Elevated NETs and Calprotectin Levels after ChAdOx1 nCoV-19 Vaccination Correlate with the Severity of Side Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Blood Collection and Processing
2.3. NETs Assays
2.4. Calprotectin Mixed Monoclonal Assay
2.5. Syndecan-1 Assay
2.6. Statistics
3. Results
3.1. Patient Characteristics
3.2. NETs, Calprotectin and Syndecan-1 Levels Are Increased
3.3. Correlations
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pavord, S.; Scully, M.; Hunt, B.J.; Lester, W.; Bagot, C.; Craven, B.; Rampotas, A.; Ambler, G.; Makris, M. Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. N. Engl. J. Med. 2021, 385, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.-H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130882. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–21010. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef]
- EMA. Renaming of AZ Vaccine: Vaxzevria (Previously COVID-19 Vaccine AstraZeneca): Link between the Vaccine and the Occurrence of Thrombosis in Combination with Thrombocytopenia. European Medicines Agency (europa.eu). Available online: https://www.ema.europa.eu/en/medicines/dhpc/vaxzevria-previously-COVID-19-vaccine-astrazeneca-link-between-vaccine-occurrence-thrombosis (accessed on 15 February 2022).
- See, I.; Lale, A.; Marquez, P.; Streiff, M.B.; Wheeler, A.P.; Tepper, N.K.; Woo, E.J.; Broder, K.R.; Edwards, K.M.; Gallego, R.; et al. Case Series of Thrombosis with Thrombocytopenia Syndrome After COVID-19 Vaccination—United States, December 2020 to August 2021. Ann. Intern. Med. 2022, 175, 513–522. [Google Scholar] [CrossRef]
- Holm, S.; Kared, H.; E Michelsen, A.; Kong, X.Y.; Dahl, T.B.; Schultz, N.H.; A Nyman, T.; Fladeby, C.; Seljeflot, I.; Ueland, T.; et al. Immune complexes, innate immunity, and NETosis in ChAdOx1 vaccine-induced thrombocytopenia. Eur. Heart J. 2021, 42, 4064–4072. [Google Scholar] [CrossRef]
- European Medicines Agency. AstraZeneca’s COVID-19 Vaccine: EMA Finds Possible Link to Very Rare Cases of Unusual Blood Clots with Low Blood Platelets. 2021. Available online: https://www.ema.europa.eu/en/news/astrazenecas-COVID-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood (accessed on 6 April 2021).
- Sørvoll, I.H.; Horvei, K.D.; Ernstsen, S.L.; Lægreid, I.J.; Lund, S.; Grønli, R.H.; Olsen, M.K.; Jacobsen, H.K.; Eriksson, A.; Halstensen, A.M.; et al. An observational study to identify the prevalence of thrombocytopenia and anti-PF4/polyanion antibodies in Norwegian health care workers after COVID-19 vaccination. J. Thromb. Haemost. 2021, 19, 1813–1818. [Google Scholar] [CrossRef]
- European Medicines Agency. AstraZeneca’s COVID-19 Vaccine Update: COVID-19 Vaccine Safety Update—Vaxzevria—9 December 2021 (europa.eu). Available online: https://www.ema.europa.eu/en/documents/COVID-19-vaccine-safety-update/COVID-19-vaccine-safety-update-vaxzevria-previously-COVID-19-vaccine-astrazeneca-9-december-2021_en.pdf (accessed on 20 January 2022).
