Report of Adverse Effects Following Population-Wide COVID-19 Vaccination: A Comparative Study between Six Different Vaccines in Baja-California, Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
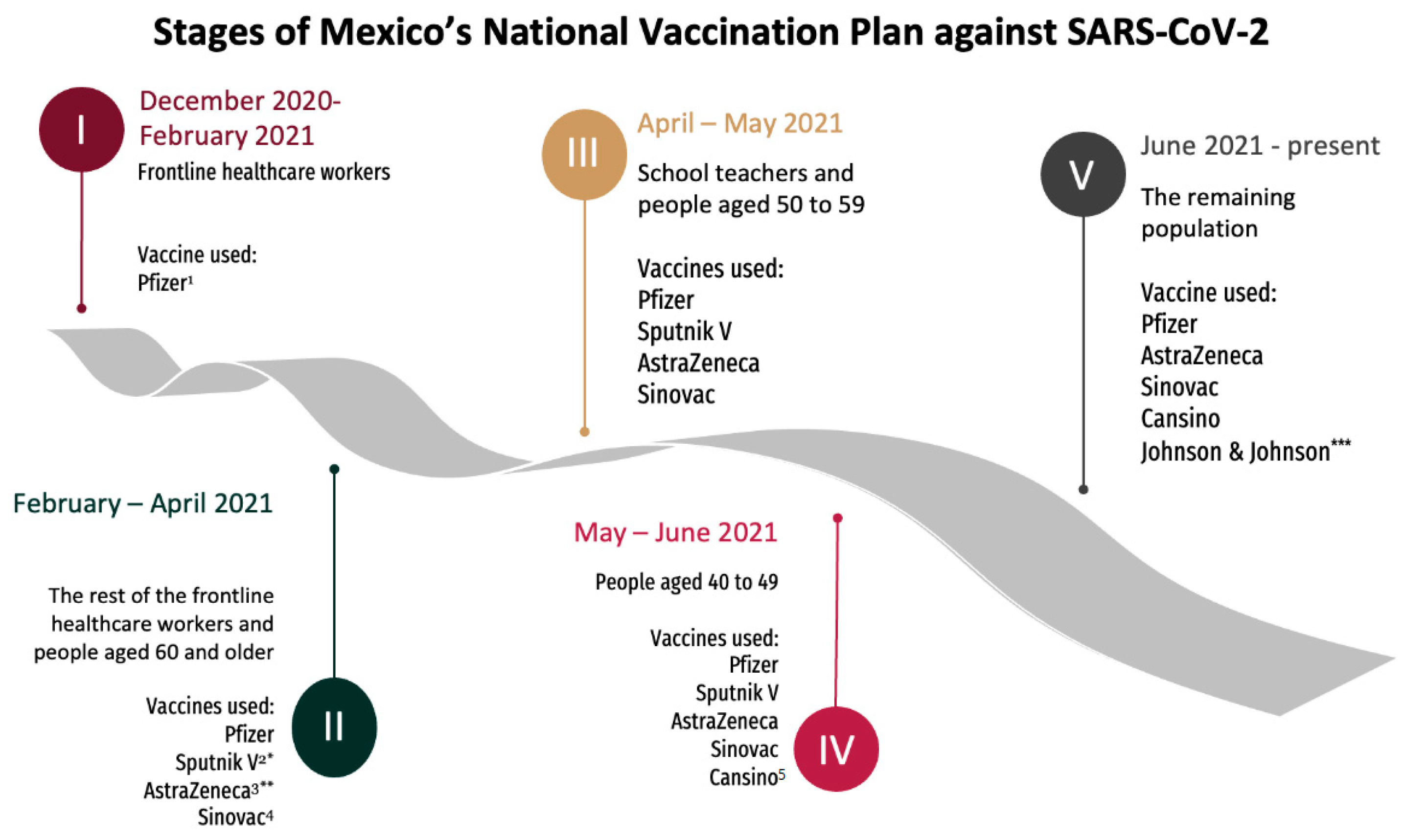
2.2. Setting and Participants
- Caused the death of the patient.
