Adjuvant Effects of a New Saponin Analog VSA-1 on Enhancing Homologous and Heterosubtypic Protection by Influenza Virus Vaccination
Abstract
:1. Introduction
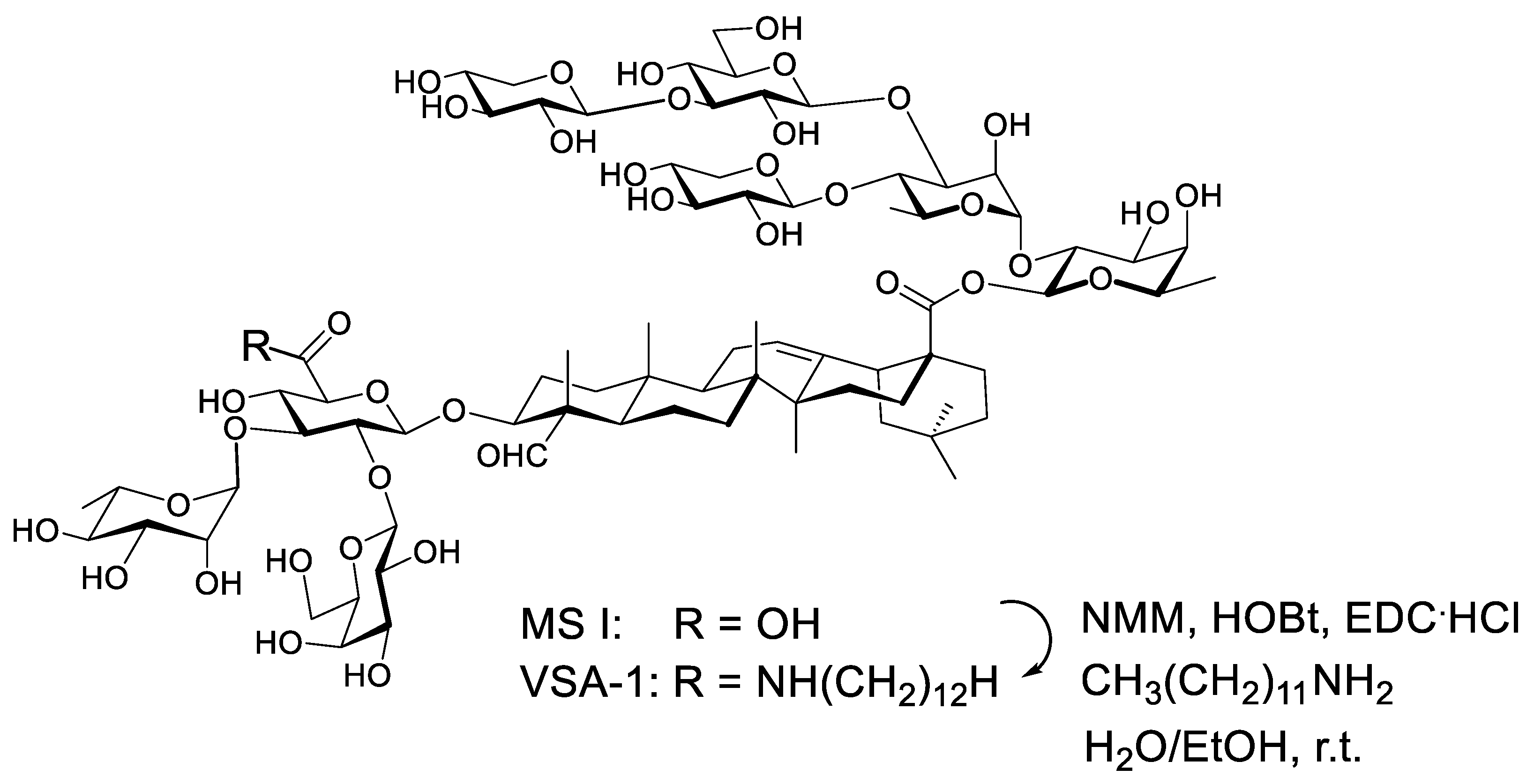
2. Materials and Methods
2.1. Animals, Reagents, and Viruses
2.2. Immunization and Virus Challenge of Mice
2.3. Antibody Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Hemagglutination Inhibition (HAI) Assay
2.5. Lung Viral Titration
2.6. Cytokine ELISA and In Vitro IgG Antibody Detection
2.7. Enzyme-Linked Immunospot (ELISpot) Assay
2.8. Flow Cytometry Analysis
2.9. In Vivo Protection Efficacy Test of Immune Sera
2.10. In Vivo Depletion of T Cells
2.11. Intraperitoneal Injection of Adjuvants
2.12. Statistical Analyses
3. Results
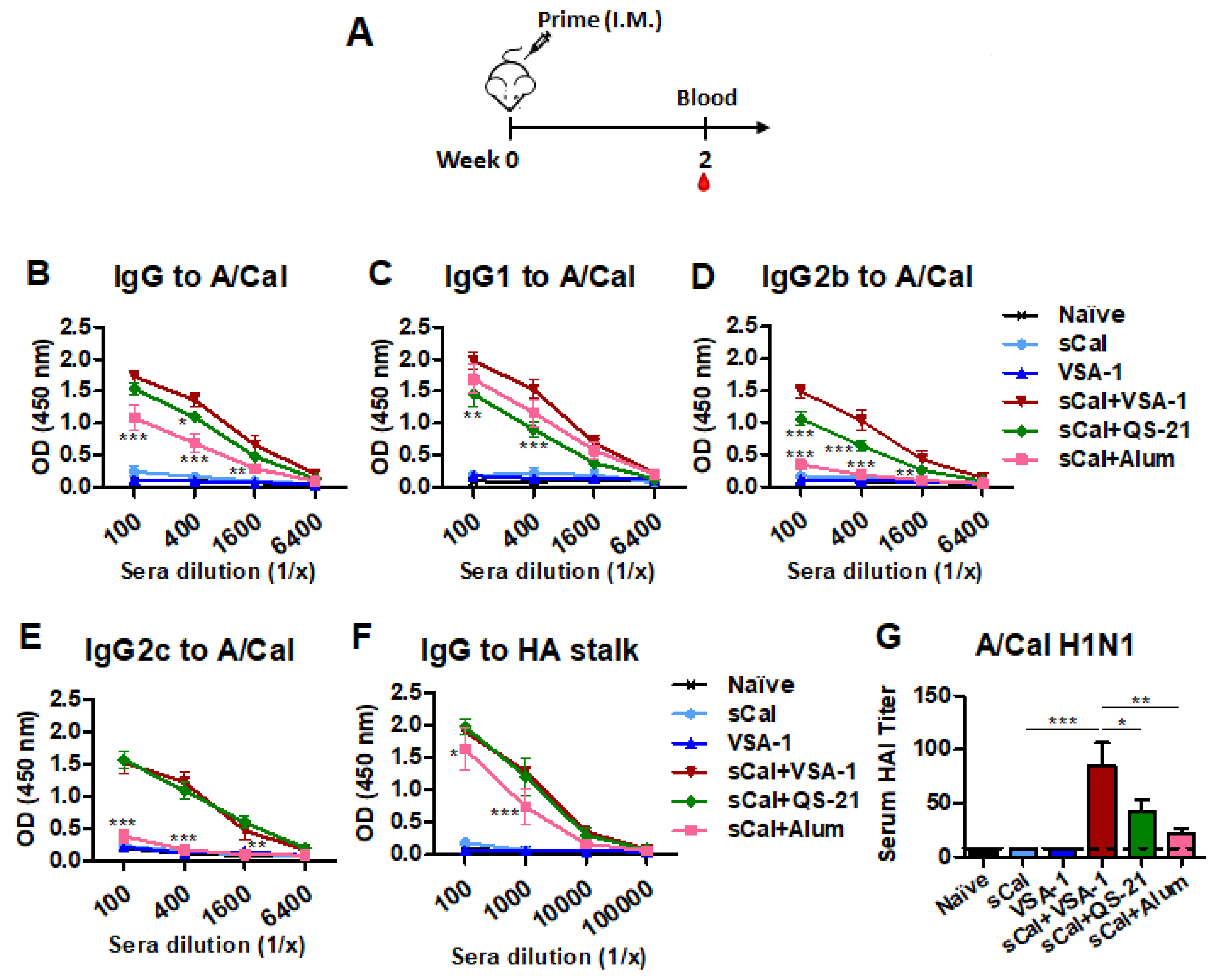
3.1. VSA-1 in Influenza Vaccination Exhibits Adjuvant Effects on Increasing the Magnitude of Virus-Specific IgG Antibodies and HAI Titers after a Single Dose
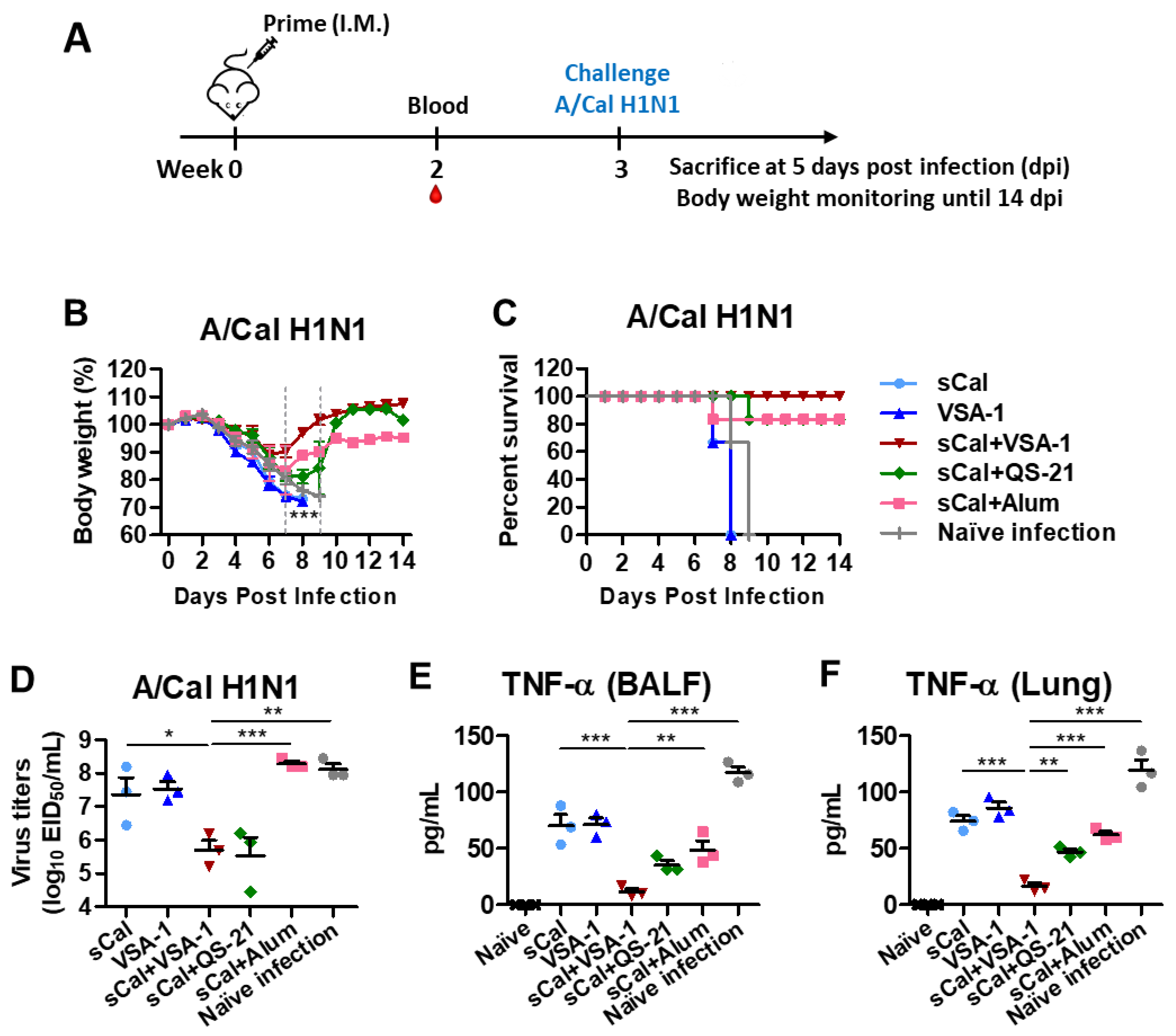
3.2. Single Dose of VSA-1-Adjuvanted Influenza Vaccination Induces Enhanced Protection against Homologous A/Cal H1N1 Virus
3.3. VSA-1-Adjuvanted Split Virus Vaccination Enhances Antibody-Secreting Cell and IFN-γ-Producing T Cell Responses
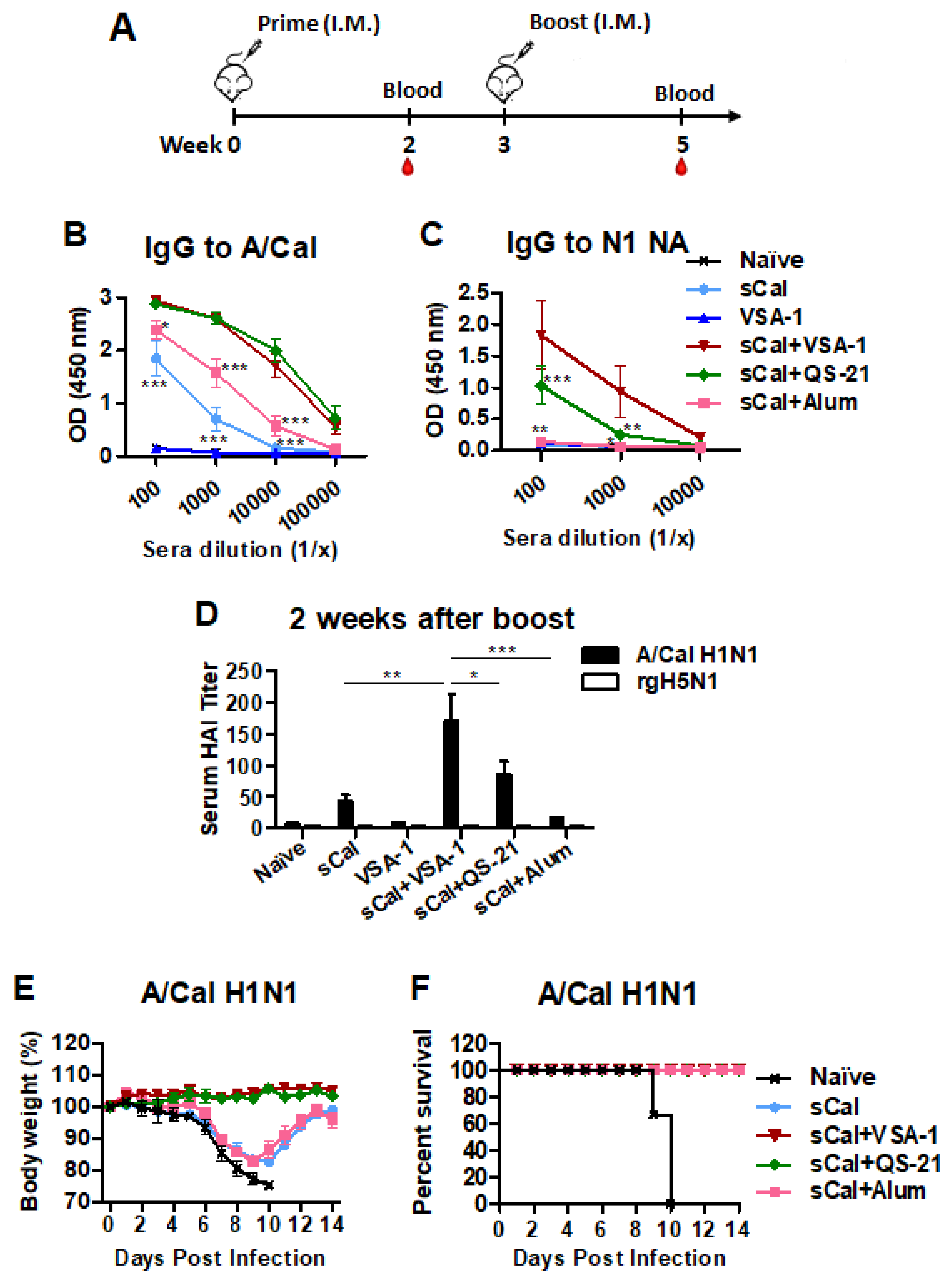
3.4. Boost Immunization with VSA-1-Adjuvanted Influenza Vaccine Further Enhances Virus-Specific IgG Antibodies, HAI Titers, and Homologous Protection
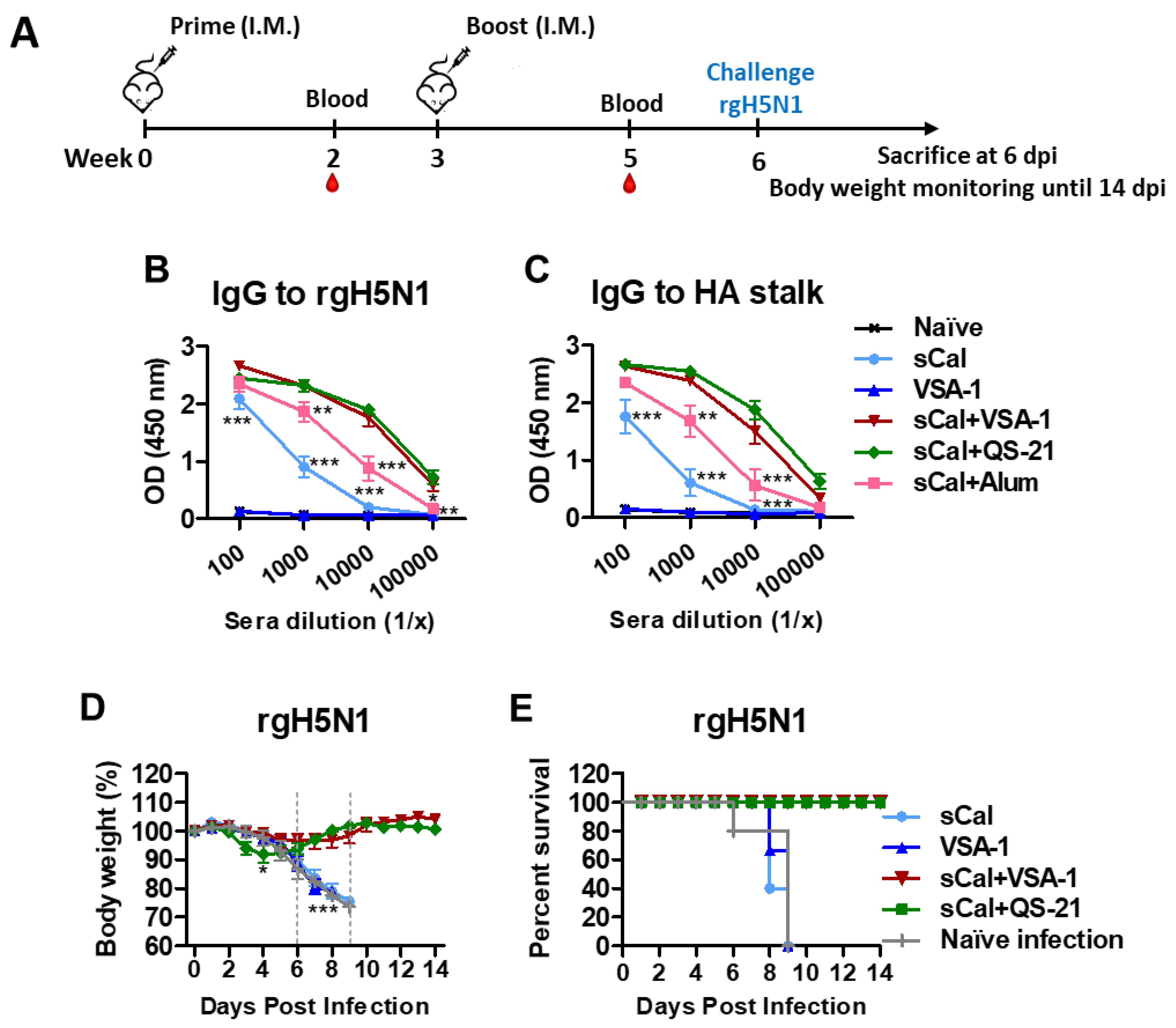
3.5. VSA-1-Adjuvanted Prime-Boost Influenza Vaccination Induces Cross-Protection against Heterosubtypic rgH5N1 Virus
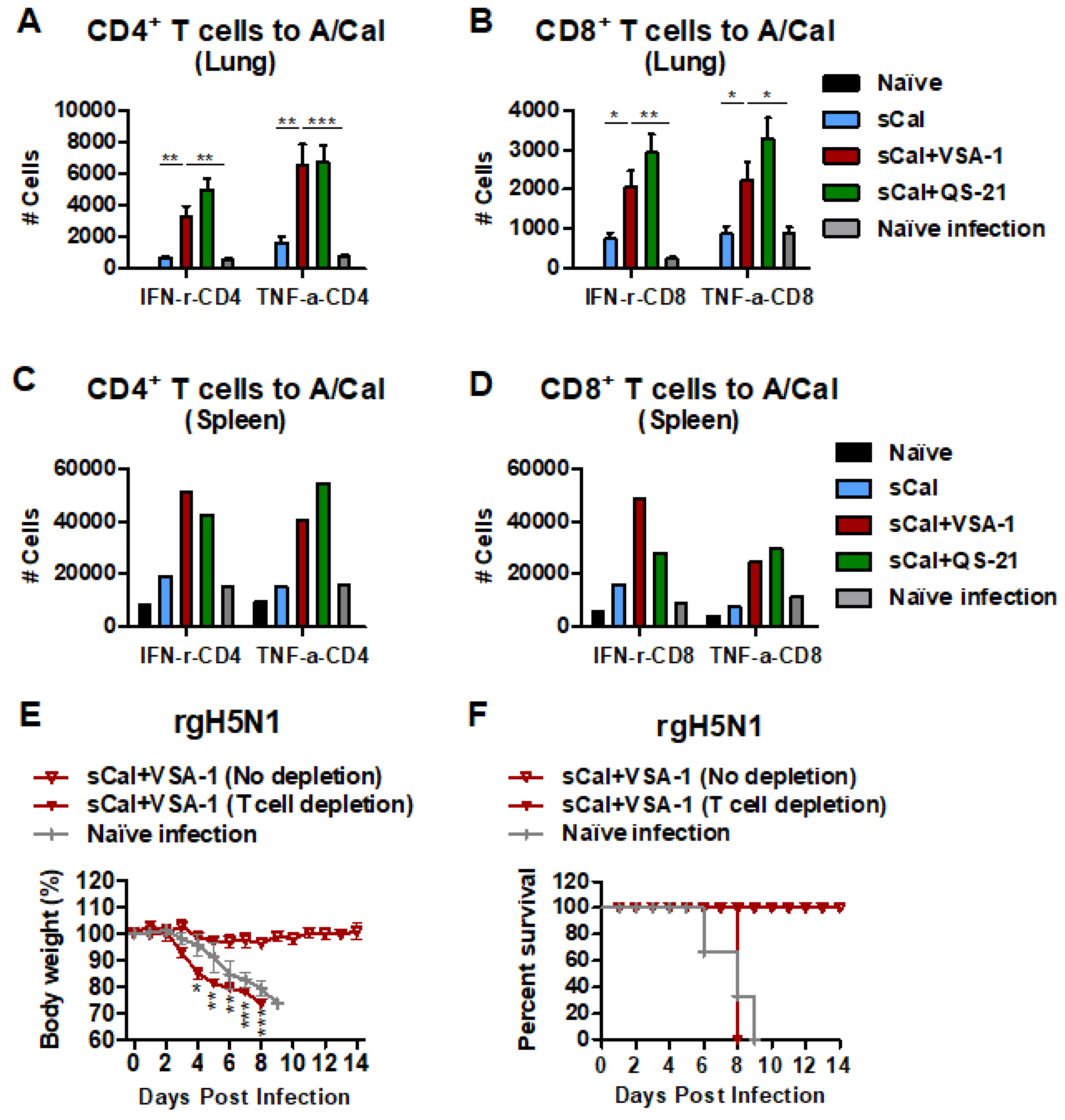
3.6. VSA-1 Adjuvanted Vaccination Induces T Cell-Dependent Protection against Heterosubtypic rgH5N1 Virus
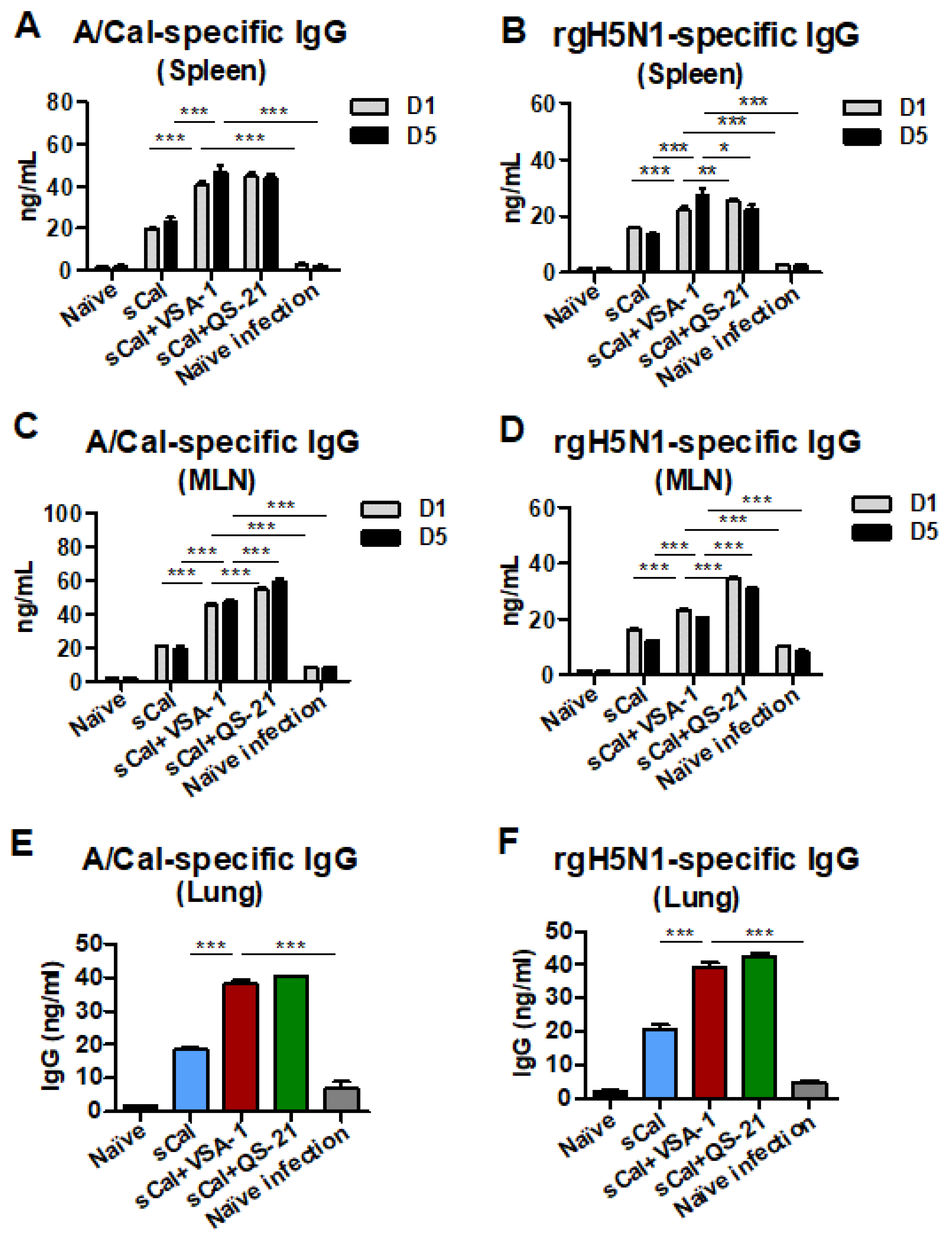
3.7. VSA-1-Adjuvanted Prime-Boost Vaccination Enhances Antibody-Secreting Cell and IFN-γ-Producing T Cell Responses upon Virus Infection
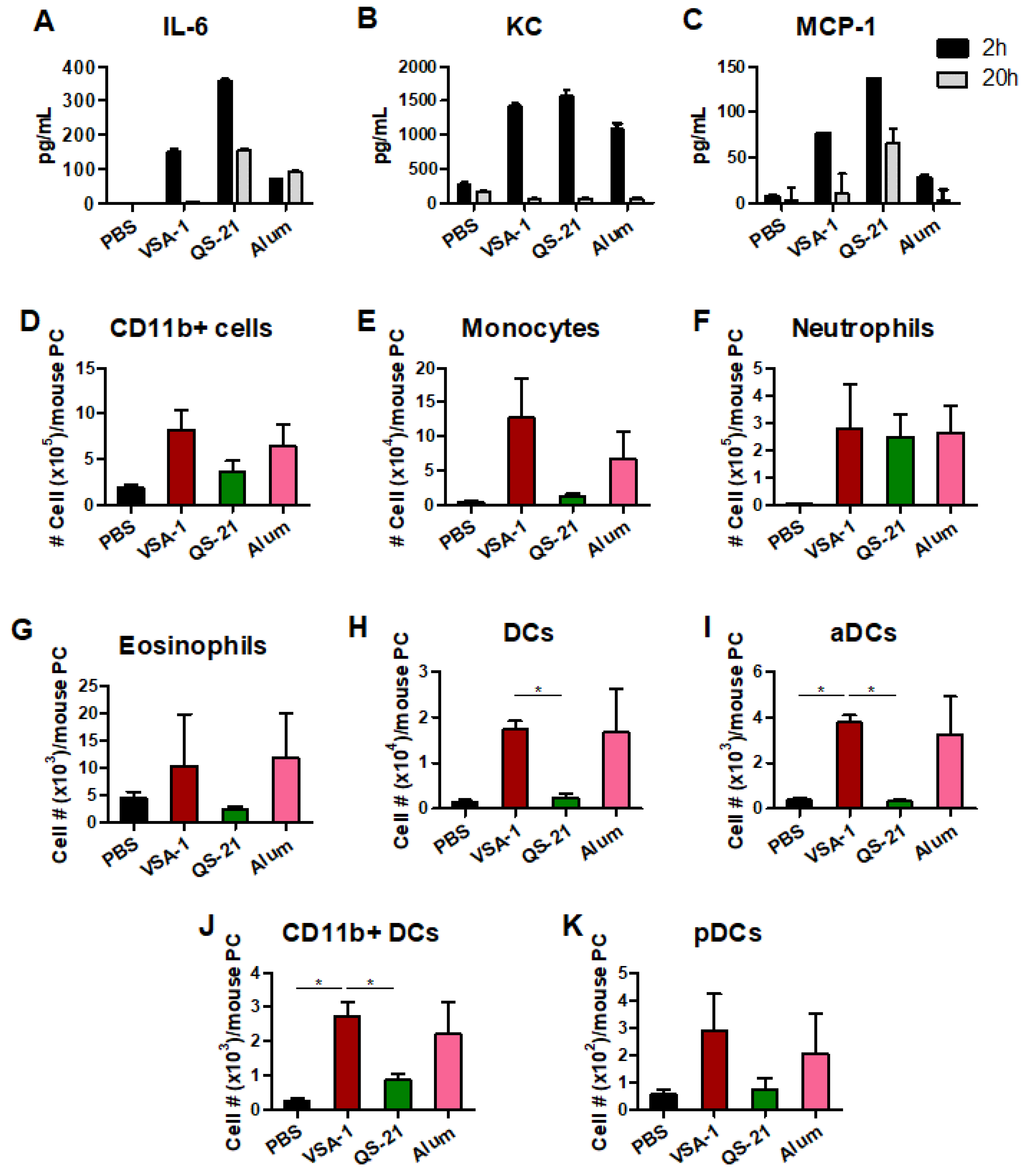
