Acute Polyserositis with Cardiac Tamponade and Bilateral Refractory Pleural Effusion after ChAdOx1 nCoV-19 Vaccination
Abstract
:1. Introduction
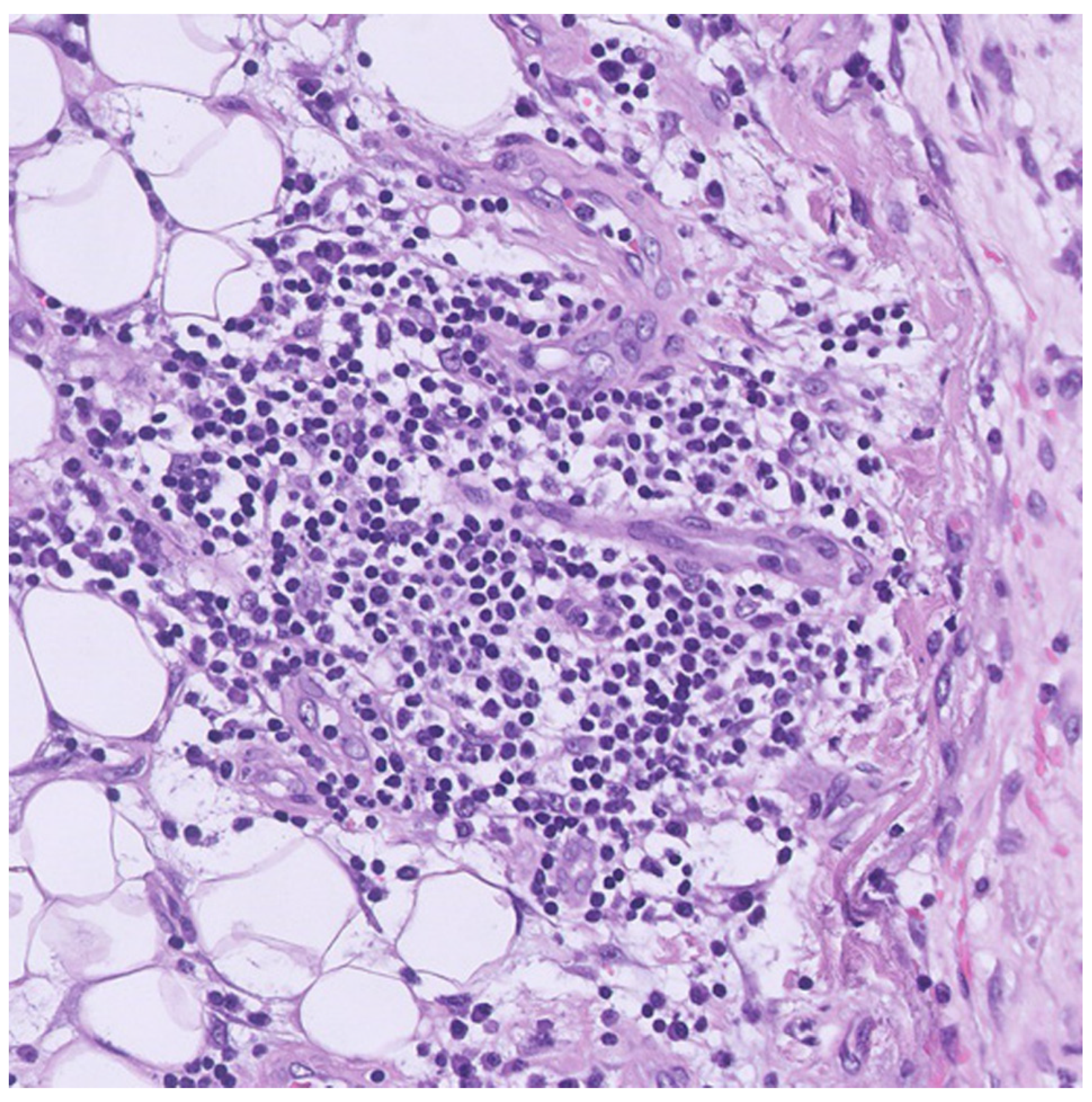
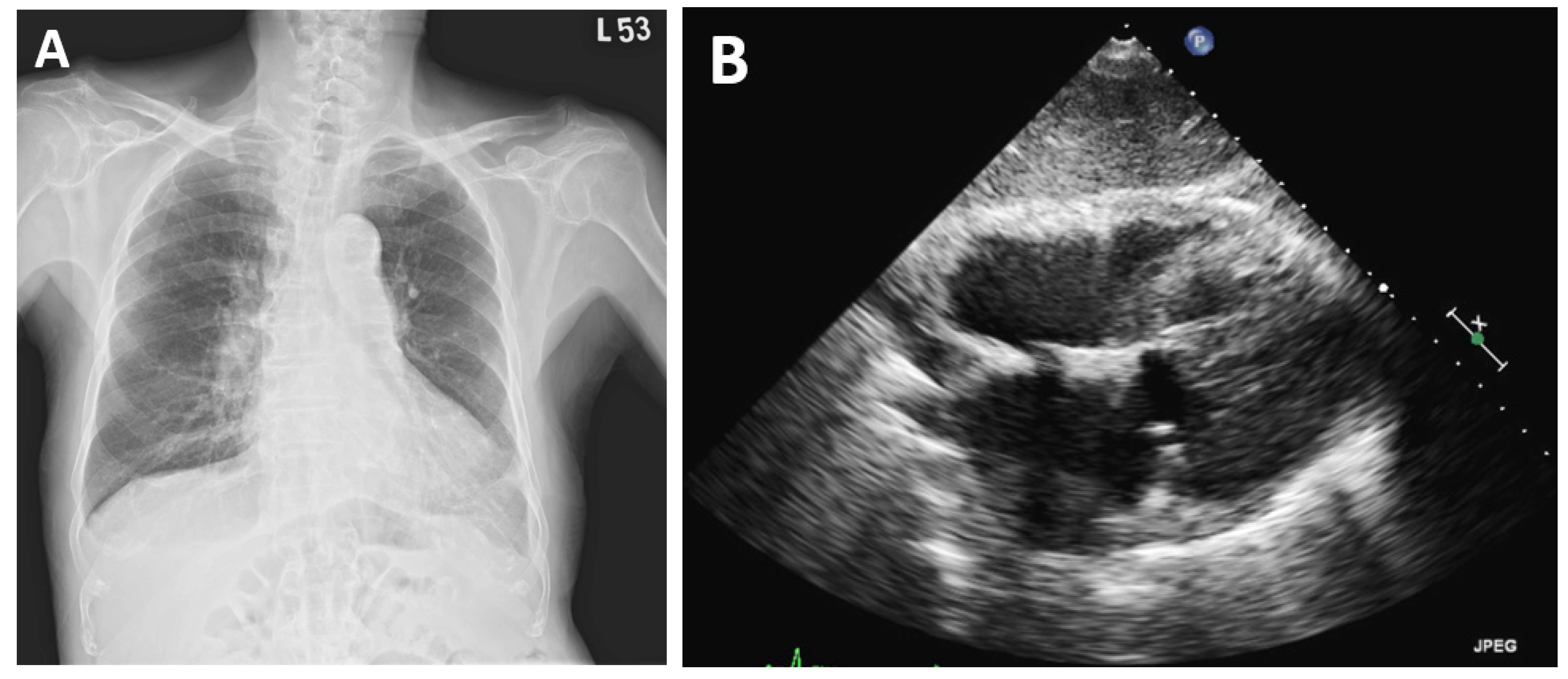
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nguyen, T.D.; Mall, G.; Westphal, J.G.; Weingärtner, O.; Möbius-Winkler, S.; Schulze, P.C. Acute myocarditis after COVID-19 vaccination with mRNA-1273 in a patient with former SARS-CoV-2 infection. ESC Heart Fail. 2021, 8, 4710–4714. [Google Scholar] [CrossRef]
- Hryniewicki, A.T.; Tolia, V.M.; Nene, R.V. Cardiac Tamponade After COVID-19 Vaccination. J. Emerg. Med. 2022, 62, 250–253. [Google Scholar] [CrossRef]
- Alami, A.; Krewski, D.; Mattison, D.; Wilson, K.; Gravel, C.A.; Villeneuve, P.J.; Farrell, P.J.; Crispo, J.A.G.; Perez-Lloret, S. Risk of Myocarditis and Pericarditis among Young Adults following mRNA COVID-19 Vaccinations. Vaccines 2022, 10, 722. [Google Scholar] [CrossRef]
- Mengesha, B.; Asenov, A.G.; Hirsh-Raccah, B.; Amir, O.; Pappo, O.; Asleh, R. Severe Acute Myocarditis after the Third (Booster) Dose of mRNA COVID-19 Vaccination. Vaccines 2022, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- Cassimatis, D.C.; Atwood, J.; Engler, R.M.; Linz, P.E.; Grabenstein, J.D.; Vernalis, M.N. Smallpox vaccination and myopericarditis: A clinical review. J. Am. Coll. Cardiol. 2004, 43, 1503–1510. [Google Scholar] [CrossRef] [Green Version]
- Mei, R.; Raschi, E.; Poluzzi, E.; Diemberger, I.; De Ponti, F. Recurrence of pericarditis after influenza vaccination: A case report and review of the literature. BMC Pharmacol. Toxicol. 2018, 19, 20. [Google Scholar] [CrossRef] [Green Version]