- Salih, F.; Schönborn, L.; Kohler, S.; Franke, C.; Möckel, M.; Dörner, T.; Bauknecht, H.C.; Pille, C.; Graw, J.A.; Alonso, A.; et al. Vaccine-Induced Thrombocytopenia with Severe Headache. N. Engl. J. Med. 2021, 385, 2103–2105. [Google Scholar] [CrossRef]
- Fagerhol, M.; Dale, I.; Andersson, T. A radioimmunoassay for a granulocyte protein as a marker in studies on the turnover of such cells. Bull. Eur. Physiopathol. Respir. 1980, 16, 273–282. [Google Scholar] [CrossRef]
- Sherwood, R.A. Faecal markers of gastrointestinal inflammation. J. Clin. Pathol. 2012, 65, 981–985. [Google Scholar] [CrossRef]
- Steinbakk, M.; Naess-Andresen, C.-F.; Fagerhol, M.; Lingaas, E.; Dale, I.; Brandtzaeg, P. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet 1990, 336, 763–765. [Google Scholar] [CrossRef]
- Yui, S.; Nakatani, Y.; Mikami, M. Calprotectin (S100A8/S100A9), an Inflammatory Protein Complex from Neutrophils with a Broad Apoptosis-Inducing Activity. Biol. Pharm. Bull. 2003, 26, 753–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Zuo, Y.; Yalavarthi, S.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Woodward, W.; Lezak, S.P.; Lugogo, N.L.; et al. Neutrophil calprotectin identifies severe pulmonary disease in COVID-19. J. Leukoc. Biol. 2020, 109, 67–72. [Google Scholar] [CrossRef]
