Safety, Efficacy, and Immunogenicity of Varying Types of COVID-19 Vaccines in Children Younger Than 18 Years: An Update of Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
2.4. Data Analysis
| Develop Outcome | Do Not Develop Outcome | |
|---|---|---|
| Exposed | a | b |
| Not Exposed | c | d |
3. Results
3.1. Characteristics of Included Studies
3.2. Safety of the COVID-19 Vaccines
3.2.1. Adverse Reactions to Different Introduction Doses
3.2.2. Adverse Reactions to Different Age Groups
3.2.3. Adverse Reactions to Different Dose Groups
3.3. Immunogenicity of the COVID-19 Vaccines
3.3.1. Humoral Immune Responses in Different Doses
3.3.2. Humoral Immune Responses in Different Ages
3.3.3. Cellular Immune Responses
3.4. Efficacy of the COVID-19 Vaccines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, P.-I.; Hu, Y.-L.; Chen, P.-Y.; Huang, Y.-C.; Hsueh, P.-R. Are children less susceptible to COVID-19? J. Microbiol. Immunol. Infect. 2020, 53, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tee, M.; Roy, A.E.; Fardin, M.A.; Srichokchatchawan, W.; Habib, H.A.; Tran, B.X.; Hussain, S.; Hoang, M.T.; Le, X.T.; et al. The impact of COVID-19 pandemic on physical and mental health of Asians: A study of seven middle-income countries in Asia. PLoS ONE 2021, 16, e0246824. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, Q.; Wang, C.; Shi, Q.; Lu, S.; Ma, Y.; Luo, X.; Xun, Y.; Li, W.; Baskota, M.; et al. Clinical characteristics of children with COVID-19: A rapid review and meta-analysis. Ann. Transl. Med. 2020, 8, 620. [Google Scholar] [CrossRef]
- de Souza, T.H.; Nadal, J.A.; Nogueira, R.J.N.; Pereira, R.M.; Brandão, M.B. Clinical manifestations of children with COVID-19: A systematic review. Pediatr. Pulmonol. 2020, 55, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch. Dis. Child. 2021, 106, 429–439. [Google Scholar] [CrossRef]
- Havers, F.P.; Whitaker, M.; Self, J.L.; Chai, S.J.; Kirley, P.D.; Alden, N.B.; Kawasaki, B.; Meek, J.; Yousey-Hindes, K.; Anderson, E.J.; et al. Hospitalization of Adolescents Aged 12–17 Years with Laboratory-Confirmed COVID-19—COVID-NET, 14 States, March 1, 2020–April 24, 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 851–857. [Google Scholar] [CrossRef]
- Jiang, L.; Tang, K.; Levin, M.; Irfan, O.; Morris, S.K.; Wilson, K.; Klein, J.D.; Bhutta, Z.A. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect. Dis. 2020, 20, e276–e288. [Google Scholar] [CrossRef]
- Penner, J.; Abdel-Mannan, O.; Grant, K.; Maillard, S.; Kucera, F.; Hassell, J.; Eyre, M.; Berger, Z.; Hacohen, Y.; Moshal, K.; et al. 6-month multidisciplinary follow-up and outcomes of patients with paediatric inflammatory multisystem syndrome (PIMS-TS) at a UK tertiary paediatric hospital: A retrospective cohort study. Lancet Child Adolesc. Health 2021, 5, 473–482. [Google Scholar] [CrossRef]
- Townsend, E. Debate: The impact of school closures and lockdown on mental health in young people. Child Adolesc. Ment. Health 2020, 25, 265–266. [Google Scholar] [CrossRef]
- O’Sullivan, K.; Clark, S.; McGrane, A.; Rock, N.; Burke, L.; Boyle, N.; Joksimovic, N.; Marshall, K. A Qualitative Study of Child and Adolescent Mental Health during the COVID-19 Pandemic in Ireland. Int. J. Environ. Res. Public Health 2021, 18, 1062. [Google Scholar] [CrossRef]
