Vaccination Strategies against Seasonal Influenza in Long Term Care Setting: Lessons from a Mathematical Modelling Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Context
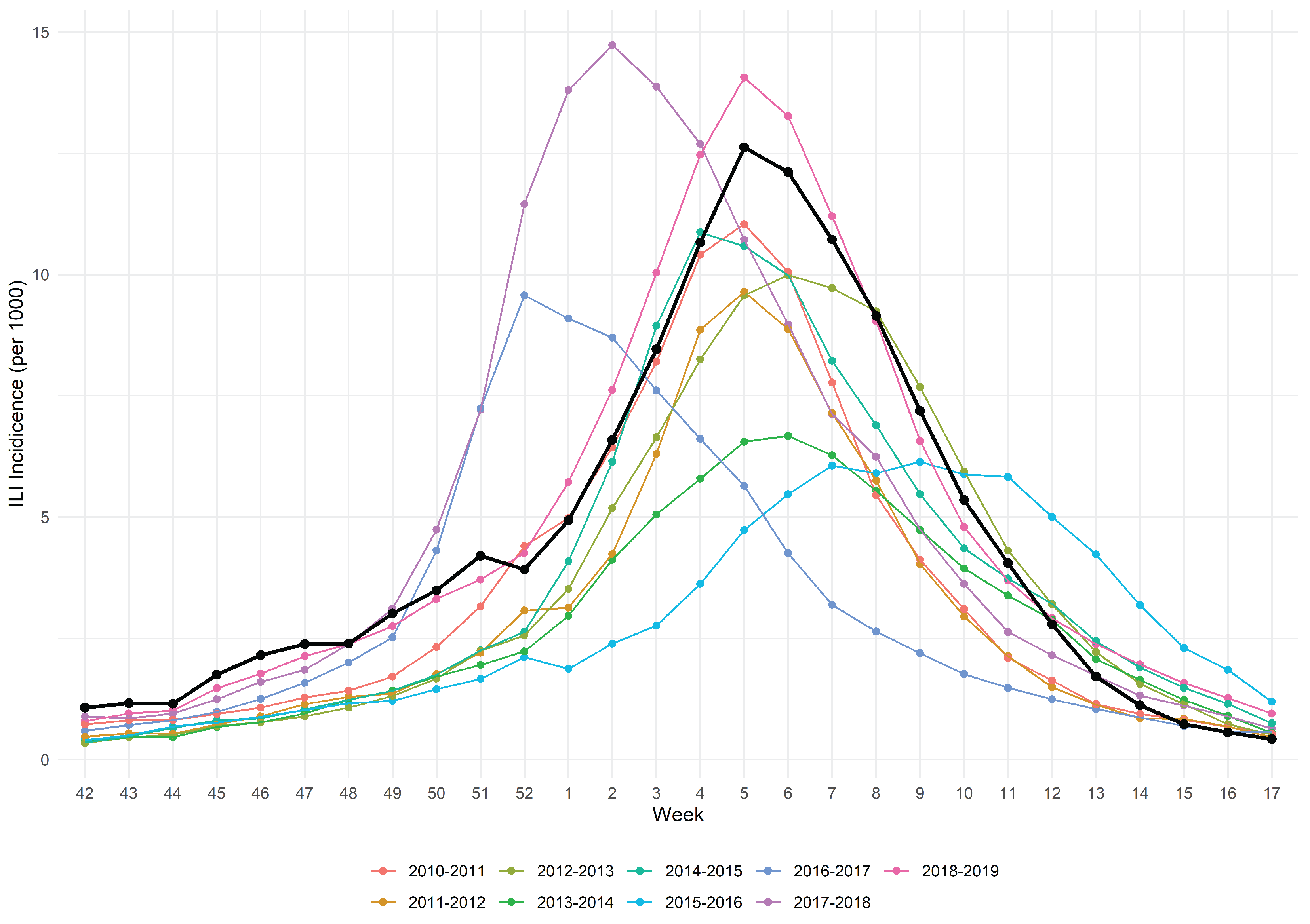
2.1.1. The 2019/2020 Influenza Season
2.1.2. The Long Term Care Nursing Home
2.2. Mathematical Model
2.2.1. Population Model
2.2.2. Stochastic Model
2.3. Key Parameters: Baseline Scenario
Model Fit
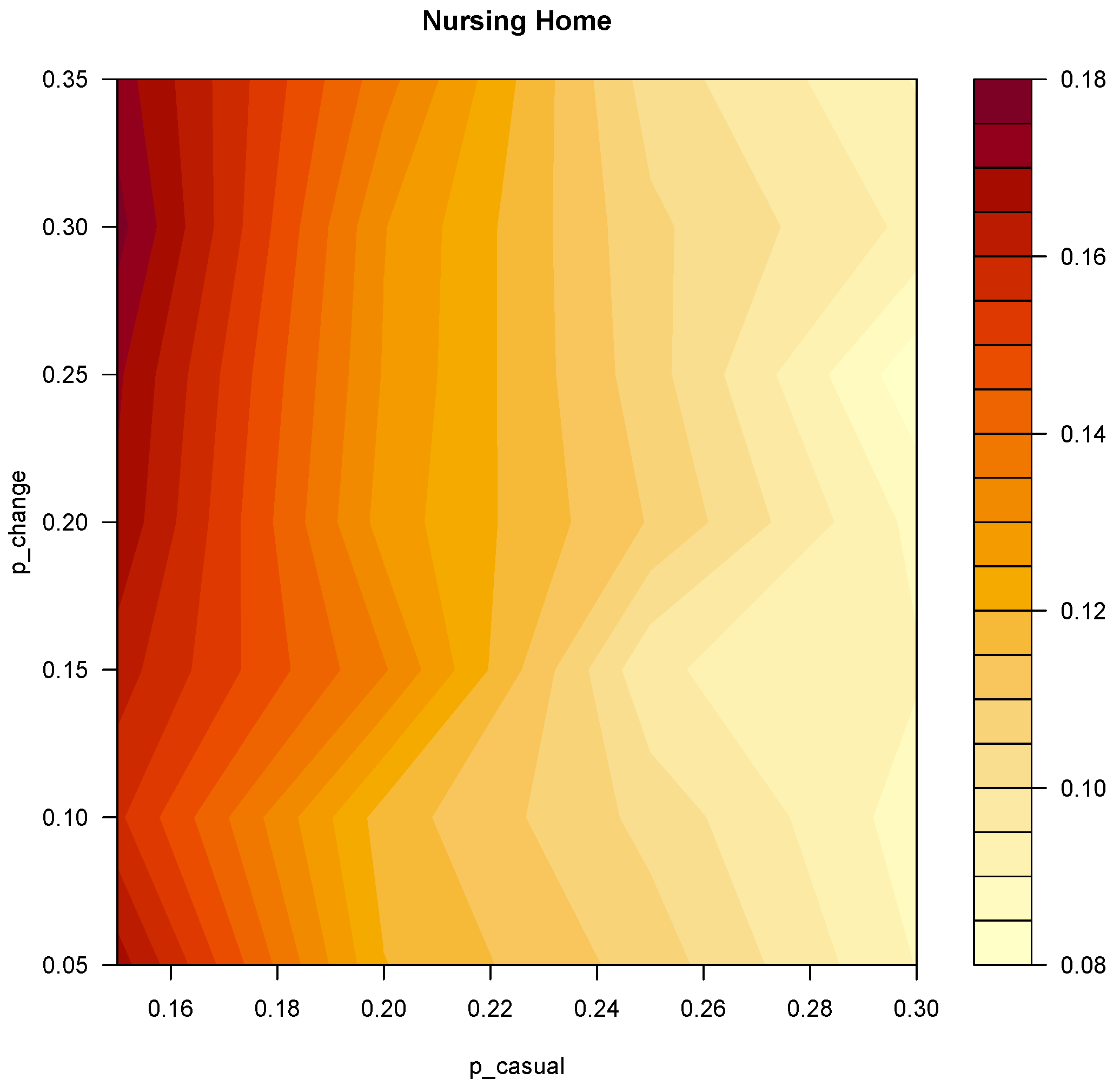
2.4. Simulation Plan
2.5. Outcome and Data Analysis
3. Results
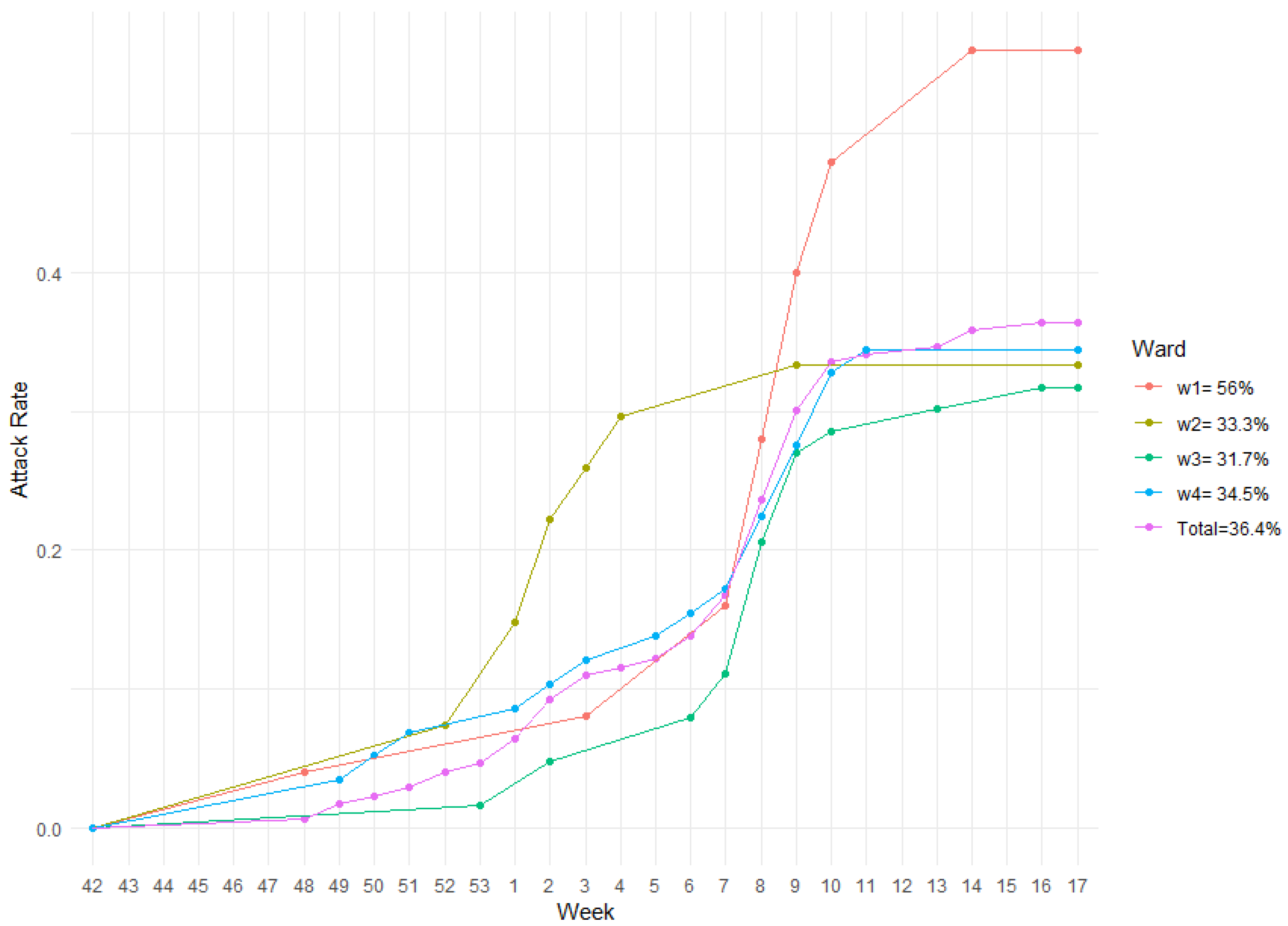
3.1. The 2019/2020 Influenza Season in the “Belletti Bona” LTC Nursing Home
3.2. Model Fit
3.3. Further Simulations
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LTC | Long Term Care |
| AR | Attack Rate |
| ILI | Influenza like illness |
| HCW | Health care workers |
References
- Hagiwara, Y.; Harada, K.; Nealon, J.; Okumura, Y.; Kimura, T.; Chaves, S.S. Seasonal influenza, its complications and related healthcare resource utilization among people 60 years and older: A descriptive retrospective study in Japan. PLoS ONE 2022, 17, e0272795. [Google Scholar] [CrossRef] [PubMed]
