Durability of ChAdOx1 nCoV-19 (Covishield®) Vaccine Induced Antibody Response in Health Care Workers
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Board on COVID 19 Disease. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 4 October 2022).
- Papoutsi, E.; Giannakoulis, V.G.; Ntella, V.; Pappa, S.; Katsaounou, P. Global burden of COVID-19 pandemic on healthcare workers. ERJ Open Res. 2020, 6, 00195-2020. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Drew, D.A.; Graham, M.S.; Joshi, A.D.; Guo, C.G.; Ma, W.; Mehta, R.S.; Warner, E.T.; Sikavi, D.R.; Lo, C.H.; et al. Risk of COVID-19 among front-line health-care workers and the general community: A prospective cohort study. Lancet Public Health 2020, 5, e475–e483. [Google Scholar] [CrossRef] [PubMed]
- Mutambudzi, M.; Niedzwiedz, C.; Macdonald, E.B.; Leyland, A.; Mair, F.; Anderson, J.; Celis-Morales, C.; Cleland, J.; Forbes, J.; Gill, J.; et al. Occupation and risk of severe COVID-19: Prospective cohort study of 120 075 UK Biobank participants. Occup. Environ. Med. 2020, 78, 307–314. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Impact of COVID-19 on Health and Care Workers: A Closer Look at Deaths; WHO: Geneva, Switzerland, 2021.
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Verma, A.; Tiwari, P.; Katiyar, H.; Aggarwal, A.; Khetan, D.; Kishore, R.V.; Kumar, P.; Singh, T.P.; Sheikh, S.; et al. Serological Immune Response Following ChAdOx1 nCoV-19 Vaccine (Covishield®) in Patients with Liver Cirrhosis. Vaccines 2022, 10, 1837. [Google Scholar]
- Bhadauria, D.S.; Katiyar, H.; Goel, A.; Tiwari, P.; Kishore, R.V.; Aggarwal, A.; Verma, A.; Khetan, D.; Kaul, A.; Yachha, M.; et al. Antibody Response to ChAdOx1 nCoV-19 (AZD1222) Vaccine in Kidney Transplant Recipients. Vaccines 2022, 10, 1693. [Google Scholar] [CrossRef]
- Raja, N.; Rajagopalan, A.; Arunachalam, J.; Prasath, A.; Durai, R.; Rajendran, M. Humoral response to viral vector COVID-19 vaccine in hemodialysis patients. Kidney Res. Clin. Pract. 2022, 41, 342–350. [Google Scholar] [CrossRef]
- Singh, A.K.; Phatak, S.R.; Singh, R.; Bhattacharjee, K.; Singh, N.K.; Gupta, A.; Sharma, A. Antibody response after first and second-dose of ChAdOx1-nCOV (CovishieldTM®) and BBV-152 (CovaxinTM®) among health care workers in India: The final results of cross-sectional coronavirus vaccine-induced antibody titre (COVAT) study. Vaccine 2021, 39, 6492–6509. [Google Scholar] [CrossRef]
