The BCG Moreau Vaccine Upregulates In Vitro the Expression of TLR4, B7-1, Dectin-1 and EP2 on Human Monocytes
Abstract
1. Background
2. Material and Methods
2.1. Study Participants
2.2. Mononuclear Cells Purification, Culture, In Vitro Infection with BCG and Phenotyping
2.3. Statistical Evaluation
3. Results
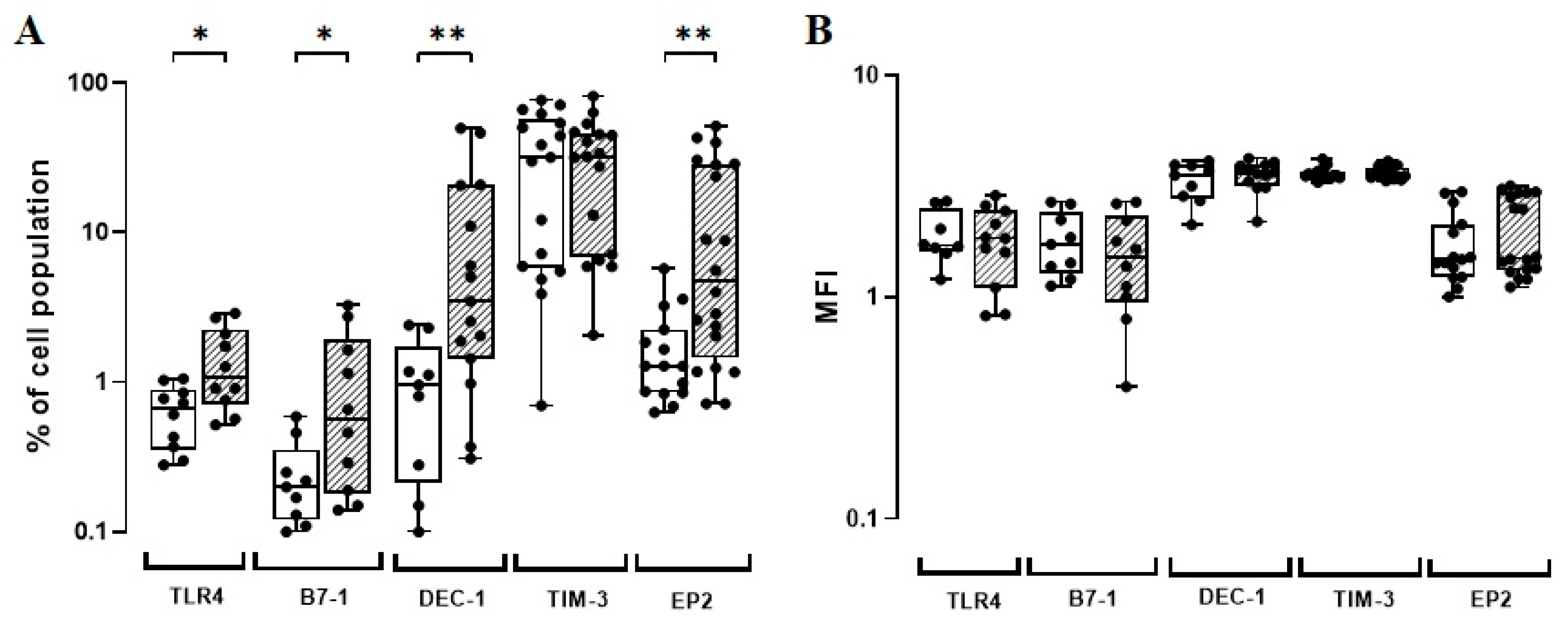
3.1. The BCG Moreau Vaccine Modulates In Vitro the Expression of TLR4, B7-1, DEC-1, and EP2 on Human Monocytes from Healthy Adult Donors
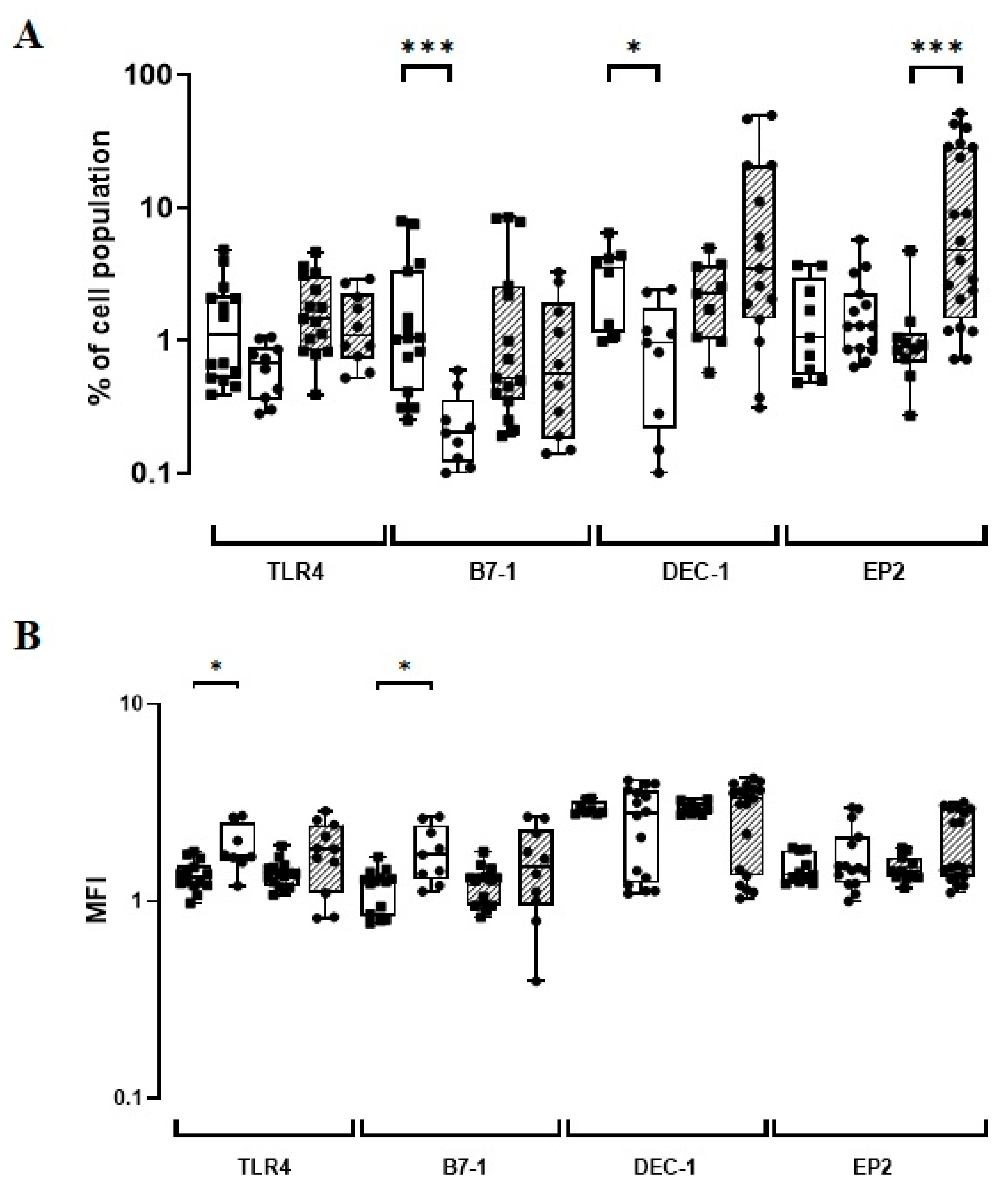
3.2. The BCG Moreau Vaccine Induces a Differential Expression of TLR4, B7-1, and DEC-1 on Human Monocytes from Neonates vs. Adults
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- WHO. Global Tuberculosis Report 2021; World Health Organization: Geneva, Switzerland, 2021.
- Calmette, A. Preventive Vaccination Against Tuberculosis with BCG. Proc. R. Soc. Med. 1931, 24, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Vanderslott, S.; Dadonaite, B.; Roser, M. Vaccination. Our World Data. 2013. Available online: https://ourworldindata.org/vaccination (accessed on 10 December 2022).