- Rosano, A.; Bella, A.; Gesualdo, F.; Acampora, A.; Pezzotti, P.; Marchetti, S.; Ricciardi, W.; Rizzo, C. Investigating the impact of influenza on excess mortality in all ages in Italy during recent seasons (2013/14–2016/17 seasons). Int. J. Infect. Dis. 2019, 88, 127–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paget, J.; Danielle Iuliano, A.; Taylor, R.J.; Simonsen, L.; Viboud, C.; Spreeuwenberg, P. Estimates of mortality associated with seasonal influenza for the European Union from the GLaMOR project. Vaccine 2022, 40, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Surveillance of COVID-19 in Long-Term Care Facilities in the EU / EEA. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-COVID-19-long-term-care-facilities-EU-EEA (accessed on 30 November 2021).
- Lansbury, L.E.; Brown, C.S.; Nguyen-Van-Tam, J.S. Influenza in long-term care facilities. Influenza Other Respir. Viruses 2017, 11, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Coles, F.B.; Balzano, G.J.; Morse, D.L. An outbreak of influenza A (H3N2) in a well immunized nursing home population. J. Am. Geriatr. Soc. 1992, 40, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Strassburg, M.A.; Greenland, S.; Sorvillo, F.J.; Lieb, L.E.; Habel, L.A. Influenza in the elderly: Report of an outbreak and a review of vaccine effectiveness reports. Vaccine 1986, 4, 38–44. [Google Scholar] [CrossRef]
- Drinka, P.J.; Gravenstein, S.; Krause, P.; Schilling, M.; Miller, B.A.; Shult, P. Outbreaks of influenza A and B in a highly immunized nursing home population. J. Fam. Pract. 1997, 45, 509–514. [Google Scholar]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Prim. 2018, 4, 3. [Google Scholar] [CrossRef]
- Reber, A.J.; Chirkova, T.; Kim, J.H.; Cao, W.; Biber, R.; Shay, D.K.; Sambhara, S. Immunosenescence and Challenges of Vaccination against Influenza in the Aging Population. Aging Dis. 2012, 3, 68–90. [Google Scholar]
- Masse, S.; Minodier, L.; Heuze, G.; Blanchon, T.; Capai, L.; Falchi, A. Influenza-like illness outbreaks in nursing homes in Corsica, France, 2014–2015: Epidemiological and molecular characterization. SpringerPlus 2016, 5, 1338. [Google Scholar] [CrossRef] [Green Version]
- Halloran, N.F.; Harries, A.D.; Ghebrehewet, S.; Cleary, P. Factors associated with influenza-like illness in care homes in Cheshire and Merseyside during the 2017–2018 influenza season. Public Health 2020, 187, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.M.; Thompson, L.H.; Nowicki, D.L.; Plourde, P.J. Outbreaks of influenza-like illness in long-term care facilities in Winnipeg, Canada. Influenza Other Respir. Viruses 2013, 7, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Regional Office for Europe. Recommendations on Influenza Vaccination during the 2018–2019 Winter Season: October 2018. Available online: https://apps.who.int/iris/handle/10665/345694 (accessed on 20 June 2021).