- Placed the life of the patient at imminent risk.
- Caused disability or persistent and significant impairment.
- Required hospitalization or extended length of hospital stay.
2.3. Measurement Instrument and Variables
2.4. Statistical Analysis
3. Results
3.1. Adverse Events Rates by Vaccine Manufacturer
3.2. Demographic Characteristics and Reported Symptoms
3.3. Severe Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Eng. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Statement on the Second Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV). Available online: https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) (accessed on 5 May 2022).
- World Health Organization. Timeline: WHO’s COVID-19 Response. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline#! (accessed on 5 May 2022).
- Li, Y.-D.; Chi, W.-Y.; Su, J.-H.; Ferrall, L.; Hung, C.-F.; Wu, T.-C. Coronavirus vaccine development: From SARS and MERS to COVID-19. J. Biomed. Sci. 2020, 27, 104. [Google Scholar] [CrossRef]
- Chilamakuri, R.; Agarwal, S. COVID-19: Characteristics and Therapeutics. Cells 2021, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, N.C.; López-Cortés, A.; González, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. NPJ Vaccines 2021, 6, 28. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatulin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Rusia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 5 May 2022).
- Secretaría de Economía. DataMéxico BETA. Available online: https://datamexico.org/en/profile/geo/baja-california-bc?PEFYears3=2019&fdiSelectorButton1=genericInvestment&fdiTimeSelector=Year&foreignYearSelector1=2019&foreignYearSelector2=2019&timeNetTradeSelector=Year&fdiYearSelector=2019 (accessed on 3 March 2022).
- Bureau of Transportation Statistics. Border Crossing/Entry Data. Available online: https://www.bts.gov/browse-statistical-products-and-data/border-crossing-data/border-crossingentry-data (accessed on 1 March 2022).
- Secretaría de Salud. Política Nacional de Vacunación contra el Virus SARS-CoV-2, para la prevención de la COVID-19 En México. Available online: https://coronavirus.gob.mx/wp-content/uploads/2021/04/28Abr2021_13h00_PNVx_COVID_19.pdf (accessed on 5 May 2022).
- Comisión Federal para la Protección Contra Riesgos Sanitarios. La COFEPRIS Otorga Autorización Para Uso de Emergencia a Vacuna para Prevenir la Enfermedad por Coronavirus (COVID-19). Available online: https://www.gob.mx/cofepris/articulos/la-cofepris-otorga-autorizacion-para-uso-de-emergencia-a-vacuna-para-prevenir-la-enfermedad-por-coronavirus-covid-19 (accessed on 5 May 2022).
- Comisión Federal Para la Protección Contra Riesgos Sanitarios. Vacunas COVID-19 Autorizadas. Available online: https://www.gob.mx/cofepris/acciones-y-programas/vacunas-covid-19-autorizadas (accessed on 5 May 2022).
- Dirección General de Epidemiología, Dirección de Vigilancia Epidemiología de Enfermedades Transmisibles. Manual de Procedimientos Estandarizados Para la Vigilancia Epidemiológica de Eventos Supuestamente Atribuibles a la Vacunación o Inmunización (ESAVI). Versión 2021. Available online: https://epidemiologia.salud.gob.mx/gobmx/salud/documentos/manuales/42_Manual_ESAVI.pdf (accessed on 5 May 2022).
- Dirección General de Epidemiología. Reporte ESAVI COVID-19 Marzo. 2022. Available online: https://www.gob.mx/cms/uploads/attachment/file/718427/REPORTEESAVIDVEETMARZO2022.pdf (accessed on 5 May 2022).
- Border COVID-19 Vaccination Plan with U.S.-Donated Shots Starts in Baja-California. Available online: https://www.latimes.com/california/story/2021-06-19/border-covid-19-vaccination-plan-us-donated-shots-baja-california (accessed on 20 May 2022).
