The Cellular and Molecular Immunotherapy in Prostate Cancer
Abstract
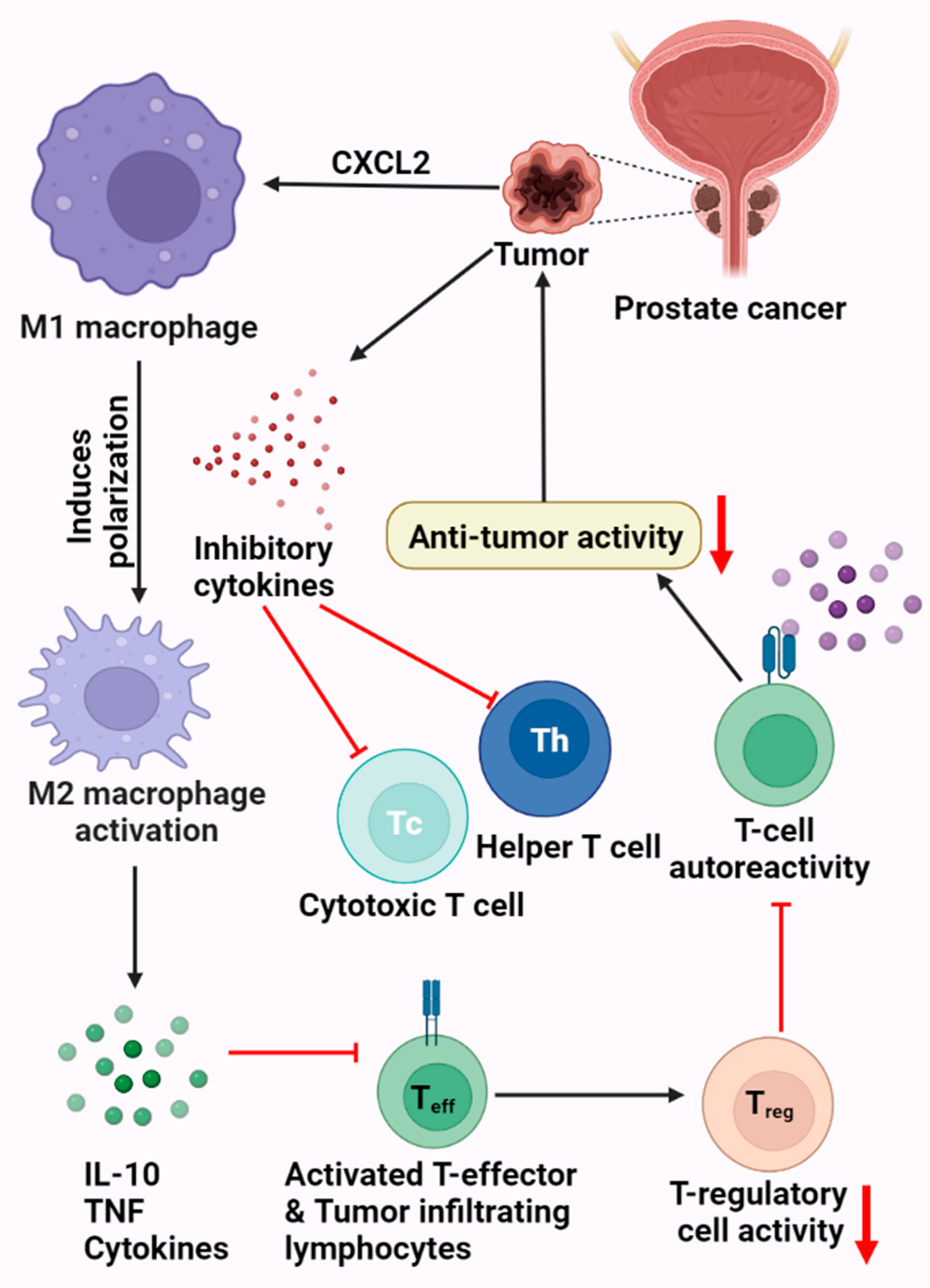
:1. Introduction
2. Epigenetic and Predictive Biomarkers for PCa
2.1. DNA Methylation
2.2. Histone Modifications
2.3. PD-L1 Expression
2.4. Indoleamine-2, 3-Dioxygenase (IDO)
3. PCa and the Immune System
Prostate Tumor Microenvironment
4. Immunotherapy Resistance of PCa
5. Immune Checkpoint Inhibition
5.1. Programmed Cell Death Protein 1 and Programmed Cell Death Protein Ligand-1 (PD-1/PD-L1)
5.2. B7-H3 Blockade
5.3. LAG-3
5.4. OX40/OX40L
5.5. 4-1BB/4-1BBL
5.6. VISTA
6. Immunotherapy for PCa
6.1. CART Cell Therapy
6.1.1. Improving CAR T Cell Persistence
6.1.2. Specificity and Safety
6.2. Experimental Prostate Cancer Vaccines
Aglatimagene Besadenovec
6.3. Vaccine-Based Therapies
6.4. DNA-Based Vaccines
6.5. Cell-Based Vaccines
6.6. Peptide-Based Vaccines
6.7. Viral Vector-Based Vaccines
7. Combination Immunotherapy
7.1. CAR T Cells Combined with Androgen Deprivation Therapy (ADT)
7.2. CAR T Cells Combined with Chemotherapy
7.3. Hormone Therapy and Immunotherapy
7.4. Chemotherapy and Immunotherapy
7.5. Vaccines and Immune Checkpoint Blockades
7.6. CTLA-4/PD-1 Combination
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef] [PubMed]
- DS, P.; Chaturvedi, P.K.; Shimokawa, T.; Kim, K.-H.; Park, W.-Y. Silencing of fused toes homolog (FTS) increases radiosensitivity to carbon-ion through downregulation of Notch signaling in cervical cancer cells. Front. Oncol. 2021, 11, 4432. [Google Scholar]
- Prabakaran, D.; Muthusami, S.; Sivaraman, T.; Yu, J.-R.; Park, W.-Y. Silencing of FTS increases radiosensitivity by blocking radiation-induced Notch1 activation and spheroid formation in cervical cancer cells. Int. J. Biol. Macromol. 2019, 126, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.-R.; Lee, J.H.; Ponnazhagan, S. Revisiting Immunotherapy: A Focus on Prostate CancerAdvances and Limitations of Immunotherapy in Prostate Cancer. Cancer Res. 2020, 80, 1615–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archer, M.; Dogra, N.; Kyprianou, N. Inflammation as a driver of prostate cancer metastasis and therapeutic resistance. Cancers 2020, 12, 2984. [Google Scholar] [CrossRef]
- Vlajnic, T.; Bubendorf, L. Molecular pathology of prostate cancer: A practical approach. Pathology 2021, 53, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Carceles-Cordon, M.; Kelly, W.K.; Gomella, L.; Knudsen, K.E.; Rodriguez-Bravo, V.; Domingo-Domenech, J. Cellular rewiring in lethal prostate cancer: The architect of drug resistance. Nat. Rev. Urol. 2020, 17, 292–307. [Google Scholar] [CrossRef]
- Schmidt-Hegemann, N.-S.; Fendler, W.P.; Ilhan, H.; Herlemann, A.; Buchner, A.; Stief, C.; Eze, C.; Rogowski, P.; Li, M.; Bartenstein, P. Outcome after PSMA PET/CT based radiotherapy in patients with biochemical persistence or recurrence after radical prostatectomy. Radiat. Oncol. 2018, 13, 37. [Google Scholar] [CrossRef] [Green Version]
- Cai, T.; Santi, R.; Tamanini, I.; Galli, I.C.; Perletti, G.; Bjerklund Johansen, T.E.; Nesi, G. Current knowledge of the potential links between inflammation and prostate cancer. Int. J. Mol. Sci. 2019, 20, 3833. [Google Scholar] [CrossRef] [Green Version]
- Muthusami, S.; Prabakaran, D.; Yu, J.-R.; Park, W.-Y. EGF-induced expression of Fused Toes Homolog (FTS) facilitates epithelial–mesenchymal transition and promotes cell migration in ME180 cervical cancer cells. Cancer Lett. 2014, 351, 252–259. [Google Scholar] [CrossRef]