- Luca, S.; Mihaescu, T. History of BCG Vaccine. Maedica 2013, 8, 53–58. [Google Scholar] [PubMed]
- Aaby, P.; Kollmann, T.R.; Benn, C.S. Nonspecific effects of neonatal and infant vaccination: Public-health, immunological and conceptual challenges. Nat. Immunol. 2014, 15, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Saadatian-Elahi, M.; Aaby, P.; Shann, F.; Netea, M.G.; Levy, O.; Louis, J.; Picot, V.; Greenberg, M.; Warren, W. Heterologous vaccine effects. Vaccine 2016, 34, 3923–3930. [Google Scholar] [CrossRef] [PubMed]
- Brook, B.; Harbeson, D.J. BCG vaccination-induced emergency granulopoiesis provides rapid protection from neonatal sepsis. Sci. Transl. Med. 2020, 12, eaax4517. [Google Scholar] [CrossRef]
- Brynjolfsson, S.F.; Bjarnarson, S.P.; Mori, E.; Del Giudice, G.; Jonsdottir, I. Concomitant administration of Mycobacterium bovis BCG with the meningococcal C conjugate vaccine to neonatal mice enhances antibody response and protective efficacy. Clin. Vaccine Immunol. CVI 2011, 18, 1936–1942. [Google Scholar] [CrossRef][Green Version]
- Leentjens, J.; Kox, M.; Stokman, R.; Gerretsen, J.; Diavatopoulos, D.A.; van Crevel, R.; Rimmelzwaan, G.F.; Pickkers, P.; Netea, M.G. BCG Vaccination Enhances the Immunogenicity of Subsequent Influenza Vaccination in Healthy Volunteers: A Randomized, Placebo-Controlled Pilot Study. J. Infect. Dis. 2015, 212, 1930–1938. [Google Scholar] [CrossRef]
- Morra, M.E.; Kien, N.D.; Elmaraezy, A.; Abdelaziz, O.A.M.; Elsayed, A.L.; Halhouli, O. Early vaccination protects against childhood leukemia: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 15986. [Google Scholar] [CrossRef]
- El-Zein, M.; Parent, M.E.; Benedetti, A.; Rousseau, M.C. Does BCG vaccination protect against the development of childhood asthma? A systematic review and meta-analysis of epidemiological studies. Int. J. Epidemiol. 2010, 39, 469–486. [Google Scholar] [CrossRef]
- Thøstesen, L.M.; Kjaergaard, J.; Pihl, G.T.; Birk, N.M.; Nissen, T.N.; Aaby, P.; Jensen, A.K.G.; Olesen, A.W.; Stensballe, L.G. Neonatal BCG vaccination and atopic dermatitis before 13 months of age: A randomized clinical trial. Allergy 2018, 73, 498–504. [Google Scholar] [CrossRef]
- Rousseau, M.C.; El-Zein, M.; Conus, F.; Legault, L.; Parent, M.E. Bacillus Calmette-Guérin (BCG) Vaccination in Infancy and Risk of Childhood Diabetes. Paediatr. Perinat. Epidemiol. 2016, 30, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Redelman-Sidi, G.; Glickman, M.S.; Bochner, B.H. The mechanism of action of BCG therapy for bladder cancer--a current perspective. Nat. Rev. Urol. 2014, 11, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Escobar, L.E.; Molina-Cruz, A. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc. Natl. Acad. Sci. USA 2020, 117, 17720–17726. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.A. Correlation between BCG genomics and protective efficacy. Scand. J. Infect. Dis. 2001, 33, 249–252. [Google Scholar] [CrossRef]
- Oettinger, T.; Jørgensen, M.; Ladefoged, A.; Hasløv, K.; Andersen, P. Development of the Mycobacterium bovis BCG vaccine: Review of the historical and biochemical evidence for a genealogical tree. Tuber. Lung Dis. 1999, 79, 243–250. [Google Scholar] [CrossRef]