- Tang, S.; Xiang, M.; Cheung, T.; Xiang, Y.-T. Mental health and its correlates among children and adolescents during COVID-19 school closure: The importance of parent-child discussion. J. Affect. Disord. 2020, 279, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Mazidi, M.; Li, K.; Li, Y.; Chen, S.; Kirwan, R.; Zhou, H.; Yan, N.; Rahman, A.; Wang, W.; et al. Prevalence of mental health problems among children and adolescents during the COVID-19 pandemic: A systematic review and meta-analysis. J. Affect. Disord. 2021, 293, 78–89. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.M.D.; Butini, L.; Pauletto, P.; Lehmkuhl, K.M.; Stefani, C.M.; Bolan, M.; Guerra, E.; Dick, B.; Canto, G.D.L.; Massignan, C. Mental health effects prevalence in children and adolescents during the COVID-19 pandemic: A systematic review. Worldviews Evid.-Based Nurs. 2022, 19, 130–137. [Google Scholar] [CrossRef]
- McKune, S.L.; Acosta, D.; Diaz, N.; Brittain, K.; Beaulieu, D.J.; Maurelli, A.T.; Nelson, E.J. Psychosocial health of school-aged children during the initial COVID-19 safer-at-home school mandates in Florida: A cross-sectional study. BMC Public Health 2021, 21, 603. [Google Scholar] [CrossRef] [PubMed]
- Engzell, P.; Frey, A.; Verhagen, M.D. Learning loss due to school closures during the COVID-19 pandemic. Proc. Natl. Acad. Sci. USA 2021, 118, e2022376118. [Google Scholar] [CrossRef]
- Larsen, L.; Helland, M.S.; Holt, T. The impact of school closure and social isolation on children in vulnerable families during COVID-19: A focus on children’s reactions. Eur. Child Adolesc. Psychiatry 2021, 31, 1–11. [Google Scholar] [CrossRef]
- Rajmil, L.; Hjern, A.; Boran, P.; Gunnlaugsson, G.; de Camargo, O.K.; Raman, S. Impact of lockdown and school closure on children’s health and well-being during the first wave of COVID-19: A narrative review. BMJ Paediatr. Open 2021, 5, e001043. [Google Scholar] [CrossRef]
- Parks, S.E.; Zviedrite, N.; Budzyn, S.E.; Panaggio, M.J.; Raible, E.; Papazian, M.; Magid, J.; Ahmed, F.; Uzicanin, A.; Barrios, L.C. COVID-19–Related School Closures and Learning Modality Changes—United States, August 1–September 17, 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1374–1376. [Google Scholar] [CrossRef]
- Dembiński, Ł.; Huss, G.; Radziewicz-Winnicki, I.; Grossman, Z.; Mazur, A.; del Torso, S.; Barak, S.; Sanz, A.C.; Hadjipanayis, A. EAP and ECPCP Statement Risks for Children’s Health During the COVID-19 Pandemic and a Call for Maintenance of Essential Pediatric Services. Front. Pediatr. 2021, 9, 679803. [Google Scholar] [CrossRef]
- Chun, J.Y.; Jeong, H.; Kim, Y. Identifying susceptibility of children and adolescents to the Omicron variant (B.1.1.529). BMC Med. 2022, 20, 451. [Google Scholar] [CrossRef]
- Goldman, E. How the unvaccinated threaten the vaccinated for COVID-19: A Darwinian perspective. Proc. Natl. Acad. Sci. USA 2021, 118, e2114279118. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Yang, R.; Chen, Z. Safety and efficacy of COVID-19 vaccines in children and adolescents: A systematic review of randomized controlled trials. J. Med. Virol. 2022, 94, 4644–4653. [Google Scholar] [CrossRef] [PubMed]