- Muthusami, S.; Prabakaran, D.; Yu, J.-R.; Park, W.-Y. FTS is responsible for radiation-induced nuclear phosphorylation of EGFR and repair of DNA damage in cervical cancer cells. J. Cancer Res. Clin. Oncol. 2015, 141, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Fay, E.K.; Graff, J.N. Immunotherapy in prostate cancer. Cancers 2020, 12, 1752. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.J.; Oladeru, O.T.; Wang, K.; Attwood, K.; Singh, A.K.; Haas-Kogan, D.A.; Neira, P.M. Prostate cancer screening patterns among sexual and gender minority individuals. Eur. Urol. 2021, 79, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Westdorp, H.; Sköld, A.E.; Snijer, B.A.; Franik, S.; Mulder, S.F.; Major, P.P.; Foley, R.; Gerritsen, W.R.; de Vries, I.J.M. Immunotherapy for prostate cancer: Lessons from responses to tumor-associated antigens. Front. Immunol. 2014, 5, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hashimi, M.M. Trends in breast cancer incidence in Iraq during the period 2000–2019. Asian Pac. J. Cancer Prev. APJCP 2021, 22, 3889. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Gastroenterol. Rev. Przegląd Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent global patterns in prostate cancer incidence and mortality rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef]
- Barsouk, A.; Padala, S.A.; Vakiti, A.; Mohammed, A.; Saginala, K.; Thandra, K.C.; Rawla, P.; Barsouk, A. Epidemiology, staging and management of prostate cancer. Med. Sci. 2020, 8, 28. [Google Scholar] [CrossRef]
- Pathirana, T.; Sequeira, R.; Del Mar, C.; Dickinson, J.A.; Armstrong, B.K.; Bell, K.J.; Glasziou, P. Trends in Prostate Specific Antigen (PSA) testing and prostate cancer incidence and mortality in Australia: A critical analysis. Cancer Epidemiol. 2022, 77, 102093. [Google Scholar] [CrossRef]
- Cheng, E.; Lee, D.H.; Tamimi, R.M.; Hankinson, S.E.; Willett, W.C.; Giovannucci, E.L.; Eliassen, A.H.; Stampfer, M.J.; Mucci, L.A.; Fuchs, C.S. Long-term survival and causes of death after diagnoses of common cancers in 3 cohorts of US health professionals. JNCI Cancer Spectr. 2022, 6, pkac021. [Google Scholar] [CrossRef]
- Shah, R.B.; Zhou, M. Recent advances in prostate cancer pathology: Gleason grading and beyond. Pathol. Int. 2016, 66, 260–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W. A contemporary prostate cancer grading system: A validated alternative to the Gleason score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wulczyn, E.; Nagpal, K.; Symonds, M.; Moran, M.; Plass, M.; Reihs, R.; Nader, F.; Tan, F.; Cai, Y.; Brown, T. Predicting prostate cancer specific-mortality with artificial intelligence-based Gleason grading. Commun. Med. 2021, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Mohler, J.L.; Antonarakis, E.S.; Armstrong, A.J.; D’Amico, A.V.; Davis, B.J.; Dorff, T.; Eastham, J.A.; Enke, C.A.; Farrington, T.A.; Higano, C.S. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 479–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Maghrabi, J.A.; Bakshi, N.A.; Farsi, H.M. Gleason grading of prostate cancer in needle core biopsies: A comparison of general and urologic pathologists. Ann. Saudi Med. 2013, 33, 40–44. [Google Scholar] [CrossRef]
- Epstein, J.I.; Allsbrook Jr, W.C.; Amin, M.B.; Egevad, L.L.; Committee, I.G. The 2005 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am. J. Surg. Pathol. 2005, 29, 1228–1242. [Google Scholar] [CrossRef] [Green Version]
- Gleason, D.F. Histologic grading of prostate cancer: A perspective. Hum. Pathol. 1992, 23, 273–279. [Google Scholar] [CrossRef]
- Munjal, A.; Leslie, S.W. Gleason Score. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kim, T.J.; Koo, K.C. Current status and future perspectives of checkpoint inhibitor immunotherapy for prostate cancer: A comprehensive review. Int. J. Mol. Sci. 2020, 21, 5484. [Google Scholar] [CrossRef]
