A Novel C-Type Lectin Receptor-Targeted α-Synuclein-Based Parkinson Vaccine Induces Potent Immune Responses and Therapeutic Efficacy in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Generation of Conjugates
2.2. Fc-Dectin-1 Binding
2.3. Activation Analysis Using Bone Marrow-Derived Dendritic Cells
2.4. Animal Experiments
2.5. Induction of Synucleinopathy in Mice
2.6. Immunohistochemistry (IHC)
2.7. Vaccine Antibody Titer Determination
2.8. Inhibition and Avidity ELISA
2.9. Thioflavin T Assay for Aggregation
2.10. Statistical Analysis
3. Results
3.1. WISIT Vaccine Design
3.2. Production of WISIT Vaccine Candidates for α-Syn
3.3. WISIT Vaccine Candidates Targeting α-Syn Are Biologically Active in DCs
3.4. WISIT PD Vaccines Elicit Higher Immune Responses Compared to Conventional PD Vaccines
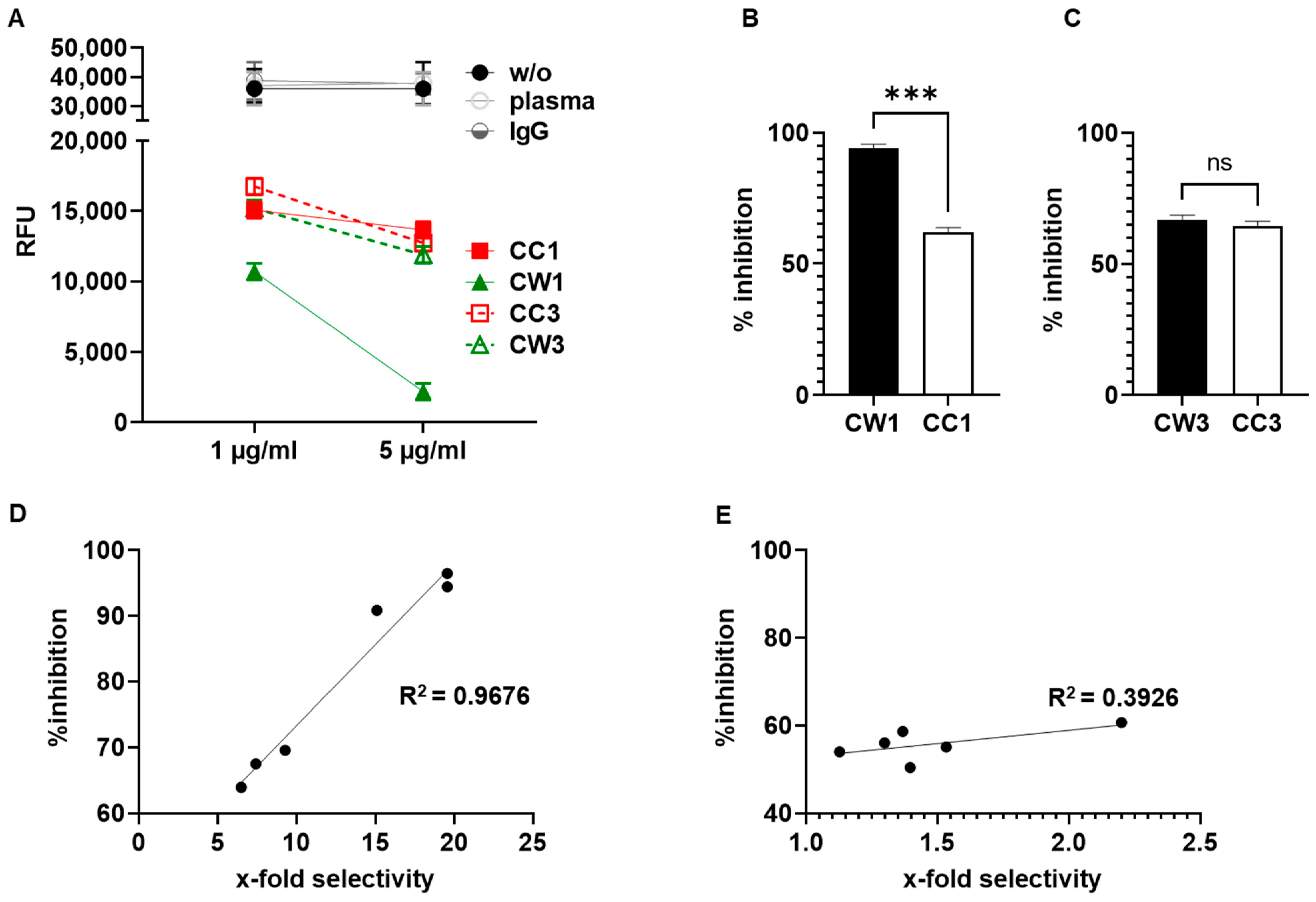
3.5. Antibodies Elicited by WISIT PD Vaccines Show Greater Binding and Selectivity to α-Syn Aggregates Than Conventional PD Vaccines
3.6. WISIT Vaccine Candidates Inhibit α-Syn Aggregation In Vitro
3.7. WISIT Vaccine CW-Type 1 Inhibits Propagation of Synucleinopathy In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A




References
- McKeith, I.G. Consensus Guidelines for the Clinical and Pathologic Diagnosis of Dementia with Lewy Bodies (DLB): Report of the Consortium on DLB International Workshop. J. Alzheimer's Dis. 2006, 9, 417–423. [Google Scholar] [CrossRef]
- Wenning, G.K.; Stefanova, N.; Jellinger, K.A.; Poewe, W.; Schlossmacher, M.G. Multiple System Atrophy: A Primary Oligodendrogliopathy: An Oligodendrogliopathy. Ann. Neurol. 2008, 64, 239–246. [Google Scholar] [CrossRef]
- Wong, Y.C.; Krainc, D. α-Synuclein Toxicity in Neurodegeneration: Mechanism and Therapeutic Strategies. Nat. Med. 2017, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- NHS Overview—Parkinson’s Disease. Available online: https://www.nhs.uk/conditions/parkinsonsdisease/It’s%20thought%20around%201%20in,get%20Parkinson’s%20disease%20than%20women (accessed on 24 June 2022).
- Valera, E.; Masliah, E. Therapeutic Approaches in Parkinson’s Disease and Related Disorders. J. Neurochem. 2016, 139, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, J.T.; Verma, A.; Dodart, J.-C.; Wang, C.Y.; Savistchenko, J.; Melki, R.; Carare, R.O.; Nicoll, J.A.R. Novel Antibodies Detect Additional α-Synuclein Pathology in Synucleinopathies: Potential Development for Immunotherapy. Alzheimer’s Res. Ther. 2020, 12, 159. [Google Scholar] [CrossRef]