- Tawfik, P.; Gertner, E.; McEvoy, C.E. Severe polyserositis induced by the 13-valent pneumococcal conjugate vaccine: A case report. J. Med. Case Rep. 2017, 11, 142. [Google Scholar] [CrossRef] [Green Version]
- Siripanthong, B.; Asatryan, B.; Hanff, T.C.; Chatha, S.R.; Khanji, M.Y.; Ricci, F.; Muser, D.; Ferrari, V.A.; Nazarian, S.; Santangeli, P.; et al. The Pathogenesis and Long-Term Consequences of COVID-19 Cardiac Injury. JACC Basic Transl. Sci. 2022, 7, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Moosmann, J.; Gentles, T.; Occleshaw, C.; Mitchelson, B. COVID Vaccine-Associated Myocarditis in Adolescent Siblings: Does It Run in the Family? Vaccines 2022, 10, 611. [Google Scholar] [CrossRef]
- Manfredi, R.; Bianco, F.; Bucciarelli, V.; Ciliberti, G.; Guerra, F.; Schicchi, N.; Tavio, M.; Berton, E.; Surace, F.C.; Colaneri, M.; et al. Clinical Profiles and CMR Findings of Young Adults and Pediatrics with Acute Myocarditis Following mRNA COVID-19 Vaccination: A Case Series. Vaccines 2022, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Natanzon, A.; Kronzon, I. Pericardial and pleural effusions in congestive heart failure-anatomical, pathophysiologic, and clinical considerations. Am. J. Med. Sci. 2009, 338, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Messina, F.; Turano, L.; Tebala, C.; Calabrese, G.; Arcadi, N. Pericardial and pleural effusion in an elderly woman with COVID-19 pneumonia: CT findings. Radiol. Case Rep. 2021, 16, 1335–1338. [Google Scholar] [CrossRef] [PubMed]