- Frontera, J.A.; Tamborska, A.A.; Doheim, M.F.; Garcia-Azorin, D.; Gezegen, H.; Guekht, A.; MMed, A.H.K.Y.K.; Santacatterina, M.; Sejvar, J.; Thakur, K.T.; et al. Neurological Events Reported after COVID-19 Vaccines: An Analysis of VAERS. Ann. Neurol. 2022, 91, 756–771. [Google Scholar] [CrossRef] [PubMed]
- Shay, D.K.; Gee, J.; Su, J.R.; Myers, T.R.; Marquez, P.; Liu, R.; Zhang, B.; Licata, C.; Clark, T.A.; Shimabukuro, T.T. Safety Monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine—United States, March–April 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 680–684. [Google Scholar] [PubMed]
- Bilotta, C.; Perrone, G.; Adelfio, V.; Spatola, G.F.; Uzzo, M.L.; Argo, A.; Zerbo, S. COVID-19 Vaccine-Related Thrombosis: A Systematic Review and Exploratory Analysis. Front. Immunol. 2021, 12, 729251. [Google Scholar] [CrossRef]
- Sharifian-Dorche, M.; Bahmanyar, M.; Sharifian-Dorche, A.; Mohammadi, P.; Nomovi, M.; Mowla, A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. J. Neurol. Sci. 2021, 428, 117607. [Google Scholar] [CrossRef]
- Pan American Health Organization. Consultation Document for Case Definitions. Available online: https://iris.paho.org/bitstream/handle/10665.2/55721/PAHOHSSMTCOVID-19210017_eng.pdf?sequence=1&isAllowed=y (accessed on 5 May 2022).
- Li, X.; Lai, L.Y.; Ostropolets, A.; Arshad, F.; Tan, E.H.; Casajust, P.; Alshammari, T.M.; Duarte-Salles, T.; Minty, E.P.; Areia, C.; et al. Bias, Precision and Timeliness of Historical (Background) Rate Comparison Methods for Vaccine Safety Monitoring: An Empirical Multi-Database Analysis. Front. Pharmacol. 2021, 12, 3307. [Google Scholar] [CrossRef]
- Montano, D. Frequency and Associations of Adverse Reactions of COVID-19 Vaccines Reported to Pharmacovigilance Systems in the European Union and the United States. Front. Public Health 2022, 9, 2237. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, C.A. Mechanisms underlying adverse reactions to vaccines. J. Comp. Pathol. 2007, 137 (Suppl. 1), S46–S50. [Google Scholar] [CrossRef] [PubMed]
- Heinz, F.X.; Stiasny, K. Distinguishing features of current COVID-19 vaccines: Knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines 2021, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Stahel, V.P. The safety of Covid-19 mRNA vaccines: A review. Patient Saf. Surg. 2021, 15, 20. [Google Scholar] [CrossRef]
- Heymans, S.; Cooper, L.T. Myocarditis after COVID-19 mRNA vaccination: Clinical observations and potential mechanisms. Nat. Rev. Cardiol. 2022, 19, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Dietmann, A.; Ripellino, P.; Humm, A.M.; Hundsberger, T.; Schreiner, B.; Théaudin, M.; Scheidegger, O. Hot Topics on COVID-19 and Its Possible Association with Guillain-Barré Syndrome. Clin. Transl. Neurosci. 2022, 6, 7. [Google Scholar] [CrossRef]
- Khalil, L.; Leary, M.; Rouphael, N.; Ofotokun, I.; Rebolledo, P.A.; Wiley, Z. Racial and Ethnic Diversity in SARS-CoV-2 Vaccine Clinical Trials Conducted in the United States. Vaccines 2022, 10, 290. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Padmapriyadarsini, C.; Vekemans, J.; Bavdekar, A.; Gupta, M.; Kulkarni, P.; Garg, B.S.; Gogtay, N.J.; Tambe, M.; Lalwani, S.; et al. A phase 2/3, participant-blind, observer-blind, randomised, controlled study to assess the safety and immunogenicity of SII-ChAdOx1 nCoV-19 (COVID-19 vaccine) in adults in India. eClinicalMedicine 2021, 42, 101218. [Google Scholar] [CrossRef]