- Benévolo-de-Andrade, T.C.; Monteiro-Maia, R.; Cosgrove, C.; Castello-Branco, L.R. BCG Moreau Rio de Janeiro: An oral vaccine against tuberculosis-review. Mem. Inst. Oswaldo Cruz 2005, 100, 459–465. [Google Scholar] [CrossRef]
- Grange, J.M.; Gibson, J.; Osborn, T.W.; Collins, C.H.; Yates, M.D. What is BCG? Tubercle 1983, 64, 129–139. [Google Scholar] [CrossRef]
- Jo, E.K.; Yang, C.S.; Choi, C.H.; Harding, C.V. Intracellular signalling cascades regulating innate immune responses to Mycobacteria: Branching out from Toll-like receptors. Cell. Microbiol. 2007, 9, 1087–1098. [Google Scholar] [CrossRef]
- Jung, S.B.; Yang, C.S.; Lee, J.S.; Shin, A.R.; Jung, S.S.; Son, J.W.; Harding, C.V.; Kim, H.J.; Park, J.K.; Paik, T.H.; et al. The mycobacterial 38-kilodalton glycolipoprotein antigen activates the mitogen-activated protein kinase pathway and release of proinflammatory cytokines through Toll-like receptors 2 and 4 in human monocytes. Infect. Immun. 2006, 74, 2686–2696. [Google Scholar] [CrossRef]
- Yu, L.; Wang, L.; Chen, S. Endogenous toll-like receptor ligands and their biological significance. J. Cell. Mol. Med. 2010, 14, 2592–2603. [Google Scholar] [CrossRef]
- Cambier, C.J.; O’Leary, S.M.; O’Sullivan, M.P.; Keane, J.; Ramakrishnan, L. Phenolic Glycolipid Facilitates Mycobacterial Escape from Microbicidal Tissue-Resident Macrophages. Immunity 2017, 47, 552–565.e4. [Google Scholar] [CrossRef] [PubMed]
- Drummond, R.A.; Brown, G.D. Signalling C-type lectins in antimicrobial immunity. PLoS Pathog 2013, 9, e1003417. [Google Scholar] [CrossRef] [PubMed]
- Hilda, J.N.; Selvaraj, A.; Das, S.D. Mycobacterium tuberculosis H37Rv is more effective compared to vaccine strains in modulating neutrophil functions: An in vitro study. FEMS Immunol. Med. Microbiol. 2012, 66, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Oosting, M.; Joosten, L.A.; Netea, M.G.; Van Crevel, R. Innate immune recognition of Mycobacterium tuberculosis. Clin. Dev. Immunol. 2011, 2011, 405310. [Google Scholar] [CrossRef]
- Lin, Y.; Feng, T.; Lan, J.; Chen, C.; Qin, Z.; Wu, Y.; Shi, H.; Ye, J.; Wei, C.; Wang, W.; et al. Expression of Toll-like Receptor 2 and Toll-like Receptor 4 in Tuberculous Pleural Effusion. Med. Chem. 2017, 13, 569–576. [Google Scholar] [CrossRef]
- Marakalala, M.J.; Graham, L.M.; Brown, G.D. The role of Syk/CARD9-coupled C-type lectin receptors in immunity to Mycobacterium tuberculosis infections. Clin. Dev. Immunol. 2010, 2010, 567571. [Google Scholar] [CrossRef]
- Rothfuchs, A.G.; Bafica, A.; Feng, C.G.; Egen, J.G.; Williams, D.L.; Brown, G.D.; Sher, A. Dectin-1 interaction with Mycobacterium tuberculosis leads to enhanced IL-12p40 production by splenic dendritic cells. J. Immunol. 2007, 179, 3463–3471. [Google Scholar] [CrossRef]
- Yadav, M.; Schorey, J.S. The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood 2006, 108, 3168–3175. [Google Scholar] [CrossRef]
- Saraav, I.; Singh, S.; Pandey, K.; Sharma, M.; Sharma, S. Mycobacterium tuberculosis MymA is a TLR2 agonist that activate macrophages and a T(H)1 response. Tuberculosis 2017, 106, 16–24. [Google Scholar] [CrossRef]
- Lemoine, S.; Jaron, B.; Tabka, S.; Ettreiki, C.; Deriaud, E.; Zhivaki, D.; Le Ray, C.; Launay, O.; Majlessi, L.; Tissieres, P.; et al. Dectin-1 activation unlocks IL12A expression and reveals the TH1 potency of neonatal dendritic cells. J. Allergy Clin. Immunol. 2015, 136, 1355–1368.e15. [Google Scholar] [CrossRef]