- Cairns, L.; Abed El Khaleq, Y.; Storrar, W.; Scheuermann-Freestone, M. COVID-19 myopericarditis with cardiac tamponade in the absence of respiratory symptoms: A case report. J. Med. Case Rep. 2021, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.B.; Kuo, M.J.; Lin, T.Y.; Chien, C.S.; Yang, Y.P.; Chou, S.J.; Leu, H.B. Cardiovascular manifestation and treatment in COVID-19. J. Chin. Med. Assoc. 2020, 83, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Fazlollahi, A.; Zahmatyar, M.; Noori, M.; Nejadghaderi, S.A.; Sullman, M.J.M.; Shekarriz-Foumani, R.; Kolahi, A.A.; Singh, K.; Safiri, S. Cardiac complications following mRNA COVID-19 vaccines: A systematic review of case reports and case series. Rev. Med. Virol. 2021, e2318. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.-Y.; Lee, C.-C.; Huang, C.-C. Acute Polyserositis with Cardiac Tamponade and Bilateral Refractory Pleural Effusion after ChAdOx1 nCoV-19 Vaccination. Vaccines 2022, 10, 1286. https://doi.org/10.3390/vaccines10081286
Li G-Y, Lee C-C, Huang C-C. Acute Polyserositis with Cardiac Tamponade and Bilateral Refractory Pleural Effusion after ChAdOx1 nCoV-19 Vaccination. Vaccines. 2022; 10(8):1286. https://doi.org/10.3390/vaccines10081286
Chicago/Turabian StyleLi, Guan-Yi, Chang-Ching Lee, and Chin-Chou Huang. 2022. "Acute Polyserositis with Cardiac Tamponade and Bilateral Refractory Pleural Effusion after ChAdOx1 nCoV-19 Vaccination" Vaccines 10, no. 8: 1286. https://doi.org/10.3390/vaccines10081286
APA StyleLi, G.-Y., Lee, C.-C., & Huang, C.-C. (2022). Acute Polyserositis with Cardiac Tamponade and Bilateral Refractory Pleural Effusion after ChAdOx1 nCoV-19 Vaccination. Vaccines, 10(8), 1286. https://doi.org/10.3390/vaccines10081286