- Lemaitre, M.; Meret, T.; Rothan-Tondeur, M.; Belmin, J.; Lejonc, J.L.; Luquel, L.; Piette, F.; Salom, M.; Verny, M.; Vetel, J.M.; et al. Effect of influenza vaccination of nursing home staff on mortality of residents: A cluster-randomized trial. J. Am. Geriatr. Soc. 2009, 57, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Wendelboe, A.M.; Avery, C.; Andrade, B.; Baumbach, J.; Landen, M.G. Importance of employee vaccination against influenza in preventing cases in long-term care facilities. Infect. Control Hosp. Epidemiol. 2011, 32, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.E.; Jefferson, T.; Lasserson, T.J. Influenza vaccination for healthcare workers who care for people aged 60 or older living in long-term care institutions. Cochrane Database Syst. Rev. 2016, 2016, CD005187. [Google Scholar] [CrossRef]
- Van Den Dool, C.; Bonten, M.J.; Hak, E.; Heijne, J.C.; Wallinga, J. The effects of influenza vaccination of health care workers in nursing homes: Insights from a mathematical model. PLoS Med. 2008, 5, 1453–1460. [Google Scholar] [CrossRef] [Green Version]
- van den Dool, C.; Bonten, M.J.; Hak, E.; Wallinga, J. Modeling the effects of influenza vaccination of health care workers in hospital departments. Vaccine 2009, 27, 6261–6267. [Google Scholar] [CrossRef]
- Wendelboe, A.M.; Grafe, C.; McCumber, M.; Anderson, M.P. Inducing Herd Immunity against Seasonal Influenza in Long-Term Care Facilities through Employee Vaccination Coverage: A Transmission Dynamics Model. Comput. Math. Methods Med. 2015, 2015, 178247. [Google Scholar] [CrossRef]
- INFLUNET Official Ministerial Influenza Surveillance Network. INFLUNET Home Page. 2020. Available online: https://www.epicentro.iss.it/influenza/influnet (accessed on 3 May 2022).
- Trentini, F.; Pariani, E.; Bella, A.; Diurno, G.; Crottogini, L.; Rizzo, C.; Merler, S.; Ajelli, M. Characterizing the transmission patterns of seasonal influenza in Italy: Lessons from the last decade. BMC Public Health 2022, 22, 19. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention, CDC. Estimated Flu-Related Illnesses, Medical visits, Hospitalizations, and Deaths in the United States—2019–2020 Flu Season. 2020. Available online: https://www.cdc.gov/flu/about/burden/2019-2020.html (accessed on 5 December 2022).
- R Core Team. R: A Language and Environment for Statistical Computing. 2022. Available online: https://www.R-project.org/ (accessed on 17 September 2022).
- Moretti, F.; Visentin, D.; Bovolenta, E.; Rimondini, M.; Majori, S.; Mazzi, M.; Poli, A.; Tardivo, S.; Torri, E. Attitudes of Nursing Home Staff Towards Influenza Vaccination: Opinions and Factors Influencing Hesitancy. Int. J. Environ. Res. Public Health 2020, 17, 1851. [Google Scholar] [CrossRef] [Green Version]
- Lorini, C.; Collini, F.; Gasparini, F.; Paolini, D.; Grazzini, M.; Ierardi, F.; Galletti, G.; Zanobini, P.; Gemmi, F.; Bonaccorsi, G. Health Literacy, Vaccine Confidence and Influenza Vaccination Uptake among Nursing Home Staff: A Cross-Sectional Study Conducted in Tuscany. Vaccines 2020, 8, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Health Ministerial Office of Italy. Vaccinazione Antinfluenzale—Coperture Vaccinali Medie—Official Ministerial Vaccination Coverage. Available online: https://www.salute.gov.it/portale/documentazione/p6_2_8_3_1.jsp?lingua=italiano&id=19 (accessed on 29 September 2022).