- Cauchemez, S.; Bosetti, P.; Kiem, C.T.; Mouro, V.; Consoli, A.; Fontanet, A. Education and mental health: Good reasons to vaccinate children. Lancet 2021, 398, 387. [Google Scholar] [CrossRef] [PubMed]
- Klass, P.; Ratner, A.J. Vaccinating Children against Covid-19—The Lessons of Measles. New Engl. J. Med. 2021, 384, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Campbell, J.D.; Creech, C.B.; Frenck, R.; Kamidani, S.; Munoz, F.M.; Nachman, S.; Spearman, P. Warp Speed for Coronavirus Disease 2019 (COVID-19) Vaccines: Why Are Children Stuck in Neutral? Clin. Infect. Dis. 2020, 73, 336–340. [Google Scholar] [CrossRef]
- Opel, D.J.; Diekema, D.S.; Ross, L.F. Should We Mandate a COVID-19 Vaccine for Children? JAMA Pediatr. 2021, 175, 125–126. [Google Scholar] [CrossRef]
- Thunström, L.; Ashworth, M.; Finnoff, D.; Newbold, S.C. Hesitancy Toward a COVID-19 Vaccine. EcoHealth 2021, 18, 44–60. [Google Scholar] [CrossRef]
- Babicki, M.; Pokorna-Kałwak, D.; Doniec, Z.; Mastalerz-Migas, A. Attitudes of Parents with Regard to Vaccination of Children against COVID-19 in Poland. A Nationwide Online Survey. Vaccines 2021, 9, 1192. [Google Scholar] [CrossRef]
- Xia, S.; Duan, K.; Zhang, Y.; Zeng, X.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; Peng, C.; Zhang, W.; et al. Safety and Immunogenicity of an Inactivated COVID-19 Vaccine, WIBP-CorV, in Healthy Children: Interim Analysis of a Randomized, Double-Blind, Controlled, Phase 1/2 Trial. Front. Immunol. 2022, 13, 196–208. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: A randomised, double-blind, controlled, phase 1/2 trial. Lancet Infect. Dis. 2022, 22, 196–208. [Google Scholar] [CrossRef]
- Ali, K.; Berman, G.; Zhou, H.; Deng, W.; Faughnan, V.; Coronado-Voges, M.; Ding, B.; Dooley, J.; Girard, B.; Hillebrand, W.; et al. Evaluation of mRNA-1273 SARS-CoV-2 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 2241–2251. [Google Scholar] [CrossRef]
- Anderson, E.J.; Creech, C.B.; Berthaud, V.; Piramzadian, A.; Johnson, K.A.; Zervos, M.; Garner, F.; Griffin, C.; Palanpurwala, K.; Turner, M.; et al. Evaluation of mRNA-1273 Vaccine in Children 6 Months to 5 Years of Age. N. Engl. J. Med. 2022, 387, 1673–1687. [Google Scholar] [CrossRef] [PubMed]
- Áñez, G.; Dunkle, L.M.; Gay, C.L.; Kotloff, K.L.; Adelglass, J.M.; Essink, B.; Campbell, J.D.; Cloney-Clark, S.; Zhu, M.; Plested, J.S.; et al. Safety, Immunogenicity and Efficacy of NVX-CoV2373 in Adolescents in PREVENT-19: A Randomized, Phase 3 Trial. medRxiv 2022. [Google Scholar] [CrossRef]
- Creech, C.B.; Anderson, E.; Berthaud, V.; Yildirim, I.; Atz, A.M.; Baez, I.M.; Finkelstein, D.; Pickrell, P.; Kirstein, J.; Yut, C.; et al. Evaluation of mRNA-1273 Covid-19 Vaccine in Children 6 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 2011–2023. [Google Scholar] [CrossRef] [PubMed]
