, 203, 312–315. [Google Scholar] [CrossRef] [Green Version]
- Pujadas, A. Varicela en un país del Cono Sur de América Latina. Situación epidemiológica y estrategias de prevención Período 2008–2018. In Proceedings of the X Congreso International de Infectología Pediátrica y Vacunas (SADIP 2021), Virtual, 28–30 April 2021. [Google Scholar]
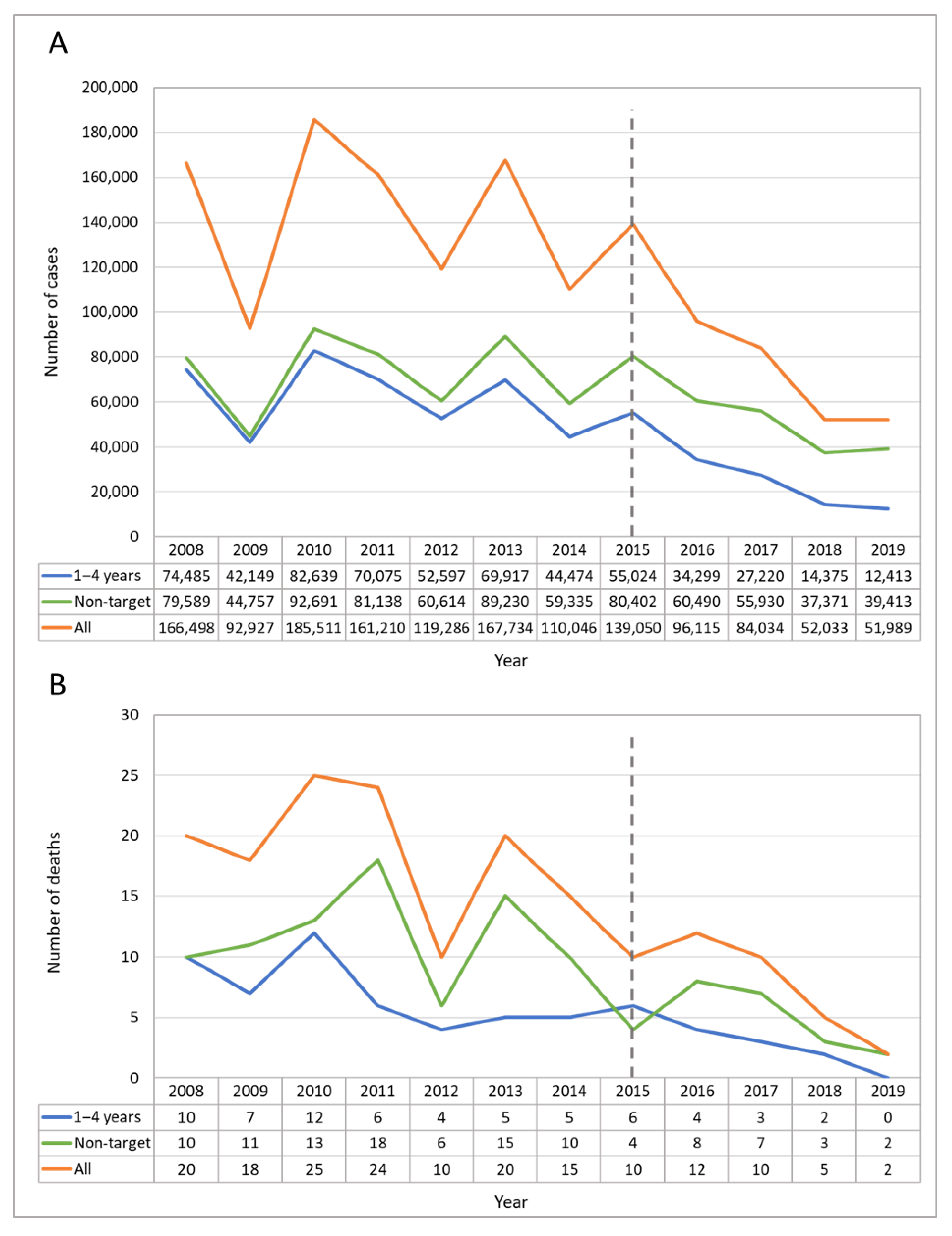

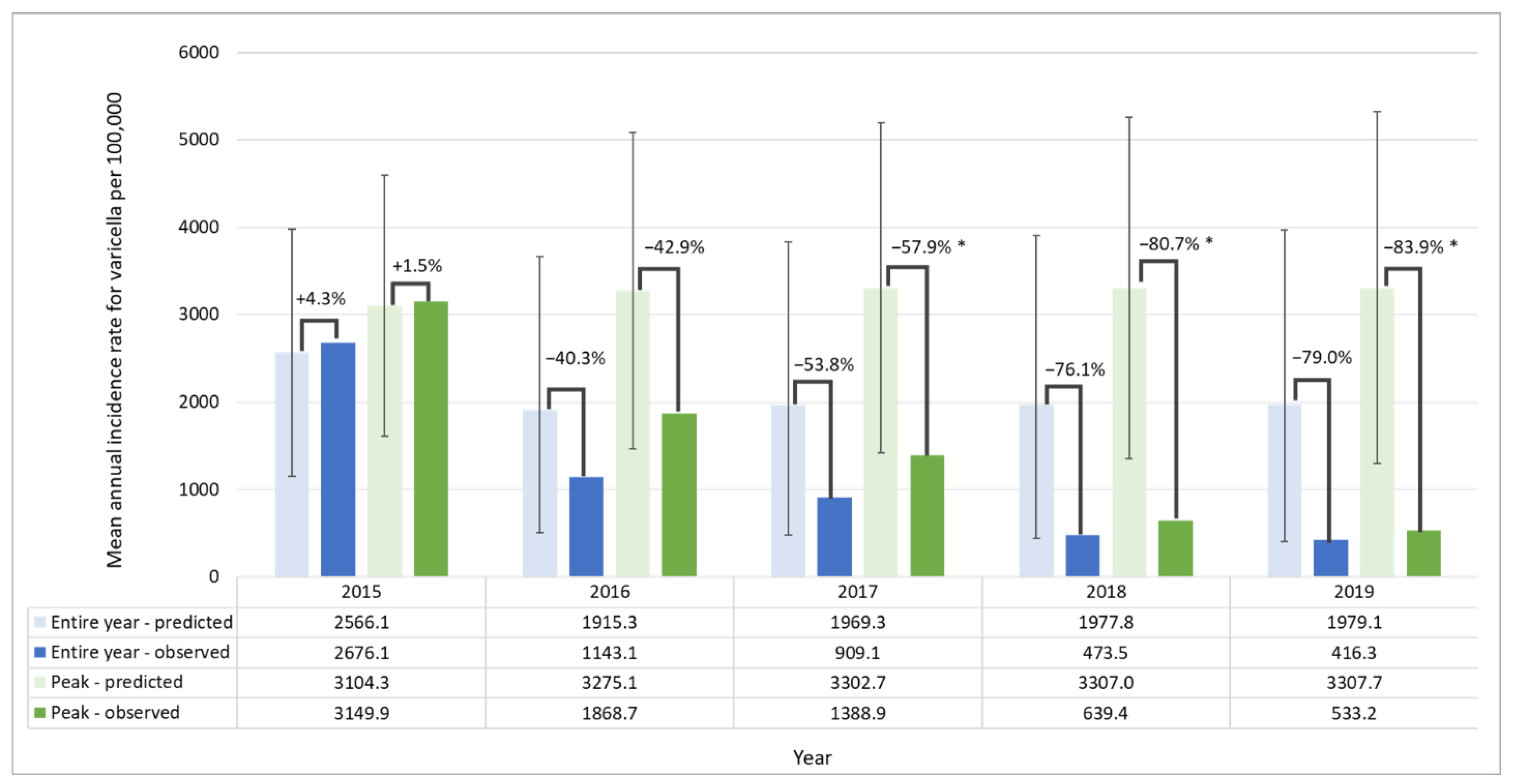

| Age Groups (Years) | Cases in the Pre-UVV Period | MAIR Pre-UVV | Cases in the Post-UVV Period | MAIR Post-UVV | % Change | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015-I | 2015-II | 2016 | 2017 | 2018 | 2019 | ||||
| <1 | 12,504 | 7091 | 13,627 | 11,382 | 8920 | 11,350 | 7690 | 2635 | 1326.2 | 6993 | 5918 | 4999 | 3215 | 3032 | 823.9 | −37.9% |
| 1–4 (target population) | 74,485 | 42,149 | 82,639 | 70,075 | 52,597 | 69,917 | 44,474 | 14,777 | 1999.1 | 40,247 | 34,299 | 27,220 | 14,375 | 12,413 | 1121.5 | −43.9% |
| 5–9 | 50,145 | 27,895 | 60,128 | 52,957 | 39,237 | 60,058 | 39,341 | 13,195 | 1264.3 | 40,445 | 40,009 | 37,354 | 24,009 | 23,917 | 1132.3 | −10.4% |
| 10–14 | 10,368 | 5766 | 12,077 | 10,765 | 8076 | 12,180 | 8421 | 2960 | 261.1 | 9061 | 10,133 | 9744 | 7022 | 8622 | 304.9 | 16.8% |