3.8. Acute Innate Immune Responses Are Differentially Modulated by VSA-1 and Other Comparing Adjuvants, QS-21, and Alum
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Nath Neerukonda, S.; Vassell, R.; Weiss, C.D. Neutralizing Antibodies Targeting the Conserved Stem Region of Influenza Hemagglutinin. Vaccines 2020, 8, 382. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, G. Unmet needs in modern vaccinology: Adjuvants to improve the immune response. Vaccine 2010, 28 (Suppl. S3), C25–C36. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Singh, M. Selection of adjuvants for enhanced vaccine potency. World J. Vaccines 2011, 1, 33–78. [Google Scholar] [CrossRef]
- Banday, A.H.; Jeelani, S.; Hruby, V.J. Cancer vaccine adjuvants—recent clinical progress and future perspectives. Immunopharmacol. Immunotoxicol. 2015, 37, 1–11. [Google Scholar] [CrossRef]
- Bastola, R.; Noh, G.; Keum, T.; Bashyal, S.; Seo, J.E.; Choi, J.; Oh, Y.; Cho, Y.; Lee, S. Vaccine adjuvants: Smart components to boost the immune system. Arch. Pharm. Res. 2017, 40, 1238–1248. [Google Scholar] [CrossRef]
- Di Pasquale, A.; Preiss, S.; Tavares Da Silva, F.; Garcon, N. Vaccine Adjuvants: From 1920 to 2015 and Beyond. Vaccines 2015, 3, 320–343. [Google Scholar] [CrossRef]
- Temizoz, B.; Kuroda, E.; Ishii, K.J. Vaccine adjuvants as potential cancer immunotherapeutics. Int. Immunol. 2016, 28, 329–338. [Google Scholar] [CrossRef]
- Bonam, S.R.; Partidos, C.D.; Halmuthur, S.K.M.; Muller, S. An overview of novel adjuvants designed for improving vaccine efficacy. Trends Pharmacol. Sci. 2017, 38, 771–793. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Friedland, L.R.; Hanon, E.; Didierlaurent, A.M. Towards an evidence based approach for the development of adjuvanted vaccines. Curr. Opin. Immunol. 2017, 47, 93–102. [Google Scholar] [CrossRef]