- Quinn, D.I.; Shore, N.D.; Egawa, S.; Gerritsen, W.R.; Fizazi, K. Immunotherapy for castration-resistant prostate cancer: Progress and new paradigms. In Urologic Oncology: Seminars and Original Investigations; Society of Urologic Oncology: Schaumburg, IL, USA, 2015; Volume 33, pp. 245–260. [Google Scholar]
- Cheng, J.C.; Jones, P.A. Epigenetic events in cancer. In Introduction to the Cellular and Molecular Biology of Cancer; Oxford University Press: Oxford, UK, 2005; pp. 78–94. [Google Scholar]
- Alshammari, E.; Zhang, Y.; Sobota, J.; Yang, Z. Aberrant DNA Methylation of Tumor Suppressor Genes and Oncogenes as Cancer Biomarkers. Genom. Epigenomic Biomark. Toxicol. Dis. Clin. Ther. Actions 2022, 257–271. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, G.; Zhou, F.; Su, B.; Li, Y. DNA methylation profiles in cancer diagnosis and therapeutics. Clin. Exp. Med. 2018, 18, 1–14. [Google Scholar] [CrossRef]
- Grasso, C.; Popovic, M.; Isaevska, E.; Lazzarato, F.; Fiano, V.; Zugna, D.; Pluta, J.; Weathers, B.; D’Andrea, K.; Almstrup, K. Association study between polymorphisms in DNA methylation-related genes and testicular germ cell tumor risk. Cancer Epidemiol. Biomark. Prev. 2022, OF1–OF11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.G.; Chen, W.S.; Li, H.; Foye, A.; Zhang, M.; Sjöström, M.; Aggarwal, R.; Playdle, D.; Liao, A.; Alumkal, J.J. The DNA methylation landscape of advanced prostate cancer. Nat. Genet. 2020, 52, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Brothman, A.R.; Swanson, G.; Maxwell, T.M.; Cui, J.; Murphy, K.J.; Herrick, J.; Speights, V.; Isaac, J.; Rohr, L.R. Global hypomethylation is common in prostate cancer cells: A quantitative predictor for clinical outcome? Cancer Genet. Cytogenet. 2005, 156, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Santourlidis, S.; Florl, A.; Ackermann, R.; Wirtz, H.C.; Schulz, W.A. High frequency of alterations in DNA methylation in adenocarcinoma of the prostate. Prostate 1999, 39, 166–174. [Google Scholar] [CrossRef]
- Schulz, W.A.; Elo, J.P.; Florl, A.R.; Pennanen, S.; Santourlidis, S.; Engers, R.; Buchardt, M.; Seifert, H.H.; Visakorpi, T. Genomewide DNA hypomethylation is associated with alterations on chromosome 8 in prostate carcinoma. Genes Chromosomes Cancer 2002, 35, 58–65. [Google Scholar] [CrossRef]
- Florl, A.; Steinhoff, C.; Müller, M.; Seifert, H.; Hader, C.; Engers, R.; Ackermann, R.; Schulz, W. Coordinate hypermethylation at specific genes in prostate carcinoma precedes LINE-1 hypomethylation. Br. J. Cancer 2004, 91, 985–994. [Google Scholar] [CrossRef] [Green Version]
- Yegnasubramanian, S.; Haffner, M.C.; Zhang, Y.; Gurel, B.; Cornish, T.C.; Wu, Z.; Irizarry, R.A.; Morgan, J.; Hicks, J.; DeWeese, T.L. DNA hypomethylation arises later in prostate cancer progression than CpG island hypermethylation and contributes to metastatic tumor heterogeneity. Cancer Res. 2008, 68, 8954–8967. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.K.; Workman, J.L. Histone acetyltransferase complexes: One size doesn’t fit all. Nat. Rev. Mol. Cell Biol. 2007, 8, 284–295. [Google Scholar] [CrossRef]
- Pomerantz, M.M.; Qiu, X.; Zhu, Y.; Takeda, D.Y.; Pan, W.; Baca, S.C.; Gusev, A.; Korthauer, K.D.; Severson, T.M.; Ha, G. Prostate cancer reactivates developmental epigenomic programs during metastatic progression. Nat. Genet. 2020, 52, 790–799. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Alumkal, J.J.; Stein, M.N.; Taplin, M.-E.; Babb, J.; Barnett, E.S.; Gomez-Pinillos, A.; Liu, X.; Moore, D.; DiPaola, R. Epigenetic Therapy with Panobinostat Combined with Bicalutamide Rechallenge in Castration-Resistant Prostate CancerEpigenetic Therapy in Antiandrogen-Resistant Prostate Cancer. Clin. Cancer Res. 2019, 25, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Ness, N.; Andersen, S.; Khanehkenari, M.R.; Nordbakken, C.V.; Valkov, A.; Paulsen, E.