| 15–24 | 3807 | 2197 | 3756 | 3301 | 2455 | 3253 | 2138 | 947 | 41.0 | 2111 | 2476 | 2220 | 1864 | 2238 | 36.8 | −10.4% |
| 25–34 | 1464 | 963 | 1532 | 1414 | 956 | 1220 | 856 | 311 | 17.8 | 708 | 904 | 755 | 557 | 596 | 12.8 | −28.4% |
| 35–44 | 628 | 387 | 741 | 634 | 459 | 549 | 394 | 162 | 9.8 | 333 | 482 | 408 | 324 | 428 | 7.7 | −21.3% |
| 45–64 | 471 | 350 | 556 | 487 | 322 | 432 | 333 | 142 | 5.1 | 232 | 375 | 303 | 283 | 369 | 4.2 | −17.5% |
| ≥65 | 202 | 108 | 274 | 198 | 189 | 188 | 162 | 62 | 4.2 | 105 | 193 | 147 | 97 | 211 | 3.5 | −16.3% |
| Non-targeted population | 79,589 | 44,757 | 92,691 | 81,138 | 60,614 | 89,230 | 59,335 | 20,414 | 178.0 | 59,988 | 60,490 | 55,930 | 37,371 | 39,413 | 153.6 | −13.7% |
| No age data | 12,424 | 6021 | 10,181 | 9997 | 6075 | 8587 | 6237 | 984 | 2640 | 1326 | 884 | 287 | 163 | NA | NA | |
| Overall | 166,498 | 92,927 | 185,511 | 161,210 | 119,286 | 167,734 | 110,046 | 36,175 | 324.9 | 102,875 | 96,115 | 84,034 | 52,033 | 51,989 | 224.1 | −31.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giglio, N.; Lasalvia, P.; Pawaskar, M.; Parellada, C.I.; Rojas, Y.G.; Micone, P.; Babic, V.V.; Neyro, S.E. Trends in Varicella Burden of Disease Following Introduction of Routine Childhood Varicella Vaccination in Argentina: A 12-Year Time Series Analysis. Vaccines 2022, 10, 1151. https://doi.org/10.3390/vaccines10071151