- Garçon, N.; Leroux-Roels, G.; Cheng, W.-F. Vaccine Adjuvants. In Understanding Modern Vaccines Perspectives in Vaccinology; Garçon, N., Stern, P.L., Cunningham, A.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 1, pp. 89–113. [Google Scholar]
- Giudice, G.D.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 2018, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhu, H.; Xia, X.; Liang, Z.; Ma, X.; Sun, B. Vaccine adjuvants: Understanding the structure and mechanism of adjuvanticity. Vaccine 2019, 37, 3167–3178. [Google Scholar] [CrossRef] [PubMed]
- Young, B.E.; Sadarangani, S.P.; Leo, Y.S. The avian influenza vaccine Emerflu. Why did it fail? Expert Rev. Vaccines 2015, 14, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, G.; Van Belle, P.; Vandepapeliere, P.; Horsmans, Y.; Janssens, M.; Carletti, I.; Garcon, N.; Wettendorff, M.; Van Mechelen, M. Vaccine Adjuvant Systems containing monophosphoryl lipid A and QS-21 induce strong humoral and cellular immune responses against hepatitis B surface antigen which persist for at least 4 years after vaccination. Vaccine 2015, 33, 1084–1091. [Google Scholar] [CrossRef]
- Tielemans, C.L.; Vlasak, J.; Kosa, D.; Billiouw, J.-M.; Verpooten, G.A.; Mezei, I.; Ryba, M.; Peeters, P.C.; Mat, O.; Jadoul, M.Y.; et al. Immunogenicity and Safety of an Investigational AS02v-Djuvanted Hepatitis B Vaccine in Patients with Renal Insufficiency Who Failed to Respond or to Maintain Antibody Levels after Prior Vaccination: Results of Two Open, Randomized, Comparative Trials. Vaccine 2011, 29, 1159–1166. [Google Scholar] [CrossRef]
- Vandepapeliere, P.; Rehermann, B.; Koutsoukos, M.; Moris, P.; Garcon, N.; Wettendorff, M.; Leroux-Roels, G. Potent Enhancement of Cellular and Humoral Immune Responses against Recombinant Hepatitis B Antigens Using AS02A Adjuvant in Healthy Adults. Vaccine 2005, 23, 2591–2601. [Google Scholar] [CrossRef]