- García-Grimshaw, M.; Ceballos-Liceaga, S.E.; Hernández-Vanegas, L.E.; Núñez, I.; Hernández-Valdivia, N.; Carrillo-García, D.A.; Michel-Chávez, A.; Galnares-Olalde, J.A.; Carbajal-Sandoval, G.; Saniger-Alba, M.D.M.; et al. Neurologic adverse events among 704,003 first-dose recipients of the BNT162b2 mRNA COVID-19 vaccine in Mexico: A nationwide descriptive study. Clin. Immunol. 2021, 229, 108786. [Google Scholar] [CrossRef] [PubMed]
- Moll, M.E.C.; Martínez, A.M.S.; Cisneros, B.T.; Onofre, J.I.G.; Floriano, G.N.; de León, M.B. Extension and Severity of Self-Reported Side Effects of Seven COVID-19 Vaccines in Mexican Population. Front. Public Health 2022, 10, 834744. [Google Scholar] [CrossRef] [PubMed]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Halperin, S.A.; Ye, L.; MacKinnon-Cameron, D.; Smith, B.; Cahn, P.E.; Ruiz-Palacios, G.M.; Ikram, A.; Lanas, F.; Guerrero, M.L.; Navarro, S.P.M.; et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: An international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet 2022, 399, 237–248. [Google Scholar]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Alhumaid, S.; Al Mutair, A.; Al Alawi, Z.; Rabaan, A.A.; Tirupathi, R.; Alomari, M.A.; Alshakhes, A.S.; Alshawi, A.M.; Ahmed, G.Y.; Almusabeh, H.M.; et al. Anaphylactic and nonanaphylactic reactions to SARS-CoV-2 vaccines: A systematic review and meta-analysis. Allergy Asthma Clin. Immunol. 2021, 17, 109. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, B.; Nuwarda, R.F.; Ramzan, I.; Kayser, V. A Narrative Review of COVID-19 Vaccines. Vaccines 2022, 10, 62. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Anastassopoulou, C.; Hatziantoniou, S.; Poland, G.A.; Tsakris, A. Anaphylaxis rates associated with COVID-19 vaccines are comparable to those of other vaccines. Vaccine 2022, 40, 183–186. [Google Scholar] [CrossRef]
- The Medicines and Healthcare Products Regulatory Agency (MHRA). Coronavirus Vaccine—Summary of Yellow Card Reporting. Available online: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting (accessed on 19 July 2022).
- Instituto de Salud Pública, Ministerio de Salud, Gobierno de Chile. Noveno Informe Estadístico “ESAVI Asociados a la Administración de Vacunas SARS-CoV-2 en Chile en Personas Desde los 18 años”. Available online: ispch.cl/wp-content/uploads/2022/05/9°-Informe-Estadistico-Poblacion-Mayor-de-18-anos-VF3.pdf (accessed on 19 July 2022).
- Gautam, A.; Patiyal, N.; Kansal, D.; Sood, A.; Chauhan, A.; Bodh, S. Pattern of adverse effects following ChAdOx1 nCoV-19 COVISHIELD vaccination in adults in tertiary healthcare institution in North India: A retrospective observational study. IJCAP 2022, 7, 91–95. [Google Scholar]
- Food and Drug Administration. Emergency Use Authorization for Vaccines to Prevent COVID-19. Available online: https://www.fda.gov/media/142749/download (accessed on 5 May 2022).