- Frenck, R.W., Jr.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Song, Y.; Li, C.; Yang, W.; Ma, Q.; Jiang, Z.; Li, M.; Lian, X.; Jiao, W.; Wang, L.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: A double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.T.C.; Chiu, C.H.; Chiu, N.C.; Tan, B.F.; Lin, C.Y.; Cheng, H.Y.; Lin, M.Y.; Lien, C.E.; Chen, C.; Huang, L.M. Safety and immunogenicity of SARS-CoV-2 vaccine MVC-COV1901 in adolescents in Taiwan: A double-blind, randomized, placebo-controlled phase 2 trial. medRxiv 2022. [Google Scholar] [CrossRef]
- Thuluva, S.; Paradkar, V.; Gunneri, S.; Yerroju, V.; Mogulla, R.R.; Suneetha, P.V.; Turaga, K.; Kyasani, M.; Manoharan, S.K.; Adabala, S.; et al. Safety, tolerability and immunogenicity of Biological E’s CORBEVAX™ vaccine in children and adolescents: A prospective, randomised, double-blind, placebo controlled, phase-2/3 study. Vaccine 2022, 40, 7130–7140. [Google Scholar] [CrossRef]
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Muñoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 35–46. [Google Scholar] [CrossRef]
- Zhu, F.; Jin, P.; Zhu, T.; Wang, W.; Ye, H.; Pan, H.; Hou, L.; Li, J.; Wang, X.; Wu, S.; et al. Safety and Immunogenicity of a Recombinant Adenovirus Type-5–Vectored Coronavirus Disease 2019 (COVID-19) Vaccine with a Homologous Prime-Boost Regimen in Healthy Participants Aged ≥6 Years: A Randomized, Double-Blind, Placebo-Controlled, Phase 2b Trial. Clin. Infect. Dis. 2021, 75, e783–e791. [Google Scholar] [CrossRef]
- Ruiz, J.B.; Bell, R.A. Parental COVID-19 Vaccine Hesitancy in the United States. Public Health Rep. 2022, 137, 1162–1169. [Google Scholar] [CrossRef]
- Russo, L.; Croci, I.; Campagna, I.; Pandolfi, E.; Villani, A.; Reale, A.; Barbieri, M.A.; Raponi, M.; Gesualdo, F.; Tozzi, A.E. Intention of Parents to Immunize Children against SARS-CoV-2 in Italy. Vaccines 2021, 9, 1469. [Google Scholar] [CrossRef]
- Wang, Q.; Xiu, S.; Zhao, S.; Wang, J.; Han, Y.; Dong, S.; Huang, J.; Cui, T.; Yang, L.; Shi, N.; et al. Vaccine Hesitancy: COVID-19 and Influenza Vaccine Willingness among Parents in Wuxi, China—A Cross-Sectional Study. Vaccines 2021, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Gu, W.; Xie, H.; Wang, X.; Cao, L.; Shan, M.; Wu, P.; Tian, Y.; Zhou, K. Parental Attitudes Towards Vaccination Against COVID-19 in China During Pandemic. Infect. Drug Resist. 2022, 15, 4541–4546. [Google Scholar] [CrossRef]
- Khatatbeh, M.; Albalas, S.; Khatatbeh, H.; Momani, W.; Melhem, O.; Al Omari, O.; Tarhini, Z.; A’Aqoulah, A.; Al-Jubouri, M.; Nashwan, A.J.; et al. Children’s rates of COVID-19 vaccination as reported by parents, vaccine hesitancy, and determinants of COVID-19 vaccine uptake among children: A multi-country study from the Eastern Mediterranean Region. BMC Public Health 2022, 22, 1375. [Google Scholar] [CrossRef]
- del Giudice, G.M.; Napoli, A.; Corea, F.; Folcarelli, L.; Angelillo, I.F. Evaluating COVID-19 Vaccine Willingness and Hesitancy among Parents of Children Aged 5–11 Years with Chronic Conditions in Italy. Vaccines 2022, 10, 396. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Choi, S.-H.; Yun, K.W. Risk Factors for Severe COVID-19 in Children: A Systematic Review and Meta-Analysis. J. Korean Med. Sci. 2022, 37, e35. [Google Scholar] [CrossRef]