- Vandepapeliere, P.; Horsmans, Y.; Moris, P.; Van Mechelen, M.; Janssens, M.; Koutsoukos, M.; Van Belle, P.; Clement, F.; Hanon, E.; Wettendorff, M.; et al. Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine 2008, 26, 1375–1386. [Google Scholar] [CrossRef]
- Martin, R.S.; Briones, R. Industrial uses and sustainable supply of Quillaja Saponaria (Rosaceae) Saponins. Econ. Bot. 1999, 53, 302–311. [Google Scholar] [CrossRef]
- Ragupathi, G.; Gardner, J.R.; Livingston, P.O.; Gin, D.Y. Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer. Expert Rev. Vaccines 2011, 10, 463–470. [Google Scholar]
- Harandi, A.M.; Medaglini, D.; Shattock, R.J. Working Group convened by EUROPRISE Vaccine adjuvants: A priority for vaccine research. Vaccine 2010, 28, 2363–2366. [Google Scholar] [CrossRef]
- Wang, P.; Dai, Q.; Thogaripally, P.; Zhang, P.; Michalek, S.M. Synthesis of QS-21-based immunoadjuvants. J. Org. Chem. 2013, 78, 11525–11534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Devalankar, D.A.; Dai, Q.; Zhang, P.; Michalek, S.M. Synthesis and Evaluation of QS-21-Based Immunoadjuvants with a Terminal-Functionalized Side Chain Incorporated in the West Wing Trisaccharide. J. Org. Chem. 2016, 81, 9560–9566. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Škalamera, Đ.; Sui, X.; Zhang, P.; Michalek, S.M. Synthesis and evaluation of a QS-17/18-based vaccine adjuvant. J. Med. Chem. 2019, 62, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Škalamera, Đ.; Sui, X.; Zhang, P.; Michalek, S.M. Synthesis and Evaluation of QS-7-Based Vaccine Adjuvants. ACS Infect. Dis. 2019, 5, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ding, X.; Kim, H.; Škalamera, Đ.; Michalek, S.M.; Zhang, P. Vaccine Adjuvants Derivatized from Momordica Saponins I and II. J. Med. Chem. 2019, 62, 9976–9982. [Google Scholar] [CrossRef]
- Iwamoto, M.; Okabe, H.; Yamauchi, T.; Tanaka, M.; Rokutani, Y.; Hara, S.; Mihashi, K.; Higuchi, R. Studies on the constituents of Momordica cochinchinensis SPRENG. I. Isolation and characterization of the seed saponins, momordica saponins I and II. Chem. Pharm. Bull. 1985, 33, 464–478. [Google Scholar] [CrossRef]