- Bakhru, P.; Sirisaengtaksin, N.; Soudani, E.; Mukherjee, S.; Khan, A.; Jagannath, C. BCG vaccine mediated reduction in the MHC-II expression of macrophages and dendritic cells is reversed by activation of Toll-like receptors 7 and 9. Cell. Immunol. 2014, 287, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Carreno, B.M.; Collins, M. The B7 family of ligands and its receptors: New pathways for costimulation and inhibition of immune responses. Annu. Rev. Immunol. 2002, 20, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.; Ling, V.; Carreno, B.M. The B7 family of immune-regulatory ligands. Genome Biol. 2005, 6, 223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khoury, S.J.; Sayegh, M.H. The roles of the new negative T cell costimulatory pathways in regulating autoimmunity. Immunity 2004, 20, 529–538. [Google Scholar] [CrossRef]
- Zha, Y.; Blank, C.; Gajewski, T.F. Negative regulation of T-cell function by PD-1. Crit. Rev. Immunol. 2004, 24, 229–237. [Google Scholar] [CrossRef]
- Boom, W.H. The role of T-cell subsets in Mycobacterium tuberculosis infection. Infect. Agents Dis. 1996, 5, 73–81. [Google Scholar]
- Flynn, J.L.; Chan, J.; Triebold, K.J.; Dalton, D.K.; Stewart, T.A.; Bloom, B.R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 1993, 178, 2249–2254. [Google Scholar] [CrossRef]
- Cheadle, E.J.; Selby, P.J.; Jackson, A.M. Mycobacterium bovis bacillus Calmette-Guérin-infected dendritic cells potently activate autologous T cells via a B7 and interleukin-12-dependent mechanism. Immunology 2003, 108, 79–88. [Google Scholar] [CrossRef]
- Chen, M.; Divangahi, M.; Gan, H.; Shin, D.S.; Hong, S.; Lee, D.M.; Serhan, C.N.; Behar, S.M.; Remold, H.G. Lipid mediators in innate immunity against tuberculosis: Opposing roles of PGE2 and LXA4 in the induction of macrophage death. J. Exp. Med. 2008, 205, 2791–2801. [Google Scholar] [CrossRef]
- Harizi, H.; Grosset, C.; Gualde, N. Prostaglandin E2 modulates dendritic cell function via EP2 and EP4 receptor subtypes. J. Leukoc. Biol. 2003, 73, 756–763. [Google Scholar] [CrossRef]
- Rangel Moreno, J.; Estrada García, I.; De La Luz García Hernández, M.; Aguilar Leon, D.; Marquez, R.; Hernández Pando, R. The role of prostaglandin E2 in the immunopathogenesis of experimental pulmonary tuberculosis. Immunology 2002, 106, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.; Siqueira, M.; Pedro, T.; Ponte, C.; Peres, L.; Marinho, S.; Castello-Branco, L.R.; Antas, P.R.Z. The role of host soluble inflammatory mediators induced by the BCG vaccine for the initiation of in vitro monocyte apoptosis in healthy Brazilian volunteers. J. Inflamm. 2015, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Poloso, N.J.; Urquhart, P.; Nicolaou, A.; Wang, J.; Woodward, D.F. PGE2 differentially regulates monocyte-derived dendritic cell cytokine responses depending on receptor usage (EP2/EP4). Mol. Immunol. 2013, 54, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Chizzolini, C.; Chicheportiche, R.; Alvarez, M.; de Rham, C.; Roux-Lombard, P.; Ferrari-Lacraz, S.; Dayer, J.M. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood 2008, 112, 3696–3703. [Google Scholar] [CrossRef]
- Boniface, K.; Bak-Jensen, K.S.; Li, Y.; Blumenschein, W.M.; McGeachy, M.J.; McClanahan, T.K.; McKenzie, B.S.; Kastelein, R.A.; Cua, D.J.; de Waal Malefyt, R. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J. Exp. Med. 2009, 206, 535–548. [Google Scholar] [CrossRef]