- Silvin, A.; Chapuis, N.; Dunsmore, G.; Goubet, A.-G.; Dubuisson, A.; Derosa, L.; Almire, C.; Hénon, C.; Kosmider, O.; Droin, N.; et al. Elevated Calprotectin and Abnormal Myeloid Cell Subsets Discriminate Severe from Mild COVID-19. Cell 2020, 182, 1401–1418.e18. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.N.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Y.; Zuo, M.; Yalavarthi, S.; Gockman, K.; Madison, J.A.; Shi, H.; Woodard, W.; Lezak, S.P.; Lugogo, N.L.; Knight, J.S.; et al. Neutrophil extracellular traps and thrombosis in COVID-19. J. Thromb. Thrombolysis 2020, 51, 446–453. [Google Scholar] [CrossRef]
- Napirei, M.; Ludwig, S.; Mezrhab, J.; Klöckl, T.; Mannherz, H.G. Murine serum nucleases - contrasting effects of plasmin and heparin on the activities of DNase1 and DNase1-like 3 (DNase1l3). FEBS J. 2009, 276, 1059–1073. [Google Scholar] [CrossRef]
- Bruschi, M.; Moroni, G.; Sinico, R.A.; Franceschini, F.; Fredi, M.; Vaglio, A.; Cavagna, L.; Petretto, A.; Pratesi, F.; Migliorini, P.; et al. Neutrophil Extracellular Traps in the Autoimmunity Context. Front. Med. 2021, 8, 614829. [Google Scholar] [CrossRef]
- Yamaoka-Tojo, M. Vascular Endothelial Glycocalyx Damage in COVID-19. Int. J. Mol. Sci. 2020, 21, 9712. [Google Scholar] [CrossRef]
- Rauch, A.; Dupont, A.; Goutay, J.; Caplan, M.; Staessens, S.; Moussa, M.; Jeanpierre, E.; Corseaux, D.; Lefevre, G.; Lassalle, F.; et al. Endotheliopathy Is Induced by Plasma from Critically Ill Patients and Associated with Organ Failure in Severe COVID-19. Circulation 2020, 142, 1881–1884. [Google Scholar] [CrossRef]