- Valera, E.; Masliah, E. Immunotherapy for Neurodegenerative Diseases: Focus on α-Synucleinopathies. Pharmacol. Ther. 2013, 138, 311–322. [Google Scholar] [CrossRef]
- Vijayakumar, D.; Jankovic, J. Slowing Parkinson’s Disease Progression with Vaccination and Other Immunotherapies. CNS Drugs 2022, 36, 327–343. [Google Scholar] [CrossRef]
- Masliah, E.; Rockenstein, E.; Adame, A.; Crews, L.; Hashimoto, M.; Seubert, P.; Lee, M.; Goldstein, J.; Chilcote, T.; Games, D.; et al. Effects of α-Synuclein Immunization in a Mouse Model of Parkinson’s Disease. Neuron 2005, 46, 857–868. [Google Scholar] [CrossRef]
- Masliah, E.; Rockenstein, E.; Mante, M.; Crews, L.; Spencer, B.; Adame, A.; Patrick, C.; Trejo, M.; Ubhi, K.; Rohn, T.T.; et al. Passive Immunization Reduces Behavioral and Neuropathological Deficits in an Alpha-Synuclein Transgenic Model of Lewy Body Disease. PLoS ONE 2011, 6, e19338. [Google Scholar] [CrossRef] [Green Version]
- Lindström, V.; Fagerqvist, T.; Nordström, E.; Eriksson, F.; Lord, A.; Tucker, S.; Andersson, J.; Johannesson, M.; Schell, H.; Kahle, P.J.; et al. Immunotherapy Targeting α-Synuclein Protofibrils Reduced Pathology in (Thy-1)-h[A30P] α-Synuclein Mice. Neurobiol. Dis. 2014, 69, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.J.; Lee, H.J.; Rockenstein, E.; Ho, D.H.; Park, E.B.; Yang, N.Y.; Desplats, P.; Masliah, E.; Lee, S.J. Antibody-Aided Clearance of Extracellular -Synuclein Prevents Cell-to-Cell Aggregate Transmission. J. Neurosci. 2012, 32, 13454–13469. [Google Scholar] [CrossRef] [PubMed]
- Games, D.; Valera, E.; Spencer, B.; Rockenstein, E.; Mante, M.; Adame, A.; Patrick, C.; Ubhi, K.; Nuber, S.; Sacayon, P.; et al. Reducing C-terminal-truncated alpha-synuclein by immunotherapy attenuates neurodegeneration and propagation in Parkinson’s disease-like models. J. Neurosci. 2014, 34, 9441–9454. [Google Scholar] [CrossRef] [PubMed]