- Kant, A.; Jansen, J.; van Balveren, L.; van Hunsel, F. Description of Frequencies of Reported Adverse Events Following Immunization Among Four Different COVID-19 Vaccine Brands. Drug Safety 2022, 45, 319–331. [Google Scholar] [CrossRef]
- AstraZeneca. Serum Institute of India Obtains Emergency Use Authorization in India for AstraZeneca’s COVID-19 Vaccine. Available online: https://www.astrazeneca.com/media-centre/press-releases/2021/serum-institute-of-india-obtains-emergency-use-authorisation-in-india-for-astrazenecas-covid-19-vaccine.html (accessed on 18 July 2022).
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, C.B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA 2022, 327, 331–340. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccines. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines (accessed on 20 May 2022).
- Drug and Vaccine Authorizations for COVID-19: List of Authorized Drugs, Vaccines and Expanded Indications. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/authorization/list-drugs.html#wb-auto-4 (accessed on 20 May 2022).

| Vaccine Platform | Vaccine Manufacturer | Administered Doses | Total AE (n) | AE per 100,000 Doses | p-Value | Mild AE (n) | Mild AE per 100,000 Doses | Severe AE (n) | Severe AE per 100,000 Doses |
|---|---|---|---|---|---|---|---|---|---|
| mRNA | Pfizer-BioNTech | 1,282,792 | 1014 | 79.05 | 1004 | 78.27 | 10 | 0.78 | |
| Viral vector | CanSinoBIO | 959,990 | 81 | 84.38 | 0.61 | 80 | 83.34 | 1 | 1.04 |
| Johnson & Johnson | 1,256,494 | 825 | 65.66 | <0.0001 | 816 | 64.94 | 9 | 0.72 | |
| AstraZeneca | 608,547 | 214 | 35.71 | <0.0001 | 209 | 34.34 | 5 | 0.82 | |
| Serum Institute of India | 23,810 | 113 | 474.59 | <0.0001 | 110 | 461.99 | 3 | 12.6 | |
| Inactivated virus | Sinovac | 248,761 | 38 | 15.28 | <0.0001 | 34 | 13.67 | 4 | 1.61 |
| Total | 3,516,394 | 2285 | 64.98 | - | 2253 | 64.07 | 32 | 0.91 |
| Adverse Events (Mild and Severe) | ||||
|---|---|---|---|---|
| Variable | Total (n = 2285) | Viral Vector/Inactivated Virus (n = 1271) | mRNA (n = 1014) | p-Value |
| Age-mean ± SD | 37.68 ±12.93 | 35.42 ± 12.98 | 40.5 ± 12.29 | <0.0001 |
| Gender | ||||
| Women—n (%) | 1532 (67.05) | 808 (63.57) | 724 (71.40) | <0.0001 |
| Pregnant—n (%) | 29 (1.27) | 20 (1.57) | 9 (0.89) | 0.11 |
| History of allergic reactions—n (%) | 326 (14.27) | 153 (12.03) | 173 (17.06) | <0.001 |
| Food | 45 (1.97) | 14 (1.10) | 31 (3.06) | <0.05 |
| Drugs | 181 (7.92) | 81 (6.37) | 100 (9.86) | <0.05 |
| Pollen | 26 (1.14) | 11 (0.87) | 15 (1.48) | 0.23 |
| Ignored | 8 (0.35) | 3 (0.24) | 5 (0.49) | 0.49 |
| Other | 25 (1.09) | 15 (1.18) | 10 (0.99) | 0.81 |
| No allergy | 1959 (85.73) | 1118 (87.96) | 841 (82.94) | <0.001 |
| Symptoms—n (%) | ||||