- Fraser, D.D.; Patterson, E.K.; Slessarev, M.; Gill, S.E.; Martin, C.; Daley, M.; Miller, M.R.; Patel, M.A.; dos Santos, C.C.; Bosma, K.J.; et al. Endothelial Injury and Glycocalyx Degradation in Critically Ill Coronavirus Disease 2019 Patients: Implications for Microvascular Platelet Aggregation. Crit. Care Explor. 2020, 2, e0194. [Google Scholar] [CrossRef] [PubMed]
- Fagerhol, M.K.; Johnson, E.; Tangen, J.; Hollan, I.; Mirlashari, M.R.; Nissen-Meyer, L.S.H.; Hetland, G. NETs analysed by novel calprotectin-based assays in blood donors and patients with multiple myeloma or rheumatoid arthritis: A pilot study. Scand. J. Immunol. 2020, 91, e12870. [Google Scholar] [CrossRef] [PubMed]
- Fagerhol, M.K.; Rugtveit, J. Heterogeneity of Fecal Calprotectin Reflecting Generation of Neutrophil Extracellular Traps (NETs) in the Gut: New Immunoassays Are Available. J. Mol. Pathol. 2022, 3, 38–51. [Google Scholar] [CrossRef]
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil Extracellular Traps Contain Calprotectin, a Cytosolic Protein Complex Involved in Host Defense against Candida albicans. PLOS Pathog. 2009, 5, e1000639. [Google Scholar] [CrossRef] [Green Version]
- Wiedmann, M.; Skattør, T.; Stray-Pedersen, A.; Romundstad, L.; Antal, E.-A.; Marthinsen, P.B.; Sørvoll, I.H.; Ernstsen, S.L.; Lund, C.G.; Holme, P.A.; et al. Vaccine Induced Immune Thrombotic Thrombocytopenia Causing a Severe Form of Cerebral Venous Thrombosis with High Fatality Rate: A Case Series. Front. Neurol. 2021, 12, 721146. [Google Scholar] [CrossRef]
- Boneschansker, L.; Inoue, Y.; Oklu, R.; Irimia, D. Capillary plexuses are vulnerable to neutrophil extracellular traps. Integr. Biol. 2016, 8, 149–155. [Google Scholar] [CrossRef] [Green Version]