- Weihofen, A.; Liu, Y.T.; Arndt, J.W.; Huy, C.; Quan, C.; Smith, B.A.; Baeriswyl, J.L.; Cavegn, N.; Senn, L.; Su, L.; et al. Development of an aggregate-selective, human-derived α-synuclein antibody BIIB054 that ameliorates disease phenotypes in Parkinson’s disease models. Neurobiol. Dis. 2019, 124, 276–288. [Google Scholar] [CrossRef]
- Tran, H.T.; Chung, C.H.-Y.; Iba, M.; Zhang, B.; Trojanowski, J.Q.; Luk, K.C.; Lee, V.M.Y. α-Synuclein Immunotherapy Blocks Uptake and Templated Propagation of Misfolded α-Synuclein and Neurodegeneration. Cell Rep. 2014, 7, 2054–2065. [Google Scholar] [CrossRef]
- Schofiled, D.J.; Irving, L.; Calo, L.; Bogstedt, A.; Rees, G.; Nuccitelli, A.; Narwal, R.; Petrone, M.; Roberts, J.; Brown, L.; et al. Preclinical development of a high affinity α-synuclein antibody, MEDI1341, that can enter the brain, sequester extracellular α-synuclein and attenuate α-synuclein spreading in vivo. Neurobiol. Dis. 2019, 132, 104582. [Google Scholar] [CrossRef]
- Pagano, G.; Boess, F.G.; Taylor, K.I.; Ricci, B.; Mollenhauer, B.; Poewe, W.; Boulany, A.; Anzures-Cabrera, J.; Vogt, A.; Marchesi, M.; et al. A Phase II Study to Evaluate the Safety and Efficacy of Prasinezumab in Early Parkinson’s Disease (PASADENA): Rationale, Design, and Baseline Data. Front. Neurol. 2021, 12, 705407. [Google Scholar] [CrossRef]
- Lang, A.E.; Siderowf, A.D.; Macklin, E.A.; Poewe, W.; Brooks, D.J.; Fernandez, H.H.; Rascol, O.; Giladi, N.; Stocchi, F.; Tanner, C.M.; et al. Trial of Cinpanemab in Early Parkinson’s Disease. N. Engl. J. Med. 2022, 387, 408–420. [Google Scholar] [CrossRef]
- Tolar, M.; Abushakra, S.; Hey, J.A.; Porsteinsson, A.; Sabbagh, M. Aducanumab, gantenerumab, BAN2401, and ALZ-801—The first wave of amyloid-targeting drugs for Alzheimer’s disease with potential for near term approval. Alzheimer’s Res. Ther. 2020, 12, 95. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Sabbagh, M.N.; Cummings, J. Open Peer Commentary to “Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE Trials as reported by Biogen December 2019”. Alzheimer’s Dement. 2021, 17, 702–703. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Taylor, K.I.; Anzures-Cabrera, J.; Marchesi, M.; Simuni, T.; Marek, M.; Postuma, R.B.; Pavese, N.; Stocchi, F.; Azulay, J.P.; et al. Trial of Prasinezumab in Early-Stage Parkinson’s Disease. N. Engl. J. Med. 2022, 387, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Mortada, I.; Farah, R.; Nabha, S.; Ojcius, D.M.; Fares, Y.; Almawi, W.Y.; Sadier, N.S. Immunotherapies for Neurodegenerative Diseases. Front. Neurol. 2021, 12, 654739. [Google Scholar] [CrossRef] [PubMed]