-E.; Nordby, Y.; Bremnes, R.M.; Donnem, T.; Busund, L.-T. The prognostic role of immune checkpoint markers programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) in a large, multicenter prostate cancer cohort. OncoTargets Ther. 2017, 8, 26789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, H.; Pinski, J.K. Association of ARV7 Expression with Molecular and Clinical Characteristics in Prostate Cancer; American Society of Clinical Oncology: Alexandria, VA, USA, 2016. [Google Scholar] [CrossRef]
- Jafari, S.; Molavi, O.; Kahroba, H.; Hejazi, M.S.; Maleki-Dizaji, N.; Barghi, S.; Kiaie, S.H.; Jadidi-Niaragh, F. Clinical application of immune checkpoints in targeted immunotherapy of prostate cancer. Cell. Mol. Life Sci. 2020, 77, 3693–3710. [Google Scholar] [CrossRef] [PubMed]
- Disis, M.L. Mechanism of action of immunotherapy. In Seminars in Oncology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 41, pp. S3–S13. [Google Scholar]
- Xie, G.; Dong, H.; Liang, Y.; Ham, J.D.; Rizwan, R.; Chen, J. CAR-NK cells: A promising cellular immunotherapy for cancer. EBioMedicine 2020, 59, 102975. [Google Scholar] [CrossRef] [PubMed]
- McGrail, D.; Pilié, P.; Rashid, N.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.; Lim, B. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.; Elkord, E. Acquired resistance to cancer immunotherapy: Role of tumor-mediated immunosuppression. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 41, pp. 13–27. [Google Scholar]
- Rausch, M.P.; Hastings, K.T. Innate and Adaptive Immune Responses to Cancer. In Fundamentals of Cancer Prevention; Springer: Berlin/Heidelberg, Germany, 2019; pp. 111–159. [Google Scholar] [CrossRef]
- Bayati, F.; Mohammadi, M.; Valadi, M.; Jamshidi, S.; Foma, A.M.; Sharif-Paghaleh, E. The therapeutic potential of regulatory T cells: Challenges and opportunities. Front. Immunol. 2021, 11, 585819. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer 2020, 19, 116. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Teng, M.W.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef]
- Zhu, S.; Luo, Z.; Li, X.; Han, X.; Shi, S.; Zhang, T. Tumor-associated macrophages: Role in tumorigenesis and immunotherapy implications. J. Cancer 2021, 12, 54. [Google Scholar] [CrossRef]
- Boettcher, A.N.; Usman, A.; Morgans, A.; VanderWeele, D.J.; Sosman, J.; Wu, J.D. Past, current, and future of immunotherapies for prostate cancer. Front. Oncol. 2019, 9, 884. [Google Scholar] [CrossRef] [Green Version]
- Shiao, S.L.; Chu, G.C.-Y.; Chung, L.W. Regulation of prostate cancer progression by the tumor microenvironment. Cancer Lett. 2016, 380, 340–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamaki, M.; Zoumpourlis, V. Immunotherapy as a precision medicine tool for the treatment of prostate cancer. Cancers 2021, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.G. Prostate cancer as a model for tumour immunotherapy. Nat. Rev. Immunol. 2010, 10, 580–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beer, T.M.; Kwon, E.D.; Drake, C.G.; Fizazi, K.; Logothetis, C.; Gravis, G.; Ganju, V.; Polikoff, J.; Saad, F.; Humanski, P. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J. Clin. Oncol. 2017, 35, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, C.H.; Antonarakis, E.S. Emerging treatments for metastatic castration-resistant prostate cancer: Immunotherapy, PARP inhibitors, and PSMA-targeted approaches. Cancer Treat. Res. Commun. 2020, 23, 100164. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Pachynski, R.K.; Narayan, V.; Flechon, A.; Gravis, G.; Galsky, M.D.; Mahammedi, H.; Patnaik, A.; Subudhi, S.K.; Ciprotti, M. Initial Results from a Phase II Study of Nivolumab (NIVO) Plus Ipilimumab (IPI) for the Treatment of Metastatic Castration-Resistant Prostate Cancer (mCRPC.; CheckMate 650); American Society of Clinical Oncology: Alexandria, VA, USA, 2019. [Google Scholar] [CrossRef]