- Khan, M.S.; Haq, I.; Qurieshi, M.A.; Majid, S.; Bhat, A.A.; Qazi, T.B.; Chowdri, I.N.; Sabah, I.; Kawoosa, M.F.; Lone, A.A.; et al. SARS-CoV-2 Seroprevalence Among Healthcare Workers by Workplace Exposure Risk in Kashmir, India. J. Hosp. Med. 2021, 16, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Wattal, C.; Oberoi, J.K.; Goel, N.; Datta, S.; Raveendran, R.; Rao, B.K.; Kumar, R. A cross-sectional study of SARS-CoV-2 seroprevalence among asymptomatic healthcare workers in a tertiary healthcare centre: Assessing the impact of PPE guidelines. Indian J. Med. Microbiol. 2021, 39, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.K.; Panda, P.K.; Bahurupi, Y.; Omar, B.; Akhil, T.; Panwar, P.; Sharma, M. Seroprevalence of Antibodies Against SARS-CoV-2 Among Health Care Workers at a Tertiary Care Hospital in Uttarakhand: A Retrospective Study. Cureus 2022, 14, e24840. [Google Scholar] [CrossRef]
- Goenka, M.K.; Afzalpurkar, S.; Goenka, U.; Das, S.S.; Mukherjee, M.; Jajodia, S.; Shah, B.B.; Patil, V.U.; Rodge, G.; Khan, U.; et al. Seroprevalence of COVID-19 Amongst Health Care Workers in a Tertiary Care Hospital of a Metropolitan City from India. J. Assoc. Physicians India 2020, 68, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Dwivedi, T.; Gajendra, S.; Sahoo, B.; Gupta, S.K.; Vikas, H.; Singh, A.R.; Mohan, A.; Bhatnagar, S.; Singh, S.; et al. Seroprevalence of antibodies to SARS-CoV-2 in healthcare workers & implications of infection control practice in India. Indian J. Med. Res. 2021, 153, 207–213. [Google Scholar]
- Varghese, S.M.; Mateethra, G.C.; George, G.; Chandran, V.S.; John, G.M.; Varghese, L.T.; Mammen, N.K.; Vinayak, V. A study on seroconversion following first & second doses of ChAdOx1 nCoV-19 vaccine in Central Kerala. Indian J. Med. Res. 2022, 155, 499–504. [Google Scholar]
- Eyre, D.W.; Lumley, S.F.; Wei, J.; Cox, S.; James, T.; Justice, A.; Jesuthasan, G.; O’Donnell, D.; Howarth, A.; Hatch, S.B.; et al. Quantitative SARS-CoV-2 anti-spike responses to Pfizer-BioNTech and Oxford-AstraZeneca vaccines by previous infection status. Clin. Microbiol. Infect. 2021, 27, 1516.e7–1516.e14. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Padmapriyadarsini, C.; Vekemans, J.; Bavdekar, A.; Gupta, M.; Kulkarni, P.; Garg, B.S.; Gogtay, N.J.; Tambe, M.; Lalwani, S.; et al. A phase 2/3, participant-blind, observer-blind, randomised, controlled study to assess the safety and immunogenicity of SII-ChAdOx1 nCoV-19 (COVID-19 vaccine) in adults in India. EClinicalMedicine 2021, 42, 101218. [Google Scholar] [CrossRef]
- Deswal, V.; Phogat, R.; Sharma, P.; Kataria, S.; Soin, A. Is a single dose of ChAdOx1 nCoV-19 vaccine (AZD1222) enough for people with prior SARS-CoV-2 infection or baseline seropositive status? Int. J. Infect. Dis. 2022, 123, 143–144. [Google Scholar] [CrossRef]