- Du, Y.; Chen, L.; Shi, Y. Safety, Immunogenicity, and Efficacy of COVID-19 Vaccines in Adolescents, Children, and Infants: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 829176. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Luo, X.; Shen, Q.; Lei, R.; Liu, X.; Liu, E.; Li, Q.; Chen, Y. Safety, Immunogenicity, and Efficacy of COVID-19 Vaccines in Children and Adolescents: A Systematic Review. Vaccines 2021, 9, 1102. [Google Scholar] [CrossRef]
- Fidel, P.L.; Noverr, M.C. Could an Unrelated Live Attenuated Vaccine Serve as a Preventive Measure To Dampen Septic Inflammation Associated with COVID-19 Infection? mBio 2020, 11, e907–e920. [Google Scholar] [CrossRef] [PubMed]
- Dhochak, N.; Singhal, T.; Kabra, S.K.; Lodha, R. Pathophysiology of COVID-19: Why Children Fare Better than Adults? Indian J. Pediatr. 2020, 87, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Piccaluga, P.P.; Malerba, G.; Navari, M.; Diani, E.; Concia, E.; Gibellini, D. Cross-Immunization Against Respiratory Coronaviruses May Protect Children From SARS-CoV2: More Than a Simple Hypothesis? Front. Pediatr. 2021, 8, 595539. [Google Scholar] [CrossRef] [PubMed]
- Price, A.M.; Olson, S.M.; Newhams, M.M.; Halasa, N.B.; Boom, J.A.; Sahni, L.C.; Pannaraj, P.S.; Irby, K.; Bline, K.E.; Maddux, A.B.; et al. BNT162b2 Protection against the Omicron Variant in Children and Adolescents. N. Engl. J. Med. 2022, 386, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.F.; Ackerson, B.K.; Luo, Y.; Sy, L.S.; Talarico, C.A.; Tian, Y.; Bruxvoort, K.J.; Tubert, J.E.; Florea, A.; Ku, J.H.; et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat. Med. 2022, 28, 1063–1071. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.J.; Hall, J.A.; Zaidi, A.; Andrews, N.J.; Dunbar, J.K.; Dabrera, G. Effect of Vaccination on Household Transmission of SARS-CoV-2 in England. N. Engl. J. Med. 2021, 385, 759–760. [Google Scholar] [CrossRef]
- Prunas, O.; Warren, J.L.; Crawford, F.W.; Gazit, S.; Patalon, T.; Weinberger, D.M.; Pitzer, V.E. Vaccination with BNT162b2 reduces transmission of SARS-CoV-2 to household contacts in Israel. Science 2022, 375, 1151–1154. [Google Scholar] [CrossRef]
- Fowlkes, A.L.; Yoon, S.K.; Lutrick, K.; Gwynn, L.; Burns, J.; Grant, L.; Phillips, A.L.; Ellingson, K.; Ferraris, M.V.; LeClair, L.B.; et al. Effectiveness of 2-Dose BNT162b2 (Pfizer BioNTech) mRNA Vaccine in Preventing SARS-CoV-2 Infection Among Children Aged 5–11 Years and Adolescents Aged 12–15 Years—PROTECT Cohort, July 2021–February 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 422–428. [Google Scholar] [CrossRef]

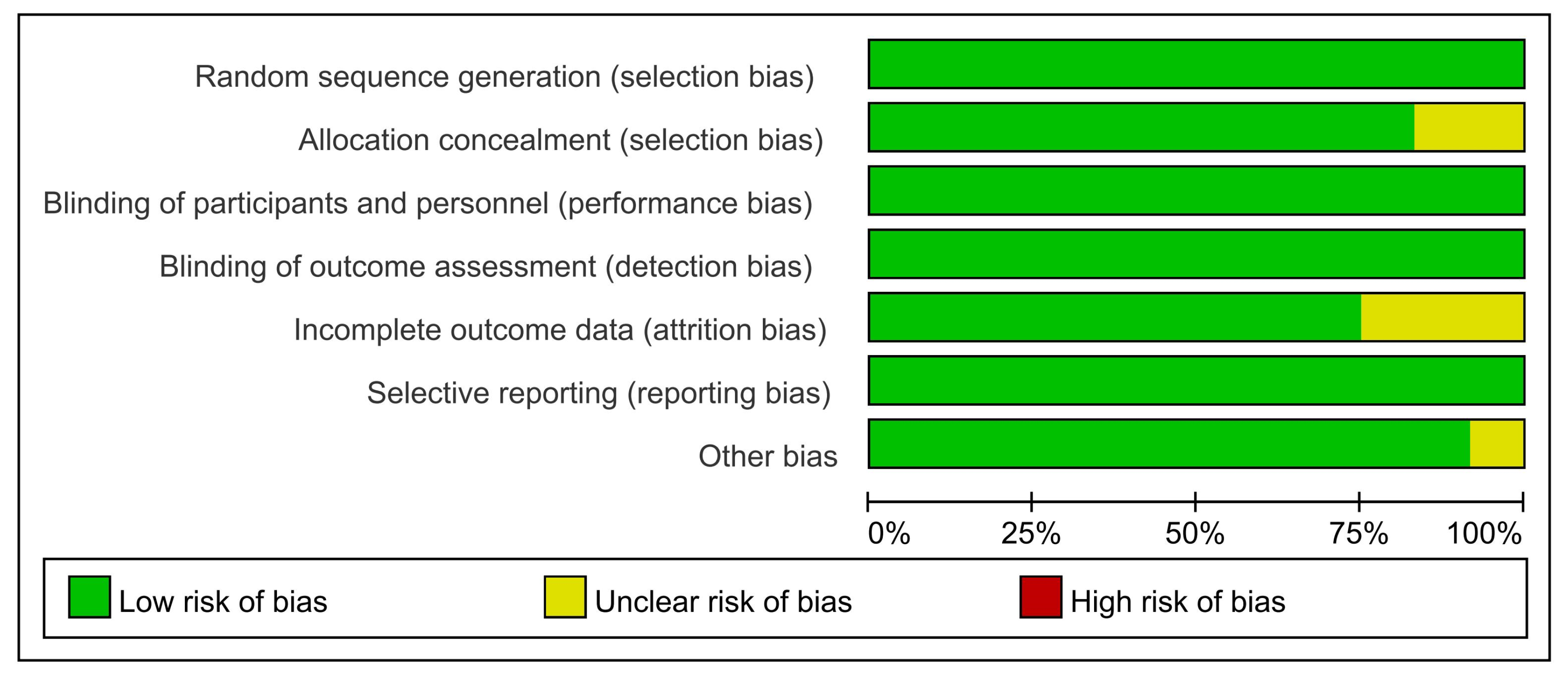
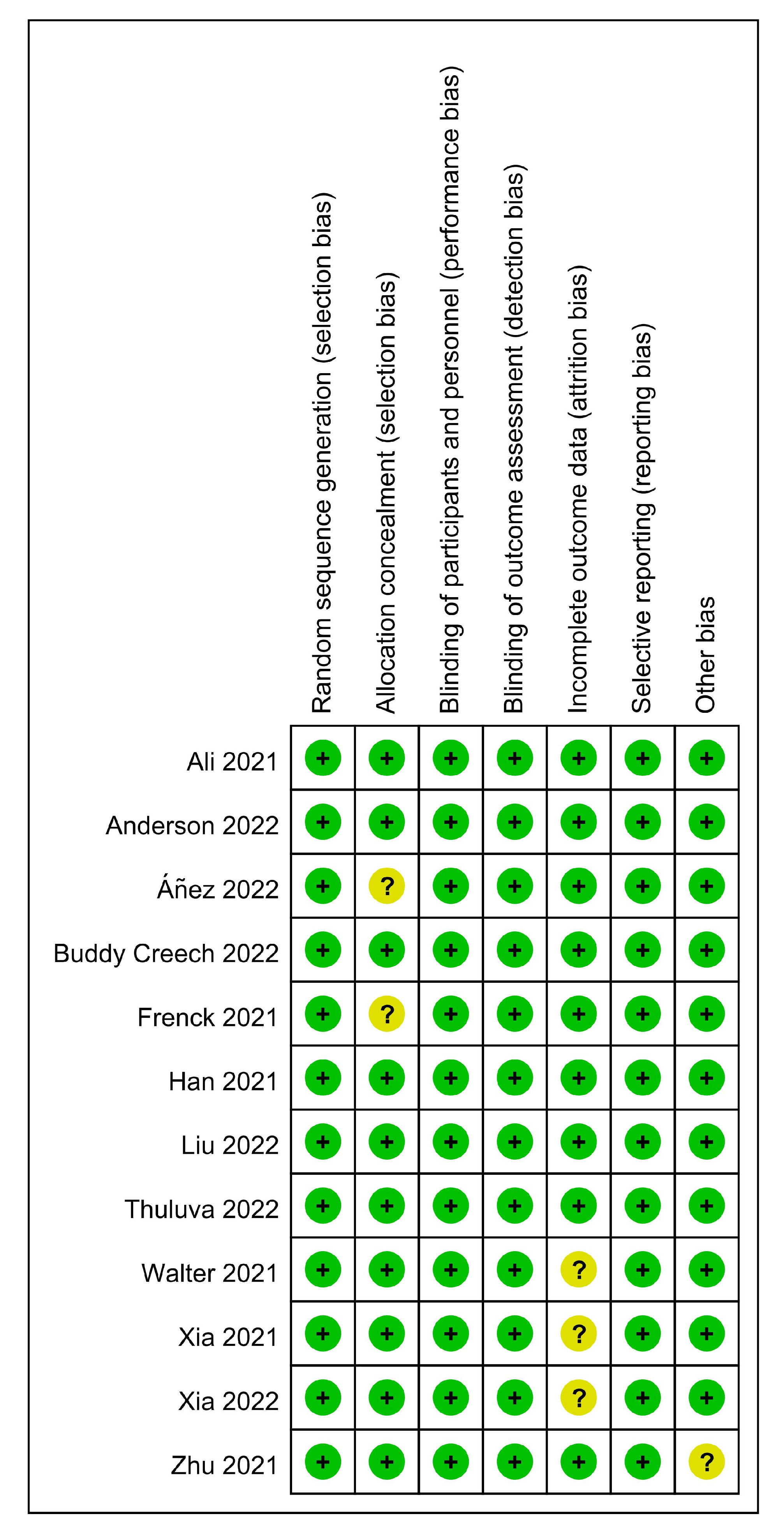
| Author, Year | Country | Phase | Age (Years) | Type of Vaccine | Dose of Administration (Per Dose) | Time of Inoculations (Days) | Control | No. of the Observation Group | No. of the Control Group |
|---|---|---|---|---|---|---|---|---|---|
| Ali et al. [31] | The USA | phase 2–3 | 12–17 | mRNA-1273 vaccine (mRNA vaccine) | 100 μg | 0, 28 | Saline | 2486 | 1240 |
| Anderson et al. [32] | the USA, Canada | phase 2–3 | 6 Months-5 | mRNA-1273 vaccine (mRNA vaccine) | 25 μg, 50 μg | 0, 28 | Saline | 5011 | 1751 |