- Narumiya, S.; Sugimoto, Y.; Ushikubi, F. Prostanoid receptors: Structures, properties, and functions. Physiol. Rev. 1999, 79, 1193–1226. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Qi, J.; Wu, F.; Guan, J.; Chen, Z.; Zhu, H. Altered PGE2-EP2 is associated with an excessive immune response in HBV-related acute-on-chronic liver failure. J. Transl. Med. 2019, 17, 93. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, Y.; Li, G.; Huang, H.; Zhang, G.; Wang, F.; Sun, J.; Yang, Q.; Zhang, X.; Lu, B. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS ONE 2012, 7, e30676. [Google Scholar] [CrossRef]
- Hastings, W.D.; Anderson, D.E.; Kassam, N.; Koguchi, K.; Greenfield, E.A.; Kent, S.C.; Zheng, X.X.; Strom, T.B.; Hafler, D.A.; Kuchroo, V.K. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur. J. Immunol. 2009, 39, 2492–2501. [Google Scholar] [CrossRef]
- Monney, L.; Sabatos, C.A.; Gaglia, J.L.; Ryu, A.; Waldner, H.; Chernova, T.; Manning, S.; Greenfield, E.A.; Coyle, A.J.; Sobel, R.A.; et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002, 415, 536–541. [Google Scholar] [CrossRef]
- Jayaraman, P.; Jacques, M.K.; Zhu, C.; Steblenko, K.M.; Stowell, B.L.; Madi, A.; Anderson, A.C.; Kuchroo, V.K.; Behar, S.M. TIM3 Mediates T Cell Exhaustion during Mycobacterium tuberculosis Infection. PLoS Pathog 2016, 12, e1005490. [Google Scholar] [CrossRef]
- Nakayama, M.; Akiba, H.; Takeda, K.; Kojima, Y.; Hashiguchi, M.; Azuma, M.; Yagita, H.; Okumura, K. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood 2009, 113, 3821–3830. [Google Scholar] [CrossRef] [PubMed]
- Antas, P.R.; Pedro, T.Q.; Santiago, E.A.; Lima, J.R.; Silva, F.C.; Melca, L.A.; Ponte, C.G. Human neonates display altered ex vivo monokine production related to healthy adults. Immunol. Lett. 2016, 170, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Hale, J.; Jaffray, J.; Li, J.; Wang, Y.; Huang, Y.; An, X.; Hillyer, C.; Wang, N.; Kinet, S.; et al. Developmental differences between neonatal and adult human erythropoiesis. Am. J. Hematol. 2018, 93, 494–503. [Google Scholar] [CrossRef]
- Simas, C.J.; Silva, D.P.; Ponte, C.G.; Castello-Branco, L.R.; Antas, P.R. Patterns of in vitro cell-death, metaloproteinase-9 and pro-inflammatory cytokines in human monocytes induced by the BCG vaccine, Moreau strain. Vaccine 2011, 29, 6446–6450. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mawa, P.A.; Webb, E.L.; Filali-Mouhim, A.; Nkurunungi, G.; Sekaly, R.P.; Lule, S.A.; Prentice, S.; Nash, S.; Dockrell, H.M.; Elliott, A.M.; et al. Maternal BCG scar is associated with increased infant proinflammatory immune responses. Vaccine 2017, 35, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Ponte, C.; Hacker, M.; Moraes, M.; Castello-Branco, L.; Silva, F.; Antas, P. The patterns of in vitro cell-death and inflammatory cytokines induced by distinct BCG vaccine strains are differentially induced in human mononuclear cells. Hum. Vaccines Immunother. 2018, 14, 28–35. [Google Scholar] [CrossRef]
- Ambarus, C.A.; Krausz, S.; van Eijk, M.; Hamann, J.; Radstake, T.R.; Reedquist, K.A.; Tak, P.P.; Baeten, D.L. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J. Immunol. Methods 2012, 375, 196–206. [Google Scholar] [CrossRef]