- Song, J.M.; Van Rooijen, N.; Bozja, J.; Compans, R.W.; Kang, S.M. Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proc. Natl. Acad. Sci. USA 2011, 108, 757–761. [Google Scholar] [CrossRef]
- Ko, E.J.; Lee, Y.; Lee, Y.T.; Kim, Y.J.; Kim, K.H.; Kang, S.M. MPL and CpG combination adjuvants promote homologous and heterosubtypic cross protection of inactivated split influenza virus vaccine. Antiviral Res. 2018, 156, 107–115. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Kim, K.H.; Subbiah, J.; Park, B.R.; Ko, E.J.; Seong, B.L.; Kang, S.M. Comparison of the effects of different potent adjuvants on enhancing the immunogenicity and cross-protection by influenza virus vaccination in young and aged mice. Antiviral Res. 2022, 197, 105229. [Google Scholar] [CrossRef]
- Ko, E.J.; Lee, Y.T.; Kim, K.H.; Jung, Y.J.; Lee, Y.; Denning, T.L.; Kang, S.M. Effects of MF59 Adjuvant on Induction of Isotype-Switched IgG Antibodies and Protection after Immunization with T-Dependent Influenza Virus Vaccine in the Absence of CD4+ T Cells. J. Virol. 2016, 90, 6976–6988. [Google Scholar] [CrossRef]
- Chae, W.; Kim, P.; Hwang, B.J.; Seong, B.L. Universal monoclonal antibody-based influenza hemagglutinin quantitative enzyme-linked immunosorbent assay. Vaccine 2019, 37, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, Y.T.; Park, S.; Jung, Y.J.; Lee, Y.; Ko, E.J.; Kim, Y.J.; Li, X.; Kang, S.M. Neuraminidase expressing virus-like particle vaccine provides effective cross protection against influenza virus. Virology 2019, 535, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Lee, Y.N.; Lee, Y.T.; Kim, M.C.; Gewirtz, A.T.; Kang, S.M. A Novel Vaccination Strategy Mediating the Induction of Lung-Resident Memory CD8 T Cells Confers Heterosubtypic Immunity against Future Pandemic Influenza Virus. J. Immunol. 