- Mandler, M.; Valera, E.; Rockenstein, E.; Weninger, H.; Patrick, C.; Adame, A.; Santic, R.; Meindl, S.; Vigl, B.; Smrzka, O.; et al. Next-Generation Active Immunization Approach for Synucleinopathies: Implications for Parkinson’s Disease Clinical Trials. Acta Neuropathol. 2014, 127, 861–879. [Google Scholar] [CrossRef] [PubMed]
- Mandler, M.; Valera, E.; Rockenstein, E.; Mante, M.; Weninger, H.; Patrick, C.; Adame, A.; Schmidhuber, S.; Santic, R.; Schneeberger, A.; et al. Active Immunization against Alpha-Synuclein Ameliorates the Degenerative Pathology and Prevents Demyelination in a Model of Multiple System Atrophy. Mol. Neurodegener. 2015, 10, 10. [Google Scholar] [CrossRef]
- Kim, C.; Hovakimyan, A.; Zagorski, K.; Antonyan, T.; Petrushina, I.; Davtyan, H.; Chailyan, G.; Hasselmann, J.; Iba, M.; Adame, A.; et al. Efficacy and Immunogenicity of MultiTEP-Based DNA Vaccines Targeting Human α-Synuclein: Prelude for IND Enabling Studies. NPJ Vaccines 2022, 7, 1. [Google Scholar] [CrossRef]
- Mandler, M.; Rockenstein, E.; Overk, C.; Mante, M.; Florio, J.; Adame, A.; Kim, C.; Santic, R.; Schneeberger, A.; Mattner, F.; et al. Effects of Single and Combined Immunotherapy Approach Targeting Amyloid β Protein and α-Synuclein in a Dementia with Lewy Bodies-like Model. Alzheimer’s Dement. 2019, 15, 1133–1148. [Google Scholar] [CrossRef]
- Doucet, M.; El-Turabi, A.; Zabel, F.; Hunn, B.H.M.; Bengoa-Vergniory, N.; Cioroch, M.; Ramm, M.; Smith, A.M.; Gomes, A.C.; Cabral de Miranda, G.; et al. Preclinical Development of a Vaccine against Oligomeric Alpha-Synuclein Based on Virus-like Particles. PLoS ONE 2017, 12, e0181844. [Google Scholar] [CrossRef]
- Barbour, R.; Elmaarouf, A.; Loannou, A.; Tam, S.; Tourino, C.; Campbell, B.; Kinney, G.; Zago, W. Development of C-Terminal α-Synuclein Vaccine for Treatment and Prevention of Parkinson’s Disease and Other Synucleinopathies. Available online: https://s29.q4cdn.com/936209790/files/doc_presentations/2022/220217_Barbour-et-al_alpha-syn-vaccine_ADPD-poster_FINAL.pdf (accessed on 27 June 2022).