- Gelanew, T.; Mulu, A.; Abebe, M.; Bates, T.A.; Wassie, L.; Teferi, M.; Fentahun, D.; Alemu, A.; Tamiru, F.; Assefa, G.; et al. A Single Dose of ChAdOx1 nCoV-19 Vaccine Elicits High Antibody Responses in Individuals with Prior SARS-CoV-2 Infection Comparable to That of Two-Dose-Vaccinated, SARS-CoV-2-Infection-Naive Individuals: A Longitudinal Study in Ethiopian Health Workers. Vaccines 2022, 10, 859. [Google Scholar] [CrossRef]
- Wanchaijiraboon, P.; Teeyapun, N.; Pakvisal, N.; Sainamthip, P.; Susiriwatananont, T.; Zungsontiporn, N.; Suntronwong, N.; Vichaiwattana, P.; Klinsawat, W.; Wanlapakorn, N.; et al. Durability of Immune Response to ChAdOx1-nCoV-19 Vaccine in Solid Cancer Patients Undergoing Anticancer Treatment. Vaccines 2022, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.P.; Hassler, H.B.; Sah, P.; Galvani, A.P.; Dornburg, A. The durability of natural infection and vaccine-induced immunity against future infection by SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2022, 119, e2204336119. [Google Scholar] [CrossRef] [PubMed]
- Katikireddi, S.V.; Cerqueira-Silva, T.; Vasileiou, E.; Robertson, C.; Amele, S.; Pan, J.; Taylor, B.; Boaventura, V.; Werneck, G.L.; Flores-Ortiz, R.; et al. Two-dose ChAdOx1 nCoV-19 vaccine protection against COVID-19 hospital admissions and deaths over time: A retrospective, population-based cohort study in Scotland and Brazil. Lancet 2022, 399, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Bobdey, S.; Kaushik, S.K.; Sahu, R.; Naithani, N.; Vaidya, R.; Sharma, M.; Vashishtha, K.; Yadav, A.K.; Sen, S.; Karade, S. Effectiveness of ChAdOx1 nCOV-19 Vaccine: Experience of a tertiary care institute. Med. J. Armed Forces India 2021, 77 (Suppl. 2), S271–S277. [Google Scholar] [CrossRef] [PubMed]
- Kaur, U.; Bala, S.; Ojha, B.; Jaiswal, S.; Kansal, S.; Chakrabarti, S.S. Occurrence of COVID-19 in priority groups receiving ChAdOx1 nCoV-19 coronavirus vaccine (recombinant): A preliminary analysis from north India. J. Med. Virol. 2022, 94, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Abhilash, K.P.; Mathiyalagan, P.; Krishnaraj, V.R.; Selvan, S.; Kanagarajan, R.; Reddy, N.P.; Rajendiran, N.; Hazra, D.; Gunasekaran, K.; Moorthy, M.; et al. Impact of prior vaccination with Covishield(TM) and Covaxin(R) on mortality among symptomatic COVID-19 patients during the second wave of the pandemic in South India during April and May 2021: A cohort study. Vaccine 2022, 40, 2107–2113. [Google Scholar] [CrossRef]
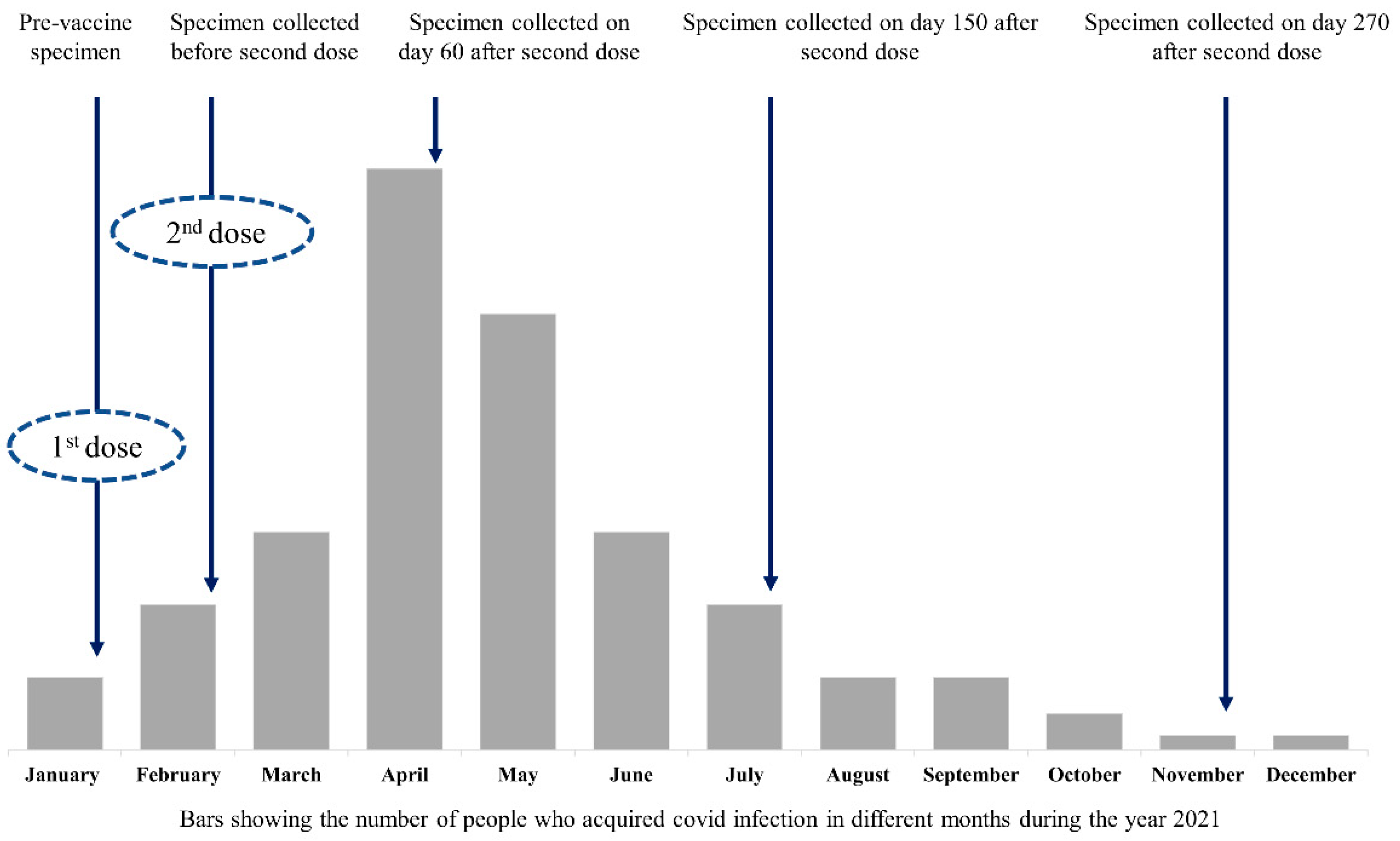


| Variable | Value |
|---|---|
| Males | 83 (61.5) |
| Age (years) | 45 (37–53) |
| Associated conditions | |
| Hypertension | 15 (11) |