- Okoli, G.N.; Racovitan, F.; Abdulwahid, T.; Righolt, C.H.; Mahmud, S.M. Variable seasonal influenza vaccine effectiveness across geographical regions, age groups and levels of vaccine antigenic similarity with circulating virus strains: A systematic review and meta-analysis of the evidence from test-negative design studies after the 2009/10 influenza pandemic. Vaccine 2021, 39, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- Dorigatti, I.; Cauchemez, S.; Pugliese, A.; Ferguson, N.M. A new approach to characterising infectious disease transmission dynamics from sentinel surveillance: Application to the Italian 2009–2010 A/H1N1 influenza pandemic. Epidemics 2012, 4, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaari, R.; Katriel, G.; Stone, L.; Mendelson, E.; Mandelboim, M.; Huppert, A. Model-based reconstruction of an epidemic using multiple datasets: Understanding influenza A/H1N1 pandemic dynamics in Israel. J. R. Soc. Interface 2016, 13, 20160099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunelli, A.; Rizzo, C.; Puzelli, S.; Bella, A.; Montomoli, E.; Rota, M.C.; Donatelli, I.; Pugliese, A. Understanding the dynamics of seasonal influenza in Italy: Incidence, transmissibility and population susceptibility in a 9-year period. Influenza Other Respir. Viruses 2013, 7, 286–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- INFLUNET Official Ministerial Influenza Surveillance Network. Rapporto Epidemiologico Influnet—Rapporto N. 24 del 8 Maggio 2020—Week of April 20th–26th 2020. 2020. Available online: https://www.salute.gov.it/portale/temi/documenti/epidemiologica/Influnet_2020_17.pdf (accessed on 7 December 2021).
- Italian National Institute of Statistics-Istat. Popolazione Italiana al 1 Gennaio—Resident Italian Population on Jan 1st. 2020. Available online: http://dati.istat.it/Index.aspx?DataSetCode=DCIS_POPRES1&Lang=en# (accessed on 15 May 2021).
- Li, M.; Wang, H.; Song, B.; Ma, J. The spread of influenza-like-illness within the household in Shanghai, China. Math. Biosci. Eng. 2020, 17, 1889–1900. [Google Scholar] [CrossRef]
- Thomas, R.E. Is influenza-like illness a useful concept and an appropriate test of influenza vaccine effectiveness? Vaccine 2014, 32, 2143–2149. [Google Scholar] [CrossRef]
- Pellegrinelli, L.; Bubba, L.; Galli, C.; Primache, V.; Anselmi, G.; Delbue, S.; Signorini, L.; Dolci, M.; Binda, S.; Pariani, E. Respiratory viruses detection among Influenza-like illness (ILI) cases in Lombardy (Northern Italy) during pre- (2018-2019) and SARS-CoV-2 pandemic (2020-2021) winter seasons. Int. J. Infect. Dis. 2022, 116, S91. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Ridda, I.; Seale, H.; Gao, Z.; Ratnamohan, V.M.; Donovan, L.; Zeng, F.; Dwyer, D.E. Respiratory viruses transmission from children to adults within a household. Vaccine 2012, 30, 3009–3014. [Google Scholar] [CrossRef]