- Volc, D.; Poewe, W.; Kutzelnigg, A.; Lührs, P.; Thun-Hohenstein, C.; Schneeberger, A.; Galabova, G.; Majbour, N.; Vaikath, N.; El-Agnaf, O.; et al. Safety and Immunogenicity of the α-Synuclein Active Immunotherapeutic PD01A in Patients with Parkinson’s Disease: A Randomised, Single-Blinded, Phase 1 Trial. Lancet Neurol. 2020, 19, 591–600. [Google Scholar] [CrossRef]
- Yu, H.J.; Thijssen, E.; Brummelen, E.; Plas, J.L.; Radanovic, I.; Moerland, M.; Hsieh, E.; Groeneveld, G.J.; Dodart, J. A Randomized First-in-Human Study With UB-312, a UBITh® A-Synuclein Peptide Vaccine. Mov. Disord. 2022, 37, mds.29016. [Google Scholar] [CrossRef]
- Weinberger, E.E.; Himly, M.; Myschik, J.; Hauser, M.; Altmann, F.; Isakovic, A.; Scheiblhofer, S.; Thalhamer, J.; Weiss, R. Generation of Hypoallergenic Neoglycoconjugates for Dendritic Cell Targeted Vaccination: A Novel Tool for Specific Immunotherapy. J. Control. Release 2013, 165, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Hu, T. Conjugation with an Inulin–Chitosan Adjuvant Markedly Improves the Immunogenicity of Mycobacterium Tuberculosis CFP10-TB10.4 Fusion Protein. Mol. Pharm. 2016, 13, 3626–3635. [Google Scholar] [CrossRef] [PubMed]
- Neimert-Andersson, T.; Binnmyr, J.; Enoksson, M.; Langebäck, J.; Zettergren, L.; Hällgren, A.-C.; Franzén, H.; Lind Enoksson, S.; Lafolie, P.; Lindberg, A.; et al. Evaluation of Safety and Efficacy as an Adjuvant for the Chitosan-Based Vaccine Delivery Vehicle ViscoGel in a Single-Blind Randomised Phase I/IIa Clinical Trial. Vaccine 2014, 32, 5967–5974. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Guo, L.; Ruan, Y.; Zhu, H.; Wang, L.; Zhou, L.; Yun, X.; Gu, J. Laminarin-Mediated Targeting to Dectin-1 Enhances Antigen-Specific Immune Responses. Biochem. Biophys. Res. Commun. 2010, 391, 958–962. [Google Scholar] [CrossRef]
- Korotchenko, E.; Schießl, V.; Scheiblhofer, S.; Schubert, M.; Dall, E.; Joubert, I.A.; Strandt, H.; Neuper, T.; Sarajlic, M.; Bauer, R.; et al. Laser-facilitated Epicutaneous Immunotherapy with Hypoallergenic Beta-glucan Neoglycoconjugates Suppresses Lung Inflammation and Avoids Local Side Effects in a Mouse Model of Allergic Asthma. Allergy 2021, 76, 210–222. [Google Scholar] [CrossRef]
- Torosantucci, A.; Bromuro, C.; Chiani, P.; De Bernardis, F.; Berti, F.; Galli, C.; Norelli, F.; Bellucci, C.; Polonelli, L.; Costantino, P.; et al. A Novel Glyco-Conjugate Vaccine against Fungal Pathogens. J. Exp. Med. 2005, 202, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.J.; Underhill, D.M. Peptidoglycan Recognition by the Innate Immune System. Nat. Rev. Immunol. 2018, 18, 243–254. [Google Scholar] [CrossRef]
- Rockenstein, E.; Ostroff, G.; Dikengil, F.; Rus, F.; Mante, M.; Florio, J.; Adame, A.; Trinh, I.; Kim, C.; Overk, C.; et al. Combined Active Humoral and Cellular Immunization Approaches for the Treatment of Synucleinopathies. J. Neurosci. 2018, 38, 1000–1014. [Google Scholar] [CrossRef]
- Wang, H.; Yang, B.; Wang, Y.; Liu, F.; Fernández-Tejada, A.; Dong, S. β-Glucan as an Immune Activator and a Carrier in the Construction of a Synthetic MUC1 Vaccine. Chem. Commun. 2019, 55, 253–256. [Google Scholar] [CrossRef]