- Tsafaras, G.P.; Ntontsi, P.; Xanthou, G. Advantages and Limitations of the Neonatal Immune System. Front. Pediatrics 2020, 8, 5. [Google Scholar] [CrossRef]
- Yan, S.R.; Qing, G.; Byers, D.M.; Stadnyk, A.W.; Al-Hertani, W.; Bortolussi, R. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect. Immun. 2004, 72, 1223–1229. [Google Scholar] [CrossRef]
- Levy, O.; Zarember, K.A.; Roy, R.M.; Cywes, C.; Godowski, P.J.; Wessels, M.R. Selective impairment of TLR-mediated innate immunity in human newborns: Neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J. Immunol. 2004, 173, 4627–4634. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Coughlin, M.; Cronstein, B.N.; Roy, R.M.; Desai, A.; Wessels, M.R. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J. Immunol. 2006, 177, 1956–1966. [Google Scholar] [CrossRef] [PubMed]
- Sugitharini, V.; Pavani, K.; Prema, A.; Berla Thangam, E. TLR-mediated inflammatory response to neonatal pathogens and co-infection in neonatal immune cells. Cytokine 2014, 69, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Yerkovich, S.T.; Wikström, M.E.; Suriyaarachchi, D.; Prescott, S.L.; Upham, J.W.; Holt, P.G. Postnatal development of monocyte cytokine responses to bacterial lipopolysaccharide. Pediatric Res. 2007, 62, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Redondo, A.C.; Ceccon, M.E.; Silveira-Lessa, A.L.; Quinello, C.; Palmeira, P.; Carvalho, W.B.; Carneiro-Sampaio, M. TLR-2 and TLR-4 expression in monocytes of newborns with late-onset sepsis. J. Pediatr. 2014, 90, 472–478. [Google Scholar] [CrossRef][Green Version]
- Förster-Waldl, E.; Sadeghi, K.; Tamandl, D.; Gerhold, B.; Hallwirth, U.; Rohrmeister, K.; Hayde, M.; Prusa, A.R.; Herkner, K.; Boltz-Nitulescu, G.; et al. Monocyte toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatric Res. 2005, 58, 121–124. [Google Scholar] [CrossRef]
- Sun, R.; Yang, X.; Sun, E.L.; Han, R.F. [TLR4 expression of human PBMC treated by BCG and its role of immune activation]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi = Chin. J. Cell. Mol. Immunol. 2011, 27, 945–948. [Google Scholar]
- Yang, Y.C.; Zhang, N.; Van Crombruggen, K.; Hu, G.H.; Hong, S.L.; Bachert, C. Transforming growth factor-beta1 in inflammatory airway disease: A key for understanding inflammation and remodeling. Allergy 2012, 67, 1193–1202. [Google Scholar] [CrossRef]
- Lichte, P.; Grigoleit, J.S.; Steiner, E.M.; Kullmann, J.S.; Schedlowski, M.; Oberbeck, R.; Kobbe, P. Low dose LPS does not increase TLR4 expression on monocytes in a human in vivo model. Cytokine 2013, 63, 74–80. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef]
- van der Meer, J.W.; Joosten, L.A.; Riksen, N.; Netea, M.G. Trained immunity: A smart way to enhance innate immune defence. Mol. Immunol. 2015, 68, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Watanabe, E.; Shimizu, M.; Negishi, Y.; Kondo, Y.; Takahashi, H. Induction of tumor-specific CD8(+) cytotoxic T lymphocytes from naïve human T cells by using Mycobacterium-derived mycolic acid and lipoarabinomannan-stimulated dendritic cells. Cancer Immunol. Immunother. 2019, 68, 1605–1619. [Google Scholar] [CrossRef] [PubMed]
- Saikolappan, S.; Estrella, J.; Sasindran, S.J.; Khan, A.; Armitige, L.Y.; Jagannath, C.; Dhandayuthapani, S. The fbpA/sapM double knock out strain of Mycobacterium tuberculosis is highly attenuated and immunogenic in macrophages. PLoS ONE 2012, 7, e36198. [Google Scholar] [CrossRef] [PubMed]