- Alemohammad, H.; Najafzadeh, B.; Asadzadeh, Z.; Baghbanzadeh, A.; Ghorbaninezhad, F.; Najafzadeh, A.; Safarpour, H.; Bernardini, R.; Brunetti, O.; Sonnessa, M. The importance of immune checkpoints in immune monitoring: A future paradigm shift in the treatment of cancer. Biomed. Pharmacother. 2022, 146, 112516. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiang, Y.; Li, F.; Yin, C.; Li, B.; Ke, X. WNT/β-catenin signaling pathway regulating T cell-inflammation in the tumor microenvironment. Front. Immunol. 2019, 10, 2293. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.T.; Lee, J.Y.; Lim, H.; Lee, S.H.; Moon, Y.J.; Pyo, H.J.; Ryu, S.E.; Shin, W.; Heo, Y.-S. Molecular mechanism of PD-1/PD-L1 blockade via anti-PD-L1 antibodies atezolizumab and durvalumab. Sci. Rep. 2017, 7, 5532. [Google Scholar] [CrossRef] [Green Version]
- Borst, J.; Busselaar, J.; Bosma, D.M.; Ossendorp, F. Mechanism of action of PD-1 receptor/ligand targeted cancer immunotherapy. Eur. J. Immunol. 2021, 51, 1911–1920. [Google Scholar] [CrossRef]
- Chapoval, A.; Chapoval, S.; Shcherbakova, N.; Shcherbakov, D. Immune checkpoints of the B7 family. Part 2. Representatives of the B7 family B7-H3, B7-H4, B7-H5, B7-H6, B7-H7, and ILDR2 and their receptors. Russ. J. Bioorganic Chem. 2019, 45, 321–334. [Google Scholar] [CrossRef]
- Kgatle, M.M.; Boshomane, T.M.; Lawal, I.O.; Mokoala, K.M.; Mokgoro, N.P.; Lourens, N.; Kairemo, K.; Zeevaart, J.R.; Vorster, M.; Sathekge, M.M. Immune checkpoints, inhibitors and radionuclides in prostate cancer: Promising combinatorial therapy approach. Int. J. Mol. Sci. 2021, 22, 4109. [Google Scholar] [CrossRef] [PubMed]
- de Lartigue, J. Early Data Make B7-H3 a Checkpoint Contender in Prostate Cancer and Beyond. OncologyLive 2022, 23. [Google Scholar]
- Zahm, C.D.; Moseman, J.E.; Delmastro, L.E.; Mcneel, D.G. PD-1 and LAG-3 blockade improve anti-tumor vaccine efficacy. Oncoimmunology 2021, 10, 1912892. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liu, Y.; Zhang, T.; Yue, L.; Xiao, Y.; Guo, C. LAG-3 is a promising inhibitory immune checkpoint for antitumor immunotherapy. Expert Rev. Anticancer. Ther. 2022, 22, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Li, X.; Zhang, J. Progress of immune checkpoint LAG-3 in immunotherapy. Oncol. Lett. 2020, 20, 207. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, S.; Zhang, X.; Jia, K.; Wang, H.; Zhou, C.; He, Y. OX40 (CD134) and OX40 ligand, important immune checkpoints in cancer. OncoTargets Ther. 2019, 12, 7347. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H.; Kim, B.S.; Lee, S.-K. Regulatory T cells in tumor microenvironment and approach for anticancer immunotherapy. Immune Netw. 2020, 20, e4. [Google Scholar] [CrossRef]
- Kovacsovics-Bankowski, M.; Chisholm, L.; Vercellini, J.; Crittenden, M.; Lary, S.; Curti, B.; Weinberg, A. Phase I/II clinical trial of anti-OX40, radiation and cyclophosphamide in patients with prostate cancer: Immunological analysis. J. ImmunoTherapy Cancer 2013, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Perez, A.G.; Perez-Trujillo, J.J.; Garza-Morales, R.; Loera-Arias, M.J.; Saucedo-Cardenas, O.; Garcia-Garcia, A.; Rodriguez-Rocha, H.; Montes-de-Oca-Luna, R. 4-1BBL as a Mediator of Cross-Talk between Innate, Adaptive, and Regulatory Immunity against Cancer. Int. J. Mol. Sci. 2021, 22, 6210. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- Youlin, K.; Li, Z.; Xiaodong, W.; Xiuheng, L.; Hengchen, Z. Combination immunotherapy with 4-1BBL and CTLA-4 blockade for the treatment of prostate cancer. Clin. Dev. Immunol. 2012, 2012, 439235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Xu, W.; Yuan, Y.; Ayithan, N.; Imai, Y.; Wu, X.; Miller, H.; Olson, M.; Feng, Y.; Huang, Y.H. Immune-checkpoint protein VISTA critically regulates the IL-23/IL-17 inflammatory axis. Sci. Rep. 2017, 7, 1485. [Google Scholar] [CrossRef] [PubMed]