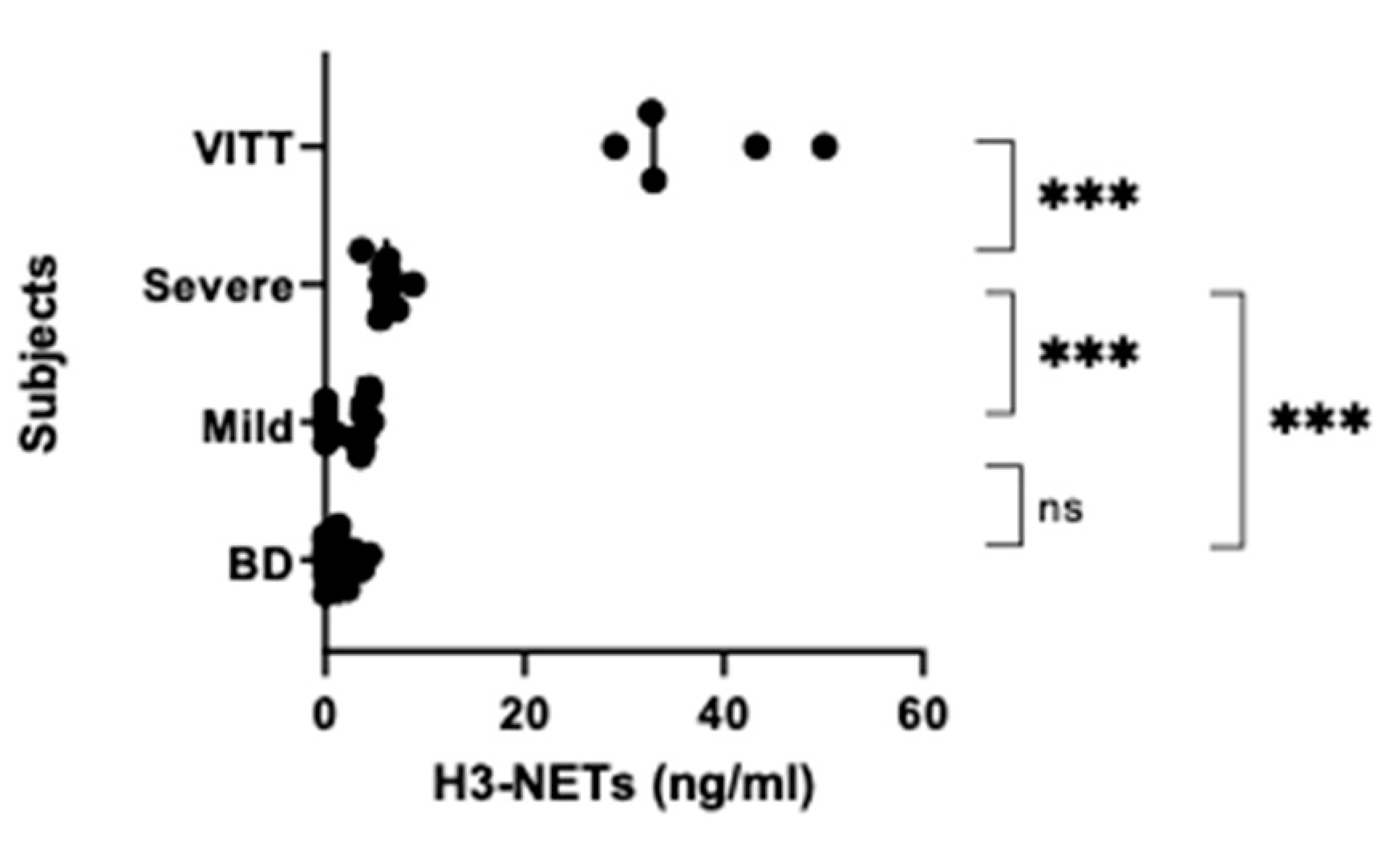
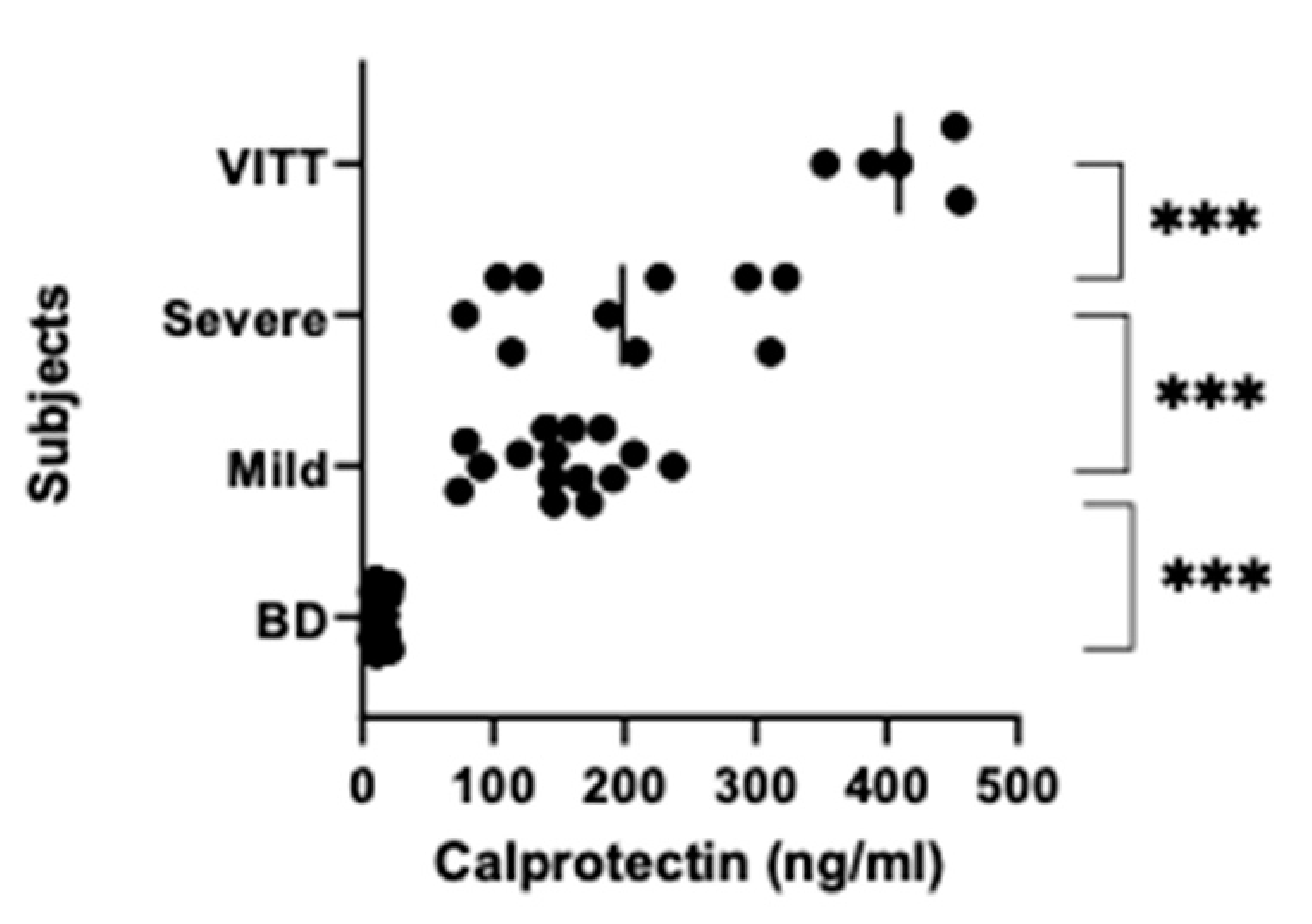
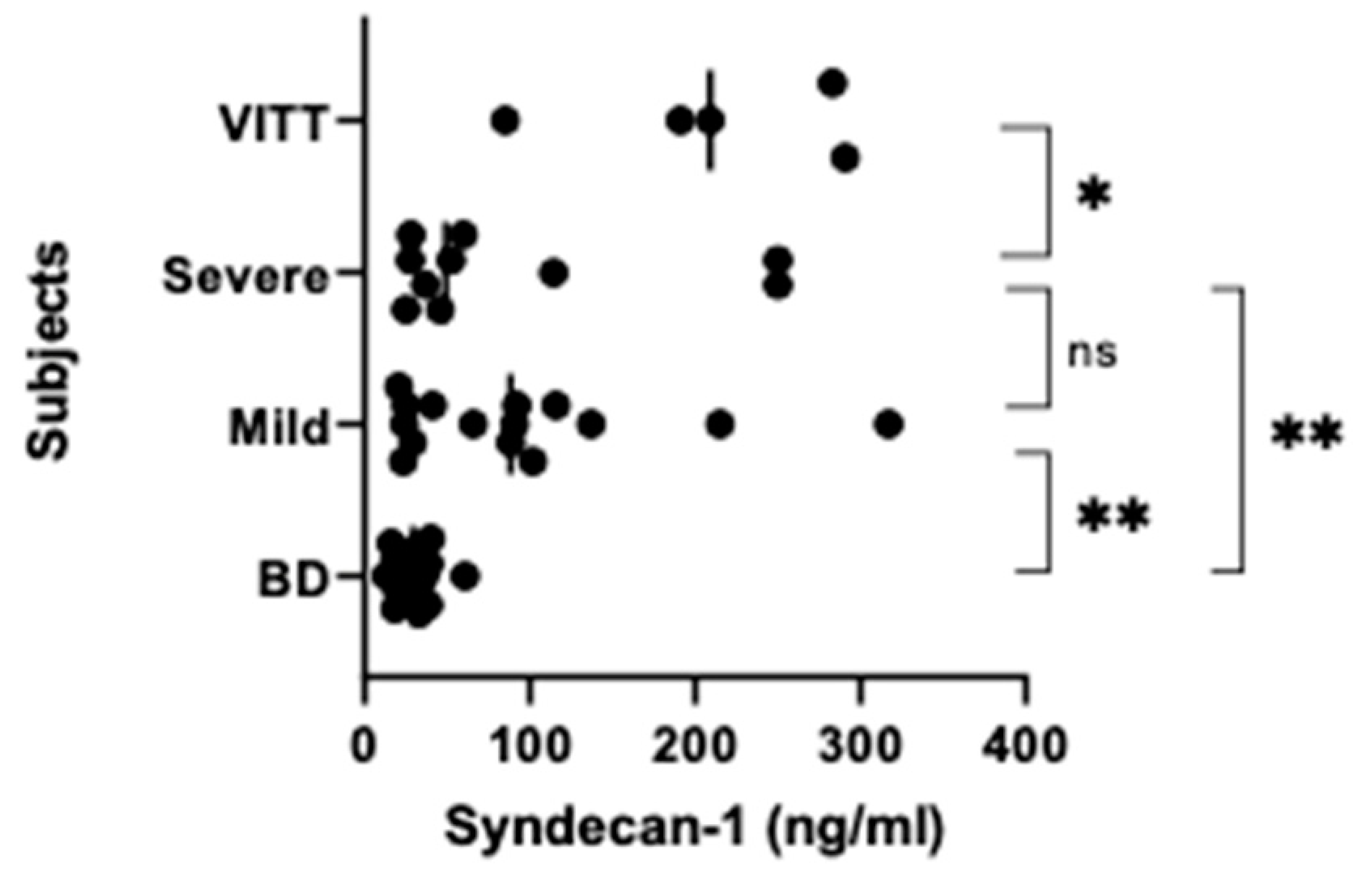
| ̶ | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|
| Age (range) | 39 (32–54) | 36 (28–48) | 38 (27–63) | 45 (24–64) |
| Sex (% women) | 80 | 100 | 80 | 50 |
| Platelet count Mean (SD) | 27 (17.2) * | 275 (88) | 303 (57) | 145-390 ** |
| Patient No | Age (Years) and Gender | Symptoms | PLT | Signs | Comments | |
|---|---|---|---|---|---|---|
| Group 1 (VITT) | 1 | 37 F | Fever, HA, visual disturbance | 22 | VITT | Fatal |
| 2 | 42 F | HA, drowsiness | 14 | VITT | Fatal | |
| 3 | 32 M | Back pain | 10 | VITT | Full recovery | |
| 4 | 39 F | HA, abdominal pain | 70 | VITT | Full recovery | |
| 5 | 54 F | HA, hemiparesis | 19 | VITT | Fatal | |
| Group 2 (Prolonged symptoms) | 6 | 43 F | Mild HA (1 wk), nausea for 2d | 149 | Large hematoma on lower extremities | ̶ |
| 7 | 40 F | Mild to moderate HA (3 wks), fever, fatigue, visual disturbance | 420 | Bruises | ̶ | |
| 8 | 32 F | Mild to moderate HA (2 wks) | 386 | Bruises | ̶ | |
| 9 | 32 F | Mild HA (2 wks) | - | Bruises | ̶ | |
| 10 | 32 F | Mild HA (4 wks) | 267 | Bruises and petechiae | ̶ | |
| 11 | 31 F | Mild to moderate HA (1 wk), muscle aches, fever | 212 | Petechiae | ̶ | |
| 12 | 28 F | Mild to strong HA (2 wks), fever | 255 | Bruises and petechiae | ̶ | |
| 13 | 45 F | Moderate to strong HA (3 wks), fever | 250 | Bruises and petechiae | ||