| Headache | 1766 (77.29) | 1042 (81.98) | 724 (71.40) | <0.0001 |
| Myalgias/arthralgias | 1464 (64.07) | 919 (72.31) | 545 (53.75) | <0.0001 |
| Fever/hyperthermia | 1069 (46.78) | 695 (54.68) | 374 (36.88) | <0.0001 |
| Chills/diaphoresis | 959 (41.97) | 631 (49.65) | 328 (32.35) | <0.0001 |
| Local injection site reactions | 1212 (53.04) | 610 (47.99) | 602 (59.37) | <0.0001 |
| Gastrointestinal symptoms * | 1023 (44.77) | 600 (47.21) | 423 (41.72) | <0.01 |
| Malaise/fatigue | 748 (32.74) | 399 (31.39) | 349 (34.42) | 0.21 |
| Dizziness | 680 (29.76) | 360 (28.32) | 320 (31.56) | 0.11 |
| Other ** | 358 (15.67) | 165 (12.98) | 193 (19.03) | <0.0001 |
| Sore or scratchy throat | 358 (15.67) | 186 (14.63) | 172 (16.96) | 0.12 |
| Cough | 336 (14.70) | 180 (14.16) | 156 (15.38) | 0.44 |
| Rhinorrhea | 342 (14.97) | 169 (13.30) | 173 (17.06) | <0.05 |
| Palpitations (tachycardia) | 211 (9.23) | 101 (7.95) | 110 (10.85) | <0.05 |
| Dyspnea/difficult breathing | 152 (6.65) | 97 (7.63) | 55 (5.42) | <0.05 |
| Rash, generalized pruritus | 180 (7.88) | 91 (7.16) | 89 (8.78) | 0.2 |
| Ocular manifestations | 113 (4.98) | 57 (4.48) | 56 (5.52) | 0.44 |
| Neurologic symptoms (peripheral) | 98 (4.29) | 43 (3.38) | 55 (5.42) | <0.05 |
| Adenopathies/lymphadenopathies | 62 (2.71) | 19 (1.49) | 43 (4.24) | <0.001 |
| Clinical Diagnosis | Time to AE (Hours) | Average Time (Hours) | Length of Stay (Days) | Outcome | Vaccine Platform | Vaccine Brand | Causality Evaluation |
|---|---|---|---|---|---|---|---|
| Acute Myocardial Infarction (n = 2) | 1 | 20.33 | - | Death | Viral Vector | Sinovac | D, E |
| 60 | 6 | Discharged | Viral Vector | AstraZeneca | A1 | ||
| Acute polyradiculopathy (n = 3) | 0 | 0.72 | 14 | Discharged | mRNA | Pfizer-BioNTech | A1 |
| 2 | 4 | Discharged | Viral Vector | SII PVT | A1, B | ||
| 0.16 | - | Death | Viral Vector | Janssen | A1 | ||
| Anaphylaxis (n = 1) | 10 | - | 7 | Discharged | mRNA | Pfizer-BioNTech | A1 |
| Auricular fibrillation (n = 1) (arrhythmia) | 7 | - | 3 | Discharged | Viral Vector | Sinovac | D, E |
| Cardiopulmonary arrest (n = 1) | 7.25 | - | 0 | Death | mRNA | Pfizer-BioNTech | A1 |
| Cavernous sinus thrombosis (n = 1) | 0.08 | - | 16 | Discharged | mRNA | Pfizer-BioNTech | A1, B |
| Encephalitis (n = 1) | 9.16 | - | 5 | Discharged | Viral Vector | Janssen | A1 |
| Exfoliative dermatitis (n = 1) | 0 | - | 6 | Discharged | Viral Vector | Janssen | A1 |
| Functional Diarrhea (n = 1) | 6 | - | 7 | Discharged | Viral Vector | CanSinoBIO | A1 |
| Guillain–Barré Syndrome (n = 7) | 0 | 4.5 | 6 | Discharged | Viral Vector | Sinovac | D |
| 3 | 4 | Discharged | mRNA | Pfizer-BioNTech | A1 | ||
| 7.5 | 7 | Discharged | Viral Vector | Janssen | B | ||
| 0 | 5 | Discharged | Viral Vector | Janssen | A1 | ||