2016, 196, 2637–2645. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Kim, K.H.; Ko, E.J.; Kim, M.C.; Lee, Y.N.; Hwang, H.S.; Lee, Y.; Jung, Y.J.; Kim, Y.J.; Santos, J.; et al. Enhancing the cross protective efficacy of live attenuated influenza virus vaccine by supplemented vaccination with M2 ectodomain virus-like particles. Virology 2019, 529, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Ko, E.J.; Lee, Y.; Kim, K.H.; Kim, M.C.; Lee, Y.N.; Kang, S.M. Intranasal vaccination with M2e5x virus-like particles induces humoral and cellular immune responses conferring cross-protection against heterosubtypic influenza viruses. PLoS ONE 2018, 13, e0190868. [Google Scholar] [CrossRef]
- Kim, M.C.; Lee, J.S.; Kwon, Y.M.; Eunju, O.; Lee, Y.J.; Choi, J.G.; Wang, B.Z.; Compans, R.W.; Kang, S.M. Multiple heterologous M2 extracellular domains presented on virus-like particles confer broader and stronger M2 immunity than live influenza A virus infection. Antiviral Res. 2013, 99, 328–335. [Google Scholar] [CrossRef]
- Park, B.R.; Kim, K.H.; Kotomina, T.; Kim, M.C.; Kwon, Y.M.; Jeeva, S.; Jung, Y.J.; Bhatnagar, N.; Isakova-Sivak, I.; Mezhenskaya, D.; et al. Broad cross protection by recombinant live attenuated influenza H3N2 seasonal virus expressing conserved M2 extracellular domain in a chimeric hemagglutinin. Sci. Rep. 2021, 11, 4151. [Google Scholar] [CrossRef]
- Ng, H.I.; Fernando, G.J.; Depelsenaire, A.C.; Kendall, M.A. Potent response of QS-21 as a vaccine adjuvant in the skin when delivered with the Nanopatch, resulted in adjuvant dose sparing. Sci. Rep. 2016, 6, 29368. [Google Scholar] [CrossRef]
- Garcon, N.; Di Pasquale, A. From discovery to licensure, the Adjuvant System story. Hum. Vaccines Immunother. 2017, 13, 19–33. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Lal, H.; Kovac, M.; Chlibek, R.; Hwang, S.J.; Díez-Domingo, J.; Godeaux, O.; Levin, M.J.; McElhaney, J.E.; Puig-Barberà, J.; et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N. Engl. J. Med. 2016, 375, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.; Cunningham, A.L.; Godeaux, O.; Chlibek, R.; Diez-Domingo, J.; Hwang, S.J.; Levin, M.J.; McElhaney, J.E.; Poder, A.; Puig-Barberà, J.; et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 2015, 372, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, N.; Poongulali, S.; Bollaerts, A.; Moris, P.; Beulah, F.E.; Ayuk, L.N.; Demoitié, M.A.; Jongert, E.; Ofori-Anyinam, O. A Randomized, Controlled Safety, and Immunogenicity Trial of the M72/AS01 Candidate Tuberculosis Vaccine in HIV-Positive Indian Adults. Medicine 2016, 95, e2459. [Google Scholar] [CrossRef] [PubMed]
- Marciani, D.J. Elucidating the Mechanisms of Action of Saponin-Derived Adjuvants. Trends Pharmacol. Sci. 2018, 39, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Lorent, J.H.; Quetin-Leclercq, J.; Mingeot-Leclercq, M.P. The amphiphilic nature of saponins and their effects on artificial and biological membranes and potential consequences for red blood and cancer cells. Org. Biomol. Chem. 2014, 12, 8803–8822. [Google Scholar] [CrossRef] [PubMed]
- Marty-Roix, R.; Vladimer, G.I.; Pouliot, K.; Weng, D.; Buglione-Corbett, R.; West, K.; MacMicking, J.D.; Chee, J.D.; Wang, S.; Lu, S.; et al. Identification of QS-21 as an Inflammasome-activating Molecular Component of Saponin Adjuvants. J. Biol. Chem. 2016, 291, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Dendouga, N.; Fochesato, M.; Lockman, L.; Mossman, S.; Giannini, S.L. Cell-mediated immune responses to a varicella-zoster virus glycoprotein E vaccine using both a TLR agonist and QS21 in mice. Vaccine 2012, 30, 3126–3135. [Google Scholar] [CrossRef]