| 14 | 48 F | Strong HA (3 wks), fever, vertigo, joint aches | - | Bruises and petechiae | ̶ | |
| 15 | 42 F | Moderate HA, fever, chills, nosebleed | 263 | Bruises and petechiae | ̶ | |
| Group 3 (Brief or no symptoms) | 16 | 38 F | Fever for 2 days, tenderness in arm for 10 days | 285 | ̶ | ̶ |
| 17 | 42 F | Fever, muscle pain, influenza-like symptoms for 1 d | 333 | ̶ | ̶ | |
| 18 | 44 F | Light symptoms for 2 d: tenderness in arms, fatigue, HA | 201 | ̶ | ̶ | |
| 19 | 35 F | Influenza-like symptoms with HA and fatigue for 1 d | 328 | ̶ | ̶ | |
| 20 | 27F | HA, dizziness, nausea for 1.5 d | 291 | ̶ | ̶ | |
| 21 | 59 F | Tenderness in arm, stiffness in joints, fatigue for 1 d | 240 | ̶ | ̶ | |
| 22 | 32 F | Fever for 1d, HA, back pain | 207 | ̶ | ̶ | |
| 23 | 32 F | Fever for 24 h | 388 | ̶ | ̶ | |
| 24 | 32 F | Fever and muscle ache 3 days | 322 | ̶ | ̶ | |
| 25 | 61 F | Fever, cold sweats, some HA for 1.5 d | 265 | ̶ | ̶ | |
| 26 | 50 F | High fever for 1d with nausea, then low fever for next day | 373 | ̶ | ̶ | |
| 27 | 35 F | Fever and HA for 1d, tenderness in arm for 1 wk | 338 | ̶ | ̶ | |
| 28 | 43 M | Fever for 1d, tired, mild HA for <1 d | 297 | ̶ | ̶ | |
| 29 | 34 M | Congestion, head 1d | 300 | ̶ | ̶ | |
| 30 | 63 M | No symptoms | 373 | ̶ | ̶ |
| Parameters | Side Effects | Platelet Count | H3-NETs | Calprotectin | Syndecan-1 |
|---|---|---|---|---|---|
| Side effects | 1 | −0.763 ** | 0.745 ** | 0.902 ** | 0.590 ** |
| Platelet count | ̶ | 1 | −0.813 ** | −0.626 ** | −0.488 * |
| H3-NETs | ̶ | ̶ | 1 | 0.818 ** | 0.427 * |