- Cubas, R.; Zhang, S.; Kwon, S.; Sevick-Muraca, E.M.; Li, M.; Chen, C.; Yao, Q. Virus-like Particle (VLP) Lymphatic Trafficking and Immune Response Generation After Immunization by Different Routes. J. Immunother. 2009, 32, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Roukens, A.H.E.; Gelinck, L.B.S.; Visser, L.G. Intradermal Vaccination to Protect Against Yellow Fever and Influenza. In Intradermal Immunization; Teunissen, M.B.M., Ed.; Current Topics in Microbiology and Immunology; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2011; Volume 351, pp. 159–179. ISBN 978-3-642-23689-1. [Google Scholar]
- De Giorgi, F.; Laferrière, F.; Zinghirino, F.; Faggiani, E.; Lends, A.; Bertoni, M.; Yu, X.; Grélard, A.; Morvan, E.; Habenstein, B.; et al. Novel Self-Replicating α-Synuclein Polymorphs That Escape ThT Monitoring Can Spontaneously Emerge and Acutely Spread in Neurons. Sci. Adv. 2020, 6, eabc4364. [Google Scholar] [CrossRef] [PubMed]
- Fagerqvist, T.; Lindström, V.; Nordström, E.; Lord, A.; Tucker, S.M.E.; Su, X.; Sahlin, C.; Kasrayan, A.; Andersson, J.; Welander, H.; et al. Monoclonal Antibodies Selective for α-Synuclein Oligomers/Protofibrils Recognize Brain Pathology in Lewy Body Disorders and α-Synuclein Transgenic Mice with the Disease-Causing A30P Mutation. J. Neurochem. 2013, 126, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Pullen, G.R.; Fitzgerald, M.G.; Hosking, C.S. Antibody Avidity Determination by ELISA Using Thiocyanate Elution. J. Immunol. Methods 1986, 86, 83–87. [Google Scholar] [CrossRef]
- MacDonald, R.A.; Hosking, C.S.; Jones, C.L. The Measurement of Relative Antibody Affinity by ELISA Using Thiocyanate Elution. J. Immunol. Methods 1988, 106, 191–194. [Google Scholar] [CrossRef]
- Wördehoff, M.; Hoyer, W. α-Synuclein Aggregation Monitored by Thioflavin T Fluorescence Assay. Bio-Protocol 2018, 8, e2941. [Google Scholar] [CrossRef]
- de Oliveira, G.A.P.; Silva, J.L. Alpha-Synuclein Stepwise Aggregation Reveals Features of an Early Onset Mutation in Parkinson’s Disease. Commun. Biol. 2019, 2, 374. [Google Scholar] [CrossRef]
- Breydo, L.; Morgan, D.; Uversky, V.N. Pseudocatalytic Antiaggregation Activity of Antibodies: Immunoglobulins Can Influence α-Synuclein Aggregation at Substoichiometric Concentrations. Mol. Neurobiol. 2016, 53, 1949–1958. [Google Scholar] [CrossRef]
- Pifferi, C.; Fuentes, R.; Fernández-Tejada, A. Natural and Synthetic Carbohydrate-Based Vaccine Adjuvants and Their Mechanisms of Action. Nat. Rev. Chem. 2021, 5, 197–216. [Google Scholar] [CrossRef]
- Höllerhage, M.; Wolff, A.; Chakroun, T.; Evsyukov, V.; Duan, L.; Chua, O.W.-H.; Tang, Q.; Koeglsperger, T.; Höglinger, G.U. Binding Stability of Antibody—α-Synuclein Complexes Predicts the Protective Efficacy of Anti-α-Synuclein Antibodies. Mol. Neurobiol. 2022, 59, 3980–3995. [Google Scholar] [CrossRef]
- Machado, Y.; Duinkerken, S.; Hoepflinger, V.; Mayr, M.; Korotchenko, E.; Kurtaj, A.; Pablos, I.; Steiner, M.; Stoecklinger, A.; Lübbers, J.; et al. Synergistic Effects of Dendritic Cell Targeting and Laser-Microporation on Enhancing Epicutaneous Skin Vaccination Efficacy. J. Control. Release 2017, 266, 87–99. [Google Scholar] [CrossRef]
- Tesfaye, D.Y.; Gudjonsson, A.; Bogen, B.; Fossum, E. Targeting Conventional Dendritic Cells to Fine-Tune Antibody Responses. Front. Immunol. 2019, 10, 1529. [Google Scholar] [CrossRef] [PubMed]