- Le Mercier, I.; Chen, W.; Lines, J.L.; Day, M.; Li, J.; Sergent, P.; Noelle, R.J.; Wang, L. VISTA regulates the development of protective antitumor immunity. Cancer Res. 2014, 74, 1933–1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagliamento, M.; Agostinetto, E.; Borea, R.; Brandão, M.; Poggio, F.; Addeo, A.; Lambertini, M. VISTA: A Promising Target for Cancer Immunotherapy? ImmunoTargets Ther. 2021, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Nowak, E.C.; Lines, J.L.; Varn, F.S.; Deng, J.; Sarde, A.; Mabaera, R.; Kuta, A.; Le Mercier, I.; Cheng, C.; Noelle, R.J. Immunoregulatory functions of VISTA. Immunol. Rev. 2017, 276, 66–79. [Google Scholar] [CrossRef]
- Yuan, L.; Tatineni, J.; Mahoney, K.M.; Freeman, G.J. VISTA: A mediator of quiescence and a promising target in cancer immunotherapy. Trends Immunol. 2021, 42, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Pan, J.; Guo, Z.; Yang, C.; Mao, L. CART cell therapy for prostate cancer: Status and promise. OncoTargets Ther. 2019, 12, 391. [Google Scholar] [CrossRef] [Green Version]
- Calderon, H.; Mamonkin, M.; Guedan, S. Analysis of CAR-mediated tonic signaling. In Chimeric Antigen Receptor T Cells; Humana: Uniondale, NY, USA, 2020; pp. 223–236. [Google Scholar] [CrossRef]
- Kloss, C.C.; Condomines, M.; Cartellieri, M.; Bachmann, M.; Sadelain, M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 2013, 31, 71–75. [Google Scholar] [CrossRef]
- He, Y.; Vlaming, M.; van Meerten, T.; Bremer, E. The Implementation of TNFRSF Co-Stimulatory Domains in CAR-T Cells for Optimal Functional Activity. Cancers 2022, 14, 299. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Z.; Ren, Z.; Li, Y. Reactions related to CAR-T cell therapy. Front. Immunol. 2021, 12, 663201. [Google Scholar] [CrossRef]
- Füchsl, F.; Krackhardt, A.M. Adoptive cellular therapy for multiple myeloma using CAR-and TCR-transgenic T cells: Response and resistance. Cells 2022, 11, 410. [Google Scholar] [CrossRef] [PubMed]
- Freen-van Heeren, J.J. Using CRISPR to enhance T cell effector function for therapeutic applications. Cytokine X 2021, 3, 100049. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Martínez, A.; Manzanera, A.G.; Sukin, S.W.; Esteban-María, J.; González-Guerrero, J.F.; Gomez-Guerra, L.; Garza-Guajardo, R.; Flores-Gutiérrez, J.P.; Elizondo Riojas, G.; Delgado-Enciso, I. Intraprostatic distribution and long-term follow-up after AdV-tk immunotherapy as neoadjuvant to surgery in patients with prostate cancer. Cancer Gene Ther. 2013, 20, 642–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilusic, M.; Madan, R.A.; Gulley, J.L. Immunotherapy of Prostate Cancer: Facts and HopesProstate Cancer Immunotherapy. Clin. Cancer Res. 2017, 23, 6764–6770. [Google Scholar] [CrossRef] [Green Version]
- Shariat, S.F.; Sadeghi, F.; Slawin, K.M. Vaccine-based immunotherapy for prostate cancer. Rev. Urol. 2000, 2, 222. [Google Scholar]
- Singh, M.A.; Shrivastava, T.P.; Gupta, M.; Sharma, A. Cancer immunotherapy: Moving forward with peptide T-cell vaccines. In Nanotherapeutics in Cancer Vaccination and Challenges; Elsevier: Amsterdam, The Netherlands, 2022; pp. 295–311. [Google Scholar]