| Áñez et al. [33] | the USA, Mexico | phase 3 | 12–17 | NVX-CoV2373 (subunit vaccine) | 0.5 mL | 0, 21 | Saline | 1487 | 745 |
| Buddy Creech et al. [34] | the USA, Canada | phase 2–3 | 6–11 | mRNA-1273 vaccine (mRNA vaccine) | 50 μg | 0, 28 | Saline | 3385 | 995 |
| Frenck et al. [35] | The USA | phase 3 | 12–15 | BNT162b2 Vaccine (mRNA vaccine) | 30 μg | 0, 21 | Saline | 1131 | 1129 |
| Han et al. [36] | China | phase 1–2 | 3–17 | CoronaVac (Inactivated vaccine) | 1.5 μg, 3.0 μg | 0, 28 | Aluminum hydroxide adjuvant | 436 | 114 |
| Liu et al. [37] | China | phase 2 | 12–17 | MVC-COV1901 (subunit vaccine) | 0.5 mL | 0, 28 | Saline | 341 | 58 |
| Thuluva et al. [38] | India | phase 2–3 | 5–17 (<12- ≥5, <18- ≥12) | CORBEVAX™ (subunit vaccine) | 0.5 mL | 0, 28 | Placebo (Not noted) | 468 | 156 |
| Walter et al. [39] | the USA, Spain, Finland, Poland | phase 2–3 | 5–11 | BNT162b2 Vaccine (mRNA vaccine) | 10 μg | 0, 21 | Saline | 1518 | 750 |
| Xia et al. [29] | China | phase 1–2 | 3–17 (3–5, 6–12, 13–17) | WBIBP-CorV (Inactivated vaccine) | 2.5 μg, 5 μg, 10 μg | 0, 28, 56 | Alum | 612 | 204 |
| Xia et al. [30] | China | phase 1–2 | 3–17 (3–5, 6–12, 13–17) | BBIBP-COV (Inactivated vaccine) | 2 μg,4 μg, 8 μg | 0, 28, 56 | Saline and aluminum hydroxide adjuvant | 756 | 252 |
| Zhu et al. [40] | China | phase 2 | 6–17 | Ad5-vectored COVID-19 vaccine (Adenovirus vaccine) | 0.3 mL | 0, 56 | Placebo containing the same excipients as the vaccine, without viral particles | 100 | 50 |
| No. of Studies | RR (95% CI) | I² | p-Value | ||
|---|---|---|---|---|---|
| Total Adverse Reactions | |||||
| mRNA vaccine | After dose 1 | 3 | 1.30 [1.07, 1.57] | 98% | <0.05 |
| After dose 2 | 3 | 1.43 [1.14, 1.79] | 98% | <0.05 | |
| Inactivated vaccine | After dose 1 | 1 | 1.27 [0.76, 2.13] | Not applicable | >0.05 |
| After dose 2 | 1 | 1.83 [0.90, 3.72] | Not applicable | >0.05 | |
| Submit vaccine | After dose 1 | 1 | 1.57 [1.17, 2.11] | Not applicable | <0.05 |
| After dose 2 | 1 | 1.94 [1.26, 2.98] | Not applicable | <0.05 | |
| Adenovirus vector vaccine | After dose 1 | 1 | 3.44 [1.78, 6.65] | Not applicable | <0.05 |
| After dose 2 | 1 | 8.25 [2.06, 33.00] | Not applicable | <0.05 | |
| Systemic adverse reactions | |||||
| mRNA vaccine | After dose 1 | 3 | 1.13 [1.02, 1.24] | 88% | <0.05 |
| After dose 2 | 3 | 1.47 [1.08, 2.01] | 99% | <0.05 | |
| Inactivated vaccine | After dose 1 | 1 | 1.32 [0.87, 2.00] | Not applicable | >0.05 |
| After dose 2 | 1 | 1.61 [0.76, 3.40] | Not applicable | >0.05 | |
| Submit vaccine | After dose 1 | 1 | 1.11 [0.78, 1.57] | Not applicable | >0.05 |
| After dose 2 | 1 | 1.22 [0.72, 2.09] | Not applicable | >0.05 | |
| Adenovirus vector vaccine | After dose 1 | 1 | 3.70 [1.55, 8.83] | Not applicable | <0.05 |
| After dose 2 | 1 | 6.00 [1.48, 24.38] | Not applicable | <0.05 | |
| Local adverse reactions | |||||
| mRNA vaccine | After dose 1 | 3 | 1.80 [1.11, 2.92] | 99% | <0.05 |
| After dose 2 | 3 | 1.93 [1.25, 2.97] | 99% | <0.05 | |
| Inactivated vaccine | After dose 1 | 1 | 6.34 [1.54, 26.10] | Not applicable | <0.05 |
| After dose 2 | 1 | 4.29 [1.03, 17.96] | Not applicable | =0.05 | |