- Coccia, M.; Collignon, C.; Hervé, C.; Chalon, A.; Welsby, I.; Detienne, S.; van Helden, M.J.; Dutta, S.; Genito, C.J.; Waters, N.C.; et al. Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNγ response promoting vaccine immunogenicity. NPJ Vaccines 2017, 2, 25. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Lodaya, R.N.; Lofano, G. The continued advance of vaccine adjuvants—‘we can work it out’. Semin. Immunol. 2020, 50, 101426. [Google Scholar] [CrossRef]
- Aydillo, T.; Escalera, A.; Strohmeier, S.; Aslam, S.; Sanchez-Cespedes, J.; Ayllon, J.; Roca-Oporto, C.; Perez-Romero, P.; Montejo, M.; Gavalda, J.; et al. Pre-existing Hemagglutinin Stalk Antibodies Correlate with Protection of Lower Respiratory Symptoms in Flu-Infected Transplant Patients. Cell Rep. Med. 2020, 1, 100130. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatnagar, N.; Kim, K.-H.; Subbiah, J.; Park, B.R.; Wang, P.; Gill, H.S.; Wang, B.-Z.; Kang, S.-M. Adjuvant Effects of a New Saponin Analog VSA-1 on Enhancing Homologous and Heterosubtypic Protection by Influenza Virus Vaccination. Vaccines 2022, 10, 1383. https://doi.org/10.3390/vaccines10091383
Bhatnagar N, Kim K-H, Subbiah J, Park BR, Wang P, Gill HS, Wang B-Z, Kang S-M. Adjuvant Effects of a New Saponin Analog VSA-1 on Enhancing Homologous and Heterosubtypic Protection by Influenza Virus Vaccination. Vaccines. 2022; 10(9):1383. https://doi.org/10.3390/vaccines10091383
Chicago/Turabian StyleBhatnagar, Noopur, Ki-Hye Kim, Jeeva Subbiah, Bo Ryoung Park, Pengfei Wang, Harvinder Singh Gill, Bao-Zhong Wang, and Sang-Moo Kang. 2022. "Adjuvant Effects of a New Saponin Analog VSA-1 on Enhancing Homologous and Heterosubtypic Protection by Influenza Virus Vaccination" Vaccines 10, no. 9: 1383. https://doi.org/10.3390/vaccines10091383
APA StyleBhatnagar, N., Kim, K.-H., Subbiah, J., Park, B. R., Wang, P., Gill, H. S., Wang, B.-Z., & Kang, S.-M. (2022). Adjuvant Effects of a New Saponin Analog VSA-1 on Enhancing Homologous and Heterosubtypic Protection by Influenza Virus Vaccination. Vaccines, 10(9), 1383. https://doi.org/10.3390/vaccines10091383