- Hunsawong, T.; Sunintaboon, P.; Warit, S.; Thaisomboonsuk, B.; Jarman, R.G.; Yoon, I.K.; Ubol, S.; Fernandez, S. Immunogenic Properties of a BCG Adjuvanted Chitosan Nanoparticle-Based Dengue Vaccine in Human Dendritic Cells. PLoS Negl. Trop. Dis. 2015, 9, e0003958. [Google Scholar] [CrossRef] [PubMed]
- Behar, S.M.; Divangahi, M.; Remold, H.G. Evasion of innate immunity by Mycobacterium tuberculosis: Is death an exit strategy? Nat. Rev. Microbiol. 2010, 8, 668–674. [Google Scholar] [CrossRef]
- Wegmann, T.G.; Lin, H.; Guilbert, L.; Mosmann, T.R. Bidirectional cytokine interactions in the maternal-fetal relationship: Is successful pregnancy a TH2 phenomenon? Immunol. Today 1993, 14, 353–356. [Google Scholar] [CrossRef]
- Willems, F.; Vollstedt, S.; Suter, M. Phenotype and function of neonatal DC. Eur. J. Immunol. 2009, 39, 26–35. [Google Scholar] [CrossRef]
- Barrios, C.; Brawand, P.; Berney, M.; Brandt, C.; Lambert, P.H.; Siegrist, C.A. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: Predominance of a Th2-biased pattern which persists after adult boosting. Eur. J. Immunol. 1996, 26, 1489–1496. [Google Scholar] [CrossRef]
- Morris, M.C.; Surendran, N. Neonatal Vaccination: Challenges and Intervention Strategies. Neonatology 2016, 109, 161–169. [Google Scholar] [CrossRef]
- Ballinger, M.N.; Peters-Golden, M.; Moore, B.B. Impaired neonatal macrophage phagocytosis is not explained by overproduction of prostaglandin E2. Respir. Res. 2011, 12, 155. [Google Scholar] [CrossRef]
- Hanekom, W.A. The immune response to BCG vaccination of newborns. Ann. N. Y. Acad. Sci. 2005, 1062, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Antal-Szalmas, P.; Strijp, J.A.; Weersink, A.J.; Verhoef, J.; Van Kessel, K.P. Quantitation of surface CD14 on human monocytes and neutrophils. J. Leukoc. Biol. 1997, 61, 721–728. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antas, P.R.Z.; da Silva, A.S.M.; Albuquerque, L.H.P.; Almeida, M.R.; Pereira, E.N.G.S.; Castello-Branco, L.R.R.; de Ponte, C.G.G. The BCG Moreau Vaccine Upregulates In Vitro the Expression of TLR4, B7-1, Dectin-1 and EP2 on Human Monocytes. Vaccines 2023, 11, 86. https://doi.org/10.3390/vaccines11010086
Antas PRZ, da Silva ASM, Albuquerque LHP, Almeida MR, Pereira ENGS, Castello-Branco LRR, de Ponte CGG. The BCG Moreau Vaccine Upregulates In Vitro the Expression of TLR4, B7-1, Dectin-1 and EP2 on Human Monocytes. Vaccines. 2023; 11(1):86. https://doi.org/10.3390/vaccines11010086
Chicago/Turabian StyleAntas, Paulo R. Z., Andreon S. M. da Silva, Lawrence H. P. Albuquerque, Matheus R. Almeida, Evelyn N. G. S. Pereira, Luiz R. R. Castello-Branco, and Carlos G. G. de Ponte. 2023. "The BCG Moreau Vaccine Upregulates In Vitro the Expression of TLR4, B7-1, Dectin-1 and EP2 on Human Monocytes" Vaccines 11, no. 1: 86. https://doi.org/10.3390/vaccines11010086
APA StyleAntas, P. R. Z., da Silva, A. S. M., Albuquerque, L. H. P., Almeida, M. R., Pereira, E. N. G. S., Castello-Branco, L. R. R., & de Ponte, C. G. G. (2023). The BCG Moreau Vaccine Upregulates In Vitro the Expression of TLR4, B7-1, Dectin-1 and EP2 on Human Monocytes. Vaccines, 11(1), 86. https://doi.org/10.3390/vaccines11010086