| Diabetes mellitus (DM) | 7 (5) |
| Hypothyroidism | 5 (4) |
| Bronchial asthma | 3 (2) |
| Coronary artery disease | 2 (2) |
| Rheumatoid arthritis | 2 (2) |
| Osteoarthritis | 1 (1) |
| Paroxysmal supraventricular tachycardia | 1 (1) |
| Bronchial asthma + hypertension | 1 (1) |
| Total | 37 (27) |
| Body mass index (Kg/M2) | 25.2 (22.9–27.6) |
| Prior COVID-19 infection | 17 (13) |
| COVID-19 Antibody | Time of Specimen Collection (Days) | |||
|---|---|---|---|---|
| After the First Dose | 60 Days after Second Dose | 150 Days after Second Dose | 270 Days after Second Dose | |
| Anti-Spike antibody | 77.2 (19.4–329.4) | 512 (114.5–9212) | 149 (51.6–2283) | 2079 (433.9–8644) |
| Neutralising antibody (%) | 22.1 (8.5–54.2) | 52.3 (7.7–92.7) | 41.1 (3.8–86.4) | 58.6 (17.6–85.1) |
| Anti-COVID Antibody | COVID-19 Antibody | Time of Specimen Collection (Days) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before Second Dose at 28 Days | 60 Days after Second Dose | 150 Days after Second Dose | 270 Days after Second Dose | ||||||
| Anti-Spike antibody (U/mL) | Anti-spike antibody was absent before vaccination (n = 99) | 40.9 (11.8–118.3) | p < 0.001 | 234.3 (96.3–9268) | p = 0.007 | 102.6 (41.4–1837) | p < 0.001 | 1858 (215.8–6784) | p = 0.333 |
| Anti-spike antibody was present before vaccination (n = 36) | 5929.5 (649.4–16,207.5) | 3395 (800.1–8852) | 1805 (201.9–4131) | 2423.5 (707.1–9552) | |||||
| Neutralising antibody (%) | Anti-spike antibody was absent before vaccination (n = 99) | 18.3 (5.9–32) | p < 0.001 | 36.6 (7.4–91.9) | p = 0.255 | 26.7 (3.6–85.5) | p = 0.155 | 63.4 (17.6–85.4) | p = 0.626 |
| Anti-spike antibody was present before vaccination (n = 36) | 80 (24.1–95.8) | 69.9 (12.6–94.2) | 62.1 (13.2–89) | 53.6 (17–84.1) | |||||
| COVID-19 Antibody | Time of Specimen Collection (Days) | |||||||
|---|---|---|---|---|---|---|---|---|
| Before the Second Dose | 60 Days after Second Dose | 150 Days after Second Dose | 270 Days after Second Dose | |||||
| Acquired COVID-19 Infection | Not Acquired COVID-19 Infection | Acquired COVID-19 Infection | Not Acquired COVID-19 Infection | Acquired COVID-19 Infection | Not Acquired COVID-19 Infection | Acquired COVID-19 Infection | Not Acquired COVID-19 Infection | |
| Anti-Spike antibody (U/mL) | 76.8 (23.4–200.4) | 78.3 (19.3–684.9) | 14,019 (4546–25,000) | 316.7 (102.3–3447) | 2062 (139–4317) | 120.7 (50.9–1584) | 2185 (1414–4611) | 2052 (215.8–10,177) |
| p value | 0.362 | <0.001 | 0.002 | 0.306 | ||||
| Neutralising antibody (%) | 18 (7.6–27.5) | 24.5 (8.7–66.6) | 86.9 (66.6–97) | 27.3 (5.7–83.1) | 79.3 (45–90.8) | 24.6 (3.3–85) | 67.7 (54–84.7) | 50.3 (12.6–85.2) |