| Parameter | Baseline Scenario Values | Reference | Values Explored with Further Simulations |
|---|---|---|---|
| Casual contact probability of transmission—p_casual | |||
| (unity: contact−1) | model fit | - | 0.3 |
| Close contact probability of transmission—p_close | |||
| (unity: contact−1) | model fit | - | 0.6 |
| Shift change probability of transmission—p_change | |||
| (unity: contact−1) | model fit | - | 0.2 |
| Initial Removed Fraction of HCWs | 0.10 | [29,30,31] | 0-0.05-0.10-0.2-0.3 |
| Initial Removed Fraction of Guests | 0.20 | [29,30,31] | 0-0.05-0.10-0.2-0.3 |
| Vaccine effectiveness on HCWs | 0.3744 | [28] | 0.1-0.2-0.37-0.5-0.6 |
| Vaccine effectiveness on Guests | 0.21 | [28] | 0.1-0.2-0.3-0.4 |
| Guests Vaccine Uptake | 0.4653 | derived from LTC facility real data | 0-0.25-0.46-0.5-0.75-1 |
| HCW Vaccine Uptake | 0.0516 | [27] | 0-0.05-0.25-0.5-0.75-1 |
| HCW Vaccine Efficacy | Ward | AR (Mean) | LCI 95% | UCI 95% | HCW Vaccine Uptake | Ward | AR (Mean) | LCI 95% | UCI 95% |
|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 1 | 0.57 | 0.05 | 0.81 | 0.00 | 1 | 0.58 | 0.37 | 0.81 |
| 0.2 | 1 | 0.59 | 0.4 | 0.8 | 0.05 | 1 | 0.59 | 0.38 | 0.81 |
| 0.37 | 1 | 0.59 | 0.38 | 0.81 | 0.25 | 1 | 0.59 | 0.38 | 0.81 |
| 0.5 | 1 | 0.58 | 0.36 | 0.81 | 0.50 | 1 | 0.56 | 0.05 | 0.77 |
| 0.6 | 1 | 0.6 | 0.37 | 0.82 | 0.75 | 1 | 0.59 | 0.36 | 0.77 |
| 1.00 | 1 | 0.56 | 0.30 | 0.77 | |||||
| 0.1 | 2 | 0.59 | 0.39 | 0.82 | 0.00 | 2 | 0.58 | 0.32 | 0.81 |
| 0.2 | 2 | 0.57 | 0.27 | 0.77 | 0.05 | 2 | 0.59 | 0.31 | 0.81 |
| 0.37 | 2 | 0.59 | 0.31 | 0.81 | 0.25 | 2 | 0.60 | 0.36 | 0.81 |
| 0.5 | 2 | 0.58 | 0.04 | 0.85 | 0.50 | 2 | 0.58 | 0.07 | 0.78 |
| 0.6 | 2 | 0.6 | 0.36 | 0.81 | 0.75 | 2 | 0.59 | 0.35 | 0.80 |
| 1.00 | 2 | 0.59 | 0.29 | 0.81 | |||||
| 0.1 | 3 | 0.37 | 0.21 | 0.53 | 0.00 | 3 | 0.36 | 0.22 | 0.50 |
| 0.2 | 3 | 0.38 | 0.23 | 0.5 | 0.05 | 3 | 0.37 | 0.22 | 0.50 |
| 0.37 | 3 | 0.37 | 0.22 | 0.5 | 0.25 | 3 | 0.37 | 0.22 | 0.53 |
| 0.5 | 3 | 0.36 | 0.22 | 0.5 | 0.50 | 3 | 0.37 | 0.21 | 0.56 |
| 0.6 | 3 | 0.35 | 0.17 | 0.49 | 0.75 | 3 | 0.38 | 0.21 | 0.54 |
| 1.00 | 3 | 0.35 | 0.21 | 0.50 | |||||
| 0.1 | 4 | 0.35 | 0.21 | 0.52 | 0.00 | 4 | 0.36 | 0.22 | 0.50 |
| 0.2 | 4 | 0.36 | 0.24 | 0.51 | 0.05 | 4 | 0.36 | 0.17 | 0.52 |
| 0.37 | 4 | 0.36 | 0.17 | 0.52 | 0.25 | 4 | 0.35 | 0.22 | 0.51 |
| 0.5 | 4 | 0.36 | 0.21 | 0.54 | 0.50 | 4 | 0.36 | 0.18 | 0.51 |
| 0.6 | 4 | 0.36 | 0.21 | 0.51 | 0.75 | 4 | 0.35 | 0.14 | 0.50 |
| 1.00 | 4 | 0.35 | 0.20 | 0.50 |
| Guests Vaccine Efficacy | Ward | AR (Mean) | LCI 95% | UCI 95% | Guests Vaccine Uptake | Ward | AR (Mean) | LCI 95% | UCI 95% |