- Yamamoto, T.N.; Kishton, R.J.; Restifo, N.P. Developing neoantigen-targeted T cell–based treatments for solid tumors. Nat. Med. 2019, 25, 1488–1499. [Google Scholar] [CrossRef]
- Supabphol, S.; Li, L.; Goedegebuure, S.P.; Gillanders, W.E. Neoantigen vaccine platforms in clinical development: Understanding the future of personalized immunotherapy. Expert Opin. Investig. Drugs 2021, 30, 529–541. [Google Scholar] [CrossRef]
- Mo, L.; Zhang, X.; Shi, X.; Wei, L.; Zheng, D.; Li, H.; Gao, J.; Li, J.; Hu, Z. Norcantharidin enhances antitumor immunity of GM-CSF prostate cancer cells vaccine by inducing apoptosis of regulatory T cells. Cancer Sci. 2018, 109, 2109–2118. [Google Scholar] [CrossRef] [Green Version]
- Small, E.J.; Sacks, N.; Nemunaitis, J.; Urba, W.J.; Dula, E.; Centeno, A.S.; Nelson, W.G.; Ando, D.; Howard, C.; Borellini, F. Granulocyte macrophage colony-stimulating factor–secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin. Cancer Res. 2007, 13, 3883–3891. [Google Scholar] [CrossRef] [Green Version]
- Shemesh, C.S.; Hsu, J.C.; Hosseini, I.; Shen, B.-Q.; Rotte, A.; Twomey, P.; Girish, S.; Wu, B. Personalized cancer vaccines: Clinical landscape, challenges, and opportunities. Mol. Ther. 2021, 29, 555–570. [Google Scholar] [CrossRef]
- Noguchi, M.; Sasada, T.; Itoh, K. Personalized peptide vaccination: A new approach for advanced cancer as therapeutic cancer vaccine. Cancer Immunol. Immunother. 2013, 62, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Lilleby, W.; Gaudernack, G.; Brunsvig, P.F.; Vlatkovic, L.; Schulz, M.; Mills, K.; Hole, K.H.; Inderberg, E.M. Phase I/IIa clinical trial of a novel hTERT peptide vaccine in men with metastatic hormone-naive prostate cancer. Cancer Immunol. Immunother. 2017, 66, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Dailey, G.P.; Crosby, E.J.; Hartman, Z.C. Cancer vaccine strategies using self-replicating RNA viral platforms. Cancer Gene Ther. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Madan, R.A.; Bilusic, M.; Heery, C.; Schlom, J.; Gulley, J.L. Clinical evaluation of TRICOM vector therapeutic cancer vaccines. In Seminars in Oncology; Elsevier: London, UK, 2012; Volume 39, pp. 296-304. [Google Scholar]
- Shaw, A.R.; Suzuki, M. Immunology of adenoviral vectors in cancer therapy. Mol. Ther.-Methods Clin. Dev. Immunol. 2019, 15, 418–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudzinski, S.O.; Cameron, B.D.; Wang, J.; Rathmell, J.C.; Giorgio, T.D.; Kirschner, A.N. Combination immunotherapy and radiotherapy causes an abscopal treatment response in a mouse model of castration resistant prostate cancer. J. Immunother. Cancer 2019, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Gamat, M.; McNeel, D.G. Androgen deprivation and immunotherapy for the treatment of prostate cancer. Endocr.-Relat. Cancer 2017, 24, T297–T310. [Google Scholar] [CrossRef]
- Wolf, P.; Alzubi, J.; Gratzke, C.; Cathomen, T. The potential of CAR T cell therapy for prostate cancer. Nat. Rev. Urol. 2021, 18, 556–571. [Google Scholar] [CrossRef]
- Salem, A.F.; Gambini, L.; Billet, S.; Sun, Y.; Oshiro, H.; Zhao, M.; Hoffman, R.M.; Bhowmick, N.A.; Pellecchia, M. Prostate cancer metastases are strongly inhibited by agonistic Epha2 ligands in an orthotopic mouse model. Cancers 2020, 12, 2854. [Google Scholar] [CrossRef]