Giglio N, Lasalvia P, Pawaskar M, Parellada CI, Rojas YG, Micone P, Babic VV, Neyro SE. Trends in Varicella Burden of Disease Following Introduction of Routine Childhood Varicella Vaccination in Argentina: A 12-Year Time Series Analysis. Vaccines. 2022; 10(7):1151. https://doi.org/10.3390/vaccines10071151
Chicago/Turabian StyleGiglio, Norberto, Pieralessandro Lasalvia, Manjiri Pawaskar, Cintia I. Parellada, Yaneth Gil Rojas, Paula Micone, Virginia V. Babic, and Silvina E. Neyro. 2022. "Trends in Varicella Burden of Disease Following Introduction of Routine Childhood Varicella Vaccination in Argentina: A 12-Year Time Series Analysis" Vaccines 10, no. 7: 1151. https://doi.org/10.3390/vaccines10071151
APA StyleGiglio, N., Lasalvia, P., Pawaskar, M., Parellada, C. I., Rojas, Y. G., Micone, P., Babic, V. V., & Neyro, S. E. (2022). Trends in Varicella Burden of Disease Following Introduction of Routine Childhood Varicella Vaccination in Argentina: A 12-Year Time Series Analysis. Vaccines, 10(7), 1151. https://doi.org/10.3390/vaccines10071151