|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 1 | 0.61 | 0.36 | 0.85 | 0.00 | 1 | 0.64 | 0.42 | 0.90 |
| 0.2 | 1 | 0.59 | 0.38 | 0.81 | 0.25 | 1 | 0.64 | 0.42 | 0.90 |
| 0.3 | 1 | 0.52 | 0.06 | 0.71 | 0.46 | 1 | 0.59 | 0.38 | 0.81 |
| 0.4 | 1 | 0.54 | 0.32 | 0.80 | 0.50 | 1 | 0.60 | 0.38 | 0.85 |
| 0.75 | 1 | 0.55 | 0.34 | 0.76 | |||||
| 1.00 | 1 | 0.52 | 0.32 | 0.76 | |||||
| 0.1 | 2 | 0.61 | 0.05 | 0.85 | 0.00 | 2 | 0.66 | 0.45 | 0.90 |
| 0.2 | 2 | 0.59 | 0.31 | 0.81 | 0.25 | 2 | 0.60 | 0.05 | 0.82 |
| 0.3 | 2 | 0.56 | 0.08 | 0.80 | 0.46 | 2 | 0.59 | 0.31 | 0.81 |
| 0.4 | 2 | 0.51 | 0.09 | 0.70 | 0.50 | 2 | 0.59 | 0.33 | 0.81 |
| 0.75 | 2 | 0.54 | 0.05 | 0.76 | |||||
| 1.00 | 2 | 0.52 | 0.20 | 0.77 | |||||
| 0.1 | 3 | 0.38 | 0.24 | 0.56 | 0.00 | 3 | 0.39 | 0.22 | 0.57 |
| 0.2 | 3 | 0.37 | 0.22 | 0.50 | 0.25 | 3 | 0.39 | 0.24 | 0.54 |
| 0.3 | 3 | 0.35 | 0.18 | 0.49 | 0.46 | 3 | 0.37 | 0.22 | 0.50 |
| 0.4 | 3 | 0.32 | 0.13 | 0.48 | 0.50 | 3 | 0.38 | 0.24 | 0.51 |
| 0.75 | 3 | 0.34 | 0.20 | 0.49 | |||||
| 1.00 | 3 | 0.33 | 0.19 | 0.52 | |||||
| 0.1 | 4 | 0.37 | 0.05 | 0.54 | 0.00 | 4 | 0.38 | 0.26 | 0.56 |
| 0.2 | 4 | 0.36 | 0.17 | 0.52 | 0.25 | 4 | 0.37 | 0.02 | 0.57 |
| 0.3 | 4 | 0.33 | 0.18 | 0.49 | 0.46 | 4 | 0.36 | 0.17 | 0.52 |
| 0.4 | 4 | 0.32 | 0.21 | 0.47 | 0.50 | 4 | 0.35 | 0.21 | 0.50 |
| 0.75 | 4 | 0.33 | 0.03 | 0.46 | |||||
| 1.00 | 4 | 0.32 | 0.21 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratti, M.; Concina, D.; Rinaldi, M.; Salinelli, E.; Di Brisco, A.M.; Ferrante, D.; Volpe, A.; Panella, M. Vaccination Strategies against Seasonal Influenza in Long Term Care Setting: Lessons from a Mathematical Modelling Study. Vaccines 2023, 11, 32. https://doi.org/10.3390/vaccines11010032
Ratti M, Concina D, Rinaldi M, Salinelli E, Di Brisco AM, Ferrante D, Volpe A, Panella M. Vaccination Strategies against Seasonal Influenza in Long Term Care Setting: Lessons from a Mathematical Modelling Study. Vaccines. 2023; 11(1):32. https://doi.org/10.3390/vaccines11010032
Chicago/Turabian StyleRatti, Matteo, Diego Concina, Maurizio Rinaldi, Ernesto Salinelli, Agnese Maria Di Brisco, Daniela Ferrante, Alessandro Volpe, and Massimiliano Panella. 2023. "Vaccination Strategies against Seasonal Influenza in Long Term Care Setting: Lessons from a Mathematical Modelling Study" Vaccines 11, no. 1: 32. https://doi.org/10.3390/vaccines11010032
APA StyleRatti, M., Concina, D., Rinaldi, M., Salinelli, E., Di Brisco, A. M., Ferrante, D., Volpe, A., & Panella, M. (2023). Vaccination Strategies against Seasonal Influenza in Long Term Care Setting: Lessons from a Mathematical Modelling Study. Vaccines, 11(1), 32. https://doi.org/10.3390/vaccines11010032