- Sanchez, C.; Chan, R.; Bajgain, P.; Rambally, S.; Palapattu, G.; Mims, M.; Rooney, C.; Leen, A.; Brenner, M.; Vera, J. Combining T-cell immunotherapy and anti-androgen therapy for prostate cancer. Prostate Cancer Prostatic Dis. 2013, 16, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Propper, D.J.; Balkwill, F.R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 2022, 19, 237–253. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, L.; Luo, C.; Jiang, M. Emerging strategies in cancer therapy combining chemotherapy with immunotherapy. Cancer Lett. 2019, 454, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Redman, J.M.; Gulley, J.L.; Madan, R.A. Combining immunotherapies for the treatment of prostate cancer. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Philadelphia, PA, USA, 2017; Volume 35, pp. 694-700. [Google Scholar]
- Vandenabeele, P.; Vandecasteele, K.; Bachert, C.; Krysko, O.; Krysko, D.V. Immunogenic apoptotic cell death and anticancer immunity. Apoptosis Cancer Pathog. Anti-Cancer Ther. 2016, 930, 133–149. [Google Scholar] [CrossRef]
- Van den Eertwegh, A.J.; Versluis, J.; Van den Berg, H.P.; Santegoets, S.J.; Van Moorselaar, R.J.A.; Van der Sluis, T.M.; Gall, H.E.; Harding, T.C.; Jooss, K.; Lowy, I. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: A phase 1 dose-escalation trial. Lancet Oncol. 2012, 13, 509–517. [Google Scholar] [CrossRef]
- McNeel, D.G.; Eickhoff, J.C.; Wargowski, E.; Zahm, C.; Staab, M.J.; Straus, J.; Liu, G. Concurrent, but not sequential, PD-1 blockade with a DNA vaccine elicits anti-tumor responses in patients with metastatic, castration-resistant prostate cancer. OncoTargets Ther. 2018, 9, 25586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNeel, D.G.; Eickhoff, J.C.; Wargowski, E.; Johnson, L.E.; Kyriakopoulos, C.E.; Emamekhoo, H.; Lang, J.M.; Brennan, M.J.; Liu, G. Phase 2 trial of T-cell activation using MVI-816 and pembrolizumab in patients with metastatic, castration-resistant prostate cancer (mCRPC). J. Immunother. Cancer 2022, 10, e004198. [Google Scholar] [CrossRef]
- Boutros, C.; Tarhini, A.; Routier, E.; Lambotte, O.; Ladurie, F.L.; Carbonnel, F.; Izzeddine, H.; Marabelle, A.; Champiat, S.; Berdelou, A. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat. Rev. Clin. Oncol. 2016, 13, 473–486. [Google Scholar] [CrossRef]
- Patel, V.G.; Oh, W.K. The evolving landscape of immunotherapy in advanced prostate cancer. Immunotherapy 2019, 11, 903–912. [Google Scholar] [CrossRef]
- Jeong, S.-h.; Kwak, C. Immunotherapy for prostate cancer: Requirements for a successful regime transfer. Investig. Clin. Urol. 2022, 63, 3. [Google Scholar] [CrossRef]
- Dorff, T.B.; Narayan, V.; Forman, S.J.; Zang, P.D.; Fraietta, J.A.; June, C.H.; Haas, N.B.; Priceman, S.J. Novel redirected T cell immunotherapies for advanced prostate cancer. Clin. Cancer Res. 2021, 28, 576–584. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukherjee, A.G.; Wanjari, U.R.; Prabakaran, D.S.; Ganesan, R.; Renu, K.; Dey, A.; Vellingiri, B.; Kandasamy, S.; Ramesh, T.; Gopalakrishnan, A.V. The Cellular and Molecular Immunotherapy in Prostate Cancer. Vaccines 2022, 10, 1370. https://doi.org/10.3390/vaccines10081370
Mukherjee AG, Wanjari UR, Prabakaran DS, Ganesan R, Renu K, Dey A, Vellingiri B, Kandasamy S, Ramesh T, Gopalakrishnan AV. The Cellular and Molecular Immunotherapy in Prostate Cancer. Vaccines. 2022; 10(8):1370. https://doi.org/10.3390/vaccines10081370
Chicago/Turabian StyleMukherjee, Anirban Goutam, Uddesh Ramesh Wanjari, D. S. Prabakaran, Raja Ganesan, Kaviyarasi Renu, Abhijit Dey, Balachandar Vellingiri, Sabariswaran Kandasamy, Thiyagarajan Ramesh, and Abilash Valsala Gopalakrishnan. 2022. "The Cellular and Molecular Immunotherapy in Prostate Cancer" Vaccines 10, no. 8: 1370. https://doi.org/10.3390/vaccines10081370
APA StyleMukherjee, A. G., Wanjari, U. R., Prabakaran, D. S., Ganesan, R., Renu, K., Dey, A., Vellingiri, B., Kandasamy, S., Ramesh, T., & Gopalakrishnan, A. V. (2022). The Cellular and Molecular Immunotherapy in Prostate Cancer. Vaccines, 10(8), 1370. https://doi.org/10.3390/vaccines10081370