- Elder, M.J.; Webster, S.J.; Chee, R.; Williams, D.L.; Hill Gaston, J.S.; Goodall, J.C. β-Glucan Size Controls Dectin-1-Mediated Immune Responses in Human Dendritic Cells by Regulating IL-1β Production. Front. Immunol. 2017, 8, 791. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Gupta, S.; Agrawal, A. Human Dendritic Cells Activated via Dectin-1 Are Efficient at Priming Th17, Cytotoxic CD8 T and B Cell Responses. PLoS ONE 2010, 5, e13418. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, C.; Longmate, J.; Lacey, S.F.; Kaltcheva, T.; Sharan, R.; Marsano, D.; Kwon, P.; Drake, J.; Williams, B.; Denison, S.; et al. Clinical Evaluation of Safety and Immunogenicity of PADRE-Cytomegalovirus (CMV) and Tetanus-CMV Fusion Peptide Vaccines with or Without PF03512676 Adjuvant. J. Infect. Dis. 2012, 205, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Snook, A.E.; Baybutt, T.R.; Xiang, B.; Abraham, T.S.; Flickinger, J.C.; Hyslop, T.; Zhan, T.; Kraft, W.K.; Sato, T.; Waldman, S.A. Split Tolerance Permits Safe Ad5-GUCY2C-PADRE Vaccine-Induced T-Cell Responses in Colon Cancer Patients. J. Immunother. Cancer 2019, 7, 104. [Google Scholar] [CrossRef] [Green Version]





| Name | B Cell Epitope | T Cell Epitope | Sugar Moiety or Adjuvant |
|---|---|---|---|
| WISIT Vaccine Candidates | |||
| CW-type 1 | α-syn 110–130 (7-mer) | PADRE | β-glucan/CLEC |
| CW-type 2 | α-syn 110–130 (8-mer) | PADRE | β-glucan/CLEC |
| CW-type 3 | α-syn 110–130 (10-mer) | PADRE | β-glucan/CLEC |
| CW-type 4 | α-syn 110–130 (11-mer) | PADRE | β-glucan/CLEC |
| CW-type 5 | α-syn 110–130 (12-mer) | PADRE | β-glucan/CLEC |
| CW-type 6 | α-syn 1–10 (8-mer) | PADRE | β-glucan/CLEC |
| Conventional Vaccine-Type Candidates | |||
| CC-type 1 | α-syn 110–130 (7-mer) | CRM 197 | aluminum oxyhydroxide |
| CC-type 2 | α-syn 110–130 (8-mer) | CRM 197 | aluminum oxyhydroxide |
| CC-type 3 | α-syn 110–130 (10-mer) | CRM 197 | aluminum oxyhydroxide |
| CC-type 4 | α-syn 110–130 (11-mer) | CRM 197 | aluminum oxyhydroxide |
| CC-type 5 | α-syn 110–130 (12-mer) | CRM 197 | aluminum oxyhydroxide |
| CC-type 6 | α-syn 1–10 (8-mer) | CRM 197 | aluminum oxyhydroxide |
| CW-Type | CC-Type | Factor | |
|---|---|---|---|
| Candidate 1 | 1.1 | 0.25 | 4.4 |
| Candidate 2 | 0.75 | 0.3 | 2.5 |
| Candidate 3 | 1.2 | 0.4 | 3 |
| Candidate 4 | 1.2 | 0.4 | 3 |
| Candidate 5 | 1.2 | 0.3 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidhuber, S.; Scheiblhofer, S.; Weiss, R.; Cserepes, M.; Tóvári, J.; Gadermaier, G.; Bezard, E.; De Giorgi, F.; Ichas, F.; Strunk, D.; et al. A Novel C-Type Lectin Receptor-Targeted α-Synuclein-Based Parkinson Vaccine Induces Potent Immune Responses and Therapeutic Efficacy in Mice. Vaccines 2022, 10, 1432. https://doi.org/10.3390/vaccines10091432
Schmidhuber S, Scheiblhofer S, Weiss R, Cserepes M, Tóvári J, Gadermaier G, Bezard E, De Giorgi F, Ichas F, Strunk D, et al. A Novel C-Type Lectin Receptor-Targeted α-Synuclein-Based Parkinson Vaccine Induces Potent Immune Responses and Therapeutic Efficacy in Mice. Vaccines. 2022; 10(9):1432. https://doi.org/10.3390/vaccines10091432
Chicago/Turabian StyleSchmidhuber, Sabine, Sandra Scheiblhofer, Richard Weiss, Mihály Cserepes, József Tóvári, Gabriele Gadermaier, Erwan Bezard, Francesca De Giorgi, François Ichas, Dirk Strunk, and et al. 2022. "A Novel C-Type Lectin Receptor-Targeted α-Synuclein-Based Parkinson Vaccine Induces Potent Immune Responses and Therapeutic Efficacy in Mice" Vaccines 10, no. 9: 1432. https://doi.org/10.3390/vaccines10091432