| Calprotectin | ̶ | ̶ | ̶ | 1 | 0.189 ns |
| Syndecan-1 | ̶ | ̶ | ̶ | ̶ | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hetland, G.; Fagerhol, M.K.; Wiedmann, M.K.H.; Søraas, A.V.L.; Mirlashari, M.R.; Nissen-Meyer, L.S.H.; Istre, M.S.; Holme, P.A.; Schultz, N.H. Elevated NETs and Calprotectin Levels after ChAdOx1 nCoV-19 Vaccination Correlate with the Severity of Side Effects. Vaccines 2022, 10, 1267. https://doi.org/10.3390/vaccines10081267
Hetland G, Fagerhol MK, Wiedmann MKH, Søraas AVL, Mirlashari MR, Nissen-Meyer LSH, Istre MS, Holme PA, Schultz NH. Elevated NETs and Calprotectin Levels after ChAdOx1 nCoV-19 Vaccination Correlate with the Severity of Side Effects. Vaccines. 2022; 10(8):1267. https://doi.org/10.3390/vaccines10081267
Chicago/Turabian StyleHetland, Geir, Magne Kristoffer Fagerhol, Markus Karl Hermann Wiedmann, Arne Vasli Lund Søraas, Mohammad Reza Mirlashari, Lise Sofie Haug Nissen-Meyer, Mette Stausland Istre, Pål Andre Holme, and Nina Haagenrud Schultz. 2022. "Elevated NETs and Calprotectin Levels after ChAdOx1 nCoV-19 Vaccination Correlate with the Severity of Side Effects" Vaccines 10, no. 8: 1267. https://doi.org/10.3390/vaccines10081267
APA StyleHetland, G., Fagerhol, M. K., Wiedmann, M. K. H., Søraas, A. V. L., Mirlashari, M. R., Nissen-Meyer, L. S. H., Istre, M. S., Holme, P. A., & Schultz, N. H. (2022). Elevated NETs and Calprotectin Levels after ChAdOx1 nCoV-19 Vaccination Correlate with the Severity of Side Effects. Vaccines, 10(8), 1267. https://doi.org/10.3390/vaccines10081267