| p value | 0.071 | <0.001 | 0.007 | 0.206 | ||||
| COVID-19 Antibody | Time of Specimen Collection (Days) | |||
|---|---|---|---|---|
| After the First Dose | 60 Days after Second Dose | 150 Days after Second Dose | 270 Days after Second Dose | |
| Anti-Spike antibody (%) | 94 (95) | 99 (100) | 99 (100) | 99 (100) |
| Neutralising antibody (%) | 46 (46.5) | 61 (61.6) | 54 (54.5) | 73 (73.4) |
| COVID-19 Antibody | Time of Specimen Collection (Days) | |||||||
|---|---|---|---|---|---|---|---|---|
| Before the Second Dose | 60 Days after Second Dose | 150 Days after Second Dose | 270 Days after Second Dose | |||||
| Acquired COVID-19 Infection | Not Acquired COVID-19 Infection | Acquired COVID-19 Infection | Not Acquired COVID-19 Infection | Acquired COVID-19 Infection | Not Acquired COVID-19 Infection | Acquired COVID-19 Infection | Not Acquired COVID-19 Infection | |
| Anti-Spike antibody (U/mL) | 76.8 (23.4–200.4) | 36.2 (10.2–98.6) | 14,019 (4546–25,000) | 147.5 (85.4–387.7) | 2062 (139–4317) | 64.3 (37.4–154.3) | 2185 (1414–4611) | 1450 (128–12,885) |
| p value | 0.052 | <0.001 | <0.001 | 0.147 | ||||
| Neutralising antibody (%) | 18 (7.6–27.5) | 18.4 (5.8–32.9) | 86.9 (66.6–97) | 21 (5.3–65.5) | 79.3 (45–90.8) | 16.1 (2.8–64.8) | 67.7 (54–84.7) | 48.7 (12.6–85.8) |
| p value | 0.936 | <0.001 | 0.001 | 0.277 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, A.; Goel, A.; Katiyar, H.; Tiwari, P.; Mayank; Sana, A.; Khetan, D.; Bhadauria, D.S.; Raja, A.; Khokher, N.; et al. Durability of ChAdOx1 nCoV-19 (Covishield®) Vaccine Induced Antibody Response in Health Care Workers. Vaccines 2023, 11, 84. https://doi.org/10.3390/vaccines11010084
Verma A, Goel A, Katiyar H, Tiwari P, Mayank, Sana A, Khetan D, Bhadauria DS, Raja A, Khokher N, et al. Durability of ChAdOx1 nCoV-19 (Covishield®) Vaccine Induced Antibody Response in Health Care Workers. Vaccines. 2023; 11(1):84. https://doi.org/10.3390/vaccines11010084
Chicago/Turabian StyleVerma, Alka, Amit Goel, Harshita Katiyar, Prachi Tiwari, Mayank, Asari Sana, Dheeraj Khetan, Dharmendra Singh Bhadauria, Ajay Raja, Neelam Khokher, and et al. 2023. "Durability of ChAdOx1 nCoV-19 (Covishield®) Vaccine Induced Antibody Response in Health Care Workers" Vaccines 11, no. 1: 84. https://doi.org/10.3390/vaccines11010084
APA StyleVerma, A., Goel, A., Katiyar, H., Tiwari, P., Mayank, Sana, A., Khetan, D., Bhadauria, D. S., Raja, A., Khokher, N., Shalimar, Singh, R. K., & Aggarwal, A. (2023). Durability of ChAdOx1 nCoV-19 (Covishield®) Vaccine Induced Antibody Response in Health Care Workers. Vaccines, 11(1), 84. https://doi.org/10.3390/vaccines11010084