| Submit vaccine | After dose 1 | 1 | 2.93 [1.76, 4.89] | Not applicable | <0.05 |
| After dose 2 | 1 | 1.99 [1.24, 3.18] | Not applicable | <0.05 | |
| Adenovirus vector vaccine | After dose 1 | 1 | 6.00 [1.94, 18.53] | Not applicable | <0.05 |
| After dose 2 | 1 | 19.69 [1.21,319.62] | Not applicable | <0.05 |
| After Dose 1 | After Dose 2 | ||||||
|---|---|---|---|---|---|---|---|
| No. of Studies | RR (95% CI) | I² | p-Value | RR (95% CI) | I² | p-Value | |
| Overall | 5 | 1.91 [1.70, 2.16] | 97 | <0.05 | 3.13 [2.73, 3.59] | 97 | <0.05 |
| Local pain | 5 | 2.32 [1.72, 3.13] | 98 | <0.05 | 2.54 [1.89, 3.42] | 98 | <0.05 |
| Erythema or Redness | 5 | 5.66 [2.75, 11.65] | 92 | <0.05 | 7.73 [3.76, 15.90] | 92 | <0.05 |
| Swelling or Hardness | 5 | 6.21 [3.14, 12.28] | 90 | <0.05 | 8.59 [4.86, 15.19] | 84 | <0.05 |
| Axillary Swelling | 3 | 1.85 [1.15, 2.98] | 93 | <0.05 | 2.90 [2.02, 4.18] | 84 | <0.05 |
| Fever | 5 | 3.31 [1.47, 7.45] | 92 | <0.05 | 7.85 [2.58, 23.91] | 96 | <0.05 |
| Headache | 4 | 1.14 [0.92, 1.43] | 94 | >0.05 | 2.04 [1.63, 2.56] | 94 | <0.05 |
| Fatigue | 4 | 1.29 [1.16, 1.43] | 79 | <0.05 | 2.08 [1.70, 2.54] | 93 | <0.05 |
| Myalgia | 4 | 1.59 [1.39, 1.81] | 43 | <0.05 | 2.87 [2.07, 3.98] | 90 | <0.05 |
| Arthralgia | 4 | 1.10 [0.84, 1.45] | 77 | >0.05 | 2.22 [1.50, 3.28] | 89 | <0.05 |
| Nausea or Vomiting | 4 | 1.41 [0.99, 1.99] | 75 | =0.05 | 2.55 [2.23, 2.92] | 0 | <0.05 |
| Chills | 4 | 1.63 [1.15, 2.33] | 89 | <0.05 | 4.37 [3.14, 6.09] | 86 | <0.05 |
| Diarrhea | 2 | 1.27 [0.96, 1.67] | 23 | >0.05 | 1.21 [0.82, 1.80] | 55 | >0.05 |
| Irritability or Crying | 1 | 1.08 [1.01, 1.16] | Not applicable | <0.05 | 1.10 [1.01, 1.19] | Not applicable | <0.05 |
| Sleepiness | 1 | 0.97 [0.86, 1.09] | Not applicable | >0.05 | 1.04 [0.90, 1.19] | Not applicable | >0.05 |
| Loss of appetite | 1 | 1.12 [0.96, 1.30] | Not applicable | >0.05 | 1.25 [1.06, 1.48] | Not applicable | <0.05 |
| After Dose 1 | After Dose 2 | ||||||
|---|---|---|---|---|---|---|---|
| No. of Studies | RR (95% CI) | I² | p-Value | RR (95% CI) | I² | p-Value | |
| mRNA vaccine | 5 | 1.91 [1.70, 2.16] | 97 | <0.05 | 3.13 [2.73, 3.59] | 97 | <0.05 |
| Inactivated vaccine | 2 | 1.76 [1.20, 2.57] | 38 | <0.05 | 2.18 [1.30, 3.67] | 0 | <0.05 |
| Subunit vaccine | 1 | 1.66 [1.26, 2.17] | 19 | <0.05 | 1.40 [1.02, 1.92] | 12 | <0.05 |
| Vectored vaccine | 1 | 5.27 [2.80, 9.91] | 0 | <0.05 | 6.21 [2.40, 16.11] | 0 | <0.05 |
| ≥12 Years | <12 Years | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | RR (95% CI) | I² | p-Value | No. of Studies | RR (95% CI) | I² | p-Value | ||
| After dose 1 | Local pain | 2 | 3.15 [2.27, 4.37] | 96 | <0.05 | 3 | 1.89 [1.44, 2.48] | 97 | <0.05 |
| Erythema or Redness | 2 | 10.74 [2.72, 43.37] | 89 | < 0.05 | 3 | 3.78 [1.96, 7.29] | 89 | <0.05 | |
| Swelling or Hardness | 2 | 10.61 [4.13, 27.28] | 81 | <0.05 | 3 | 4.39 [2.24, 8.58] | 85 | <0.05 | |
| Fever | 2 | 5.00 [1.40, 17.82] | 89 | <0.05 | 3 | 2.50 [1.02, 6.17] | 90 | =0.05 | |
| Headache | 2 | 1.35 [1.00, 1.82] | 96 | =0.05 | 2 | 0.98 [0.89, 1.07] | 0 | >0.05 | |
| Fatigue | 2 | 1.38 [1.24, 1.55] | 71 | <0.05 | 2 | 1.19 [1.03, 1.38] | 72 | <0.05 | |