| 21 | - | Discharged | Viral Vector | Janssen | A1 | ||
| 0 | 5 | Discharged | Viral Vector | Janssen | A1 | ||
| 0 | 10 | Discharged | Viral Vector | AstraZeneca | C | ||
| Ischemic stroke (n = 1) | 8.66 | - | 3 | Discharged | mRNA | Pfizer-BioNTech | A1, C |
| Lower extremities thrombosis (n = 1) | 0 | - | 6 | Discharged | Viral Vector | Sinovac | A1 |
| Lower right extremity acute neuropathy (n = 1) | 1.5 | - | 3 | Discharged | Viral Vector | Janssen | A1 |
| Myelopathy (n = 1) | 15 | - | 1 | Discharged | Viral Vector | SII PVT | B |
| Neurologic deterioration and weakness (n = 1) | 16 | - | 3 | Discharged | Viral Vector | Janssen | A1, A3 |
| Polyneuropathy (n = 1) | 0 | - | 8 | Discharged | mRNA | Pfizer-BioNTech | A1, B |
| Rhabdomyolysis (n = 1) | 9 | - | 4 | Discharged | mRNA | Pfizer-BioNTech | A1 |
| Septic shock (n = 1) | 0 | - | 2 | Death | mRNA | Pfizer-BioNTech | A1 |
| Stevens–Johnson Syndrome (n = 1) | 48 | - | - | Discharged | Viral Vector | AstraZeneca | A1 |
| Transverse myelitis (n = 2) | 15.91 | 8.21 | 15 | Discharged | mRNA | Pfizer-BioNTech | A1 |
| 0.5 | 7 | Discharged | mRNA | Pfizer-BioNTech | B | ||
| Unspecified Radiculopathy (n = 1) | ND | ND | 10 | Discharged | Viral Vector | SII PVT | A1 |
| Unspecified thrombocytopenia (n = 1) | 23 | - | 11 | Discharged | Viral Vector | AstraZeneca | A1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendez-Lizarraga, C.A.; Chacon-Cruz, E.; Carrillo-Meza, R.; Hernández-Milán, N.S.; Inustroza-Sánchez, L.C.; Ovalle-Marroquín, D.F.; Machado-Contreras, J.R.; Ceballos Zuñiga, O.; Bejarano-Ramírez, V.; Aguilar-Aguayo, C.; et al. Report of Adverse Effects Following Population-Wide COVID-19 Vaccination: A Comparative Study between Six Different Vaccines in Baja-California, Mexico. Vaccines 2022, 10, 1196. https://doi.org/10.3390/vaccines10081196
Mendez-Lizarraga CA, Chacon-Cruz E, Carrillo-Meza R, Hernández-Milán NS, Inustroza-Sánchez LC, Ovalle-Marroquín DF, Machado-Contreras JR, Ceballos Zuñiga O, Bejarano-Ramírez V, Aguilar-Aguayo C, et al. Report of Adverse Effects Following Population-Wide COVID-19 Vaccination: A Comparative Study between Six Different Vaccines in Baja-California, Mexico. Vaccines. 2022; 10(8):1196. https://doi.org/10.3390/vaccines10081196
Chicago/Turabian StyleMendez-Lizarraga, Cesar A., Enrique Chacon-Cruz, Ricardo Carrillo-Meza, Néstor Saúl Hernández-Milán, Leslie C. Inustroza-Sánchez, Diego F. Ovalle-Marroquín, Jesús René Machado-Contreras, Omar Ceballos Zuñiga, Verónica Bejarano-Ramírez, Cipriano Aguilar-Aguayo, and et al. 2022. "Report of Adverse Effects Following Population-Wide COVID-19 Vaccination: A Comparative Study between Six Different Vaccines in Baja-California, Mexico" Vaccines 10, no. 8: 1196. https://doi.org/10.3390/vaccines10081196