| Myalgia | 2 | 1.70 [1.52, 1.90] | 14 | <0.05 | 2 | 1.40 [1.18, 1.67] | 0 | <0.05 | |
| Arthralgia | 2 | 1.33 [1.15, 1.55] | 0 | <0.05 | 2 | 0.84 [0.45, 1.56] | 85 | >0.05 | |
| Nausea or Vomiting | 2 | 1.78 [0.82, 3.86] | 81 | >0.05 | 2 | 1.24 [0.67, 2.27] | 62 | >0.05 | |
| Chills | 2 | 2.08 [1.31, 3.30] | 92 | <0.05 | 2 | 1.25 [0.83, 1.87] | 69 | >0.05 | |
| After dose 2 | Local pain | 2 | 3.64 [2.55, 5.19] | 95 | <0.05 | 3 | 1.99 [1.70, 2.34] | 92 | <0.05 |
| Erythema or Redness | 2 | 10.16 [2.05, 50.29] | 93 | <0.05 | 3 | 6.45 [2.90, 14.31] | 91 | <0.05 | |
| Swelling or Hardness | 2 | 10.00 [2.11, 47.24] | 93 | <0.05 | 3 | 7.71 [4.33, 13.72] | 78 | <0.05 | |
| Fever | 2 | 15.28 [10.11, 23.11 | 4 | <0.05 | 3 | 5.07 [1.14, 22.44] | 97 | <0.05 | |
| Headache | 2 | 2.50 [2.14, 2.91] | 79 | <0.05 | 2 | 1.66 [1.35, 2.04] | 77 | <0.05 | |
| Fatigue | 2 | 2.47 [2.20, 2.78] | 62 | <0.05 | 2 | 1.75 [1.55, 1.98] | 55 | <0.05 | |
| Myalgia | 2 | 3.80 [3.35, 4.31] | 0 | <0.05 | 2 | 2.11 [1.41, 3.16] | 81 | <0.05 | |
| Arthralgia | 2 | 3.14 [2.68, 3.66] | 0 | <0.05 | 2 | 1.57 [1.11, 2.22] | 57 | <0.05 | |
| Nausea or Vomiting | 2 | 2.78 [2.31, 3.36] | 0 | <0.05 | 2 | 2.33 [1.92, 2.82] | 0 | <0.05 | |
| Chills | 2 | 5.85 [5.03, 6.79] | 0 | <0.05 | 2 | 4.37 [3.14, 6.09] | 72 | <0.05 | |
| After Dose 1 | After Dose 2 | ||||||
|---|---|---|---|---|---|---|---|
| No. of Studies | RR (95% CI) | I² | p-Value | RR (95% CI) | I² | p-Value | |
| Overall | 1 | 0.74 [0.71, 0.77] | 97 | <0.05 | 0.80 [0.77, 0.83] | 98 | <0.05 |
| Any local adverse reactions | 1 | 0.70 [0.66, 0.74] | Not applicable | <0.05 | 0.73 [0.70, 0.77] | Not applicable | <0.05 |
| Local pain | 1 | 0.60 [0.57, 0.64] | Not applicable | <0.05 | 0.64 [0.60, 0.67] | Not applicable | <0.05 |
| Erythema or Redness | 1 | 1.53 [1.24, 1.89] | Not applicable | <0.05 | 1.53 [1.29, 1.80] | Not applicable | <0.05 |
| Swelling or Hardness | 1 | 1.85 [1.49, 2.31] | Not applicable | <0.05 | 1.83 [1.55, 2.15] | Not applicable | <0.05 |
| Axillary swelling | 1 | 0.91 [0.73, 1.13] | Not applicable | >0.05 | 1.02 [0.85, 1.23] | Not applicable | >0.05 |
| Included Studies | Type of Vaccine | Days | Age (Years) | Dose | Participants | GMT (IU/mL) | GMR | Serologic Response | The Difference in Serologic Response | Noninferiority |
|---|---|---|---|---|---|---|---|---|---|---|
| Ali et al. [31] | mRNA-1273 vaccine (mRNA vaccine) | 57 | 12–17 | 100 µg | 340 | 1401.7 (1276.3, 1539.5) | 1.08 (0.94, 1.25) | 336/340 (97.0, 99.7) | 0.2 (−1.8, 2.4) | Yes |
| 18–25 | NA | 296 | 1301.3 (1177.0, 1438.8) | 292/296 (96.6, 99.6) | ||||||
| Anderson et al. [32] | mRNA-1273 vaccine (mRNA vaccine) | 57 | 6–23 months | 25 μg | 230 | 1781 (1616, 1962) | 1.3 (1.1, 1.5) | 230/230 (98.4, 100.0) | 0.7 (−1.0, 2.5) | Yes |
| 2–5 | 25 μg | 264 | 1410 (1272, 1563) | 1.0 (0.9, 1.2) | 261/264 (96.7, 99.8) | −0.4 (−2.7, 1.5) | Yes | |||
| 18–25 | 100 μg | 294 | 1391 (1263, 1531) | / | 289/291 (97.5, 99.9) | / | / | |||
| Áñez et al. [33] | NVX-CoV2373 (subunit vaccine) | 35 | 12–17 | 0.5 mL | 390 | 3860 (3423, 4352) | 1.5 (1.3, 1.7) | -/390 (98.7%) (97.0, 99.6) | −1.0 (−2.8, 0.2) | Yes |
| 18–25 | NA | 416 | 2634 (2398, 2904) | -/416 (99.8%) (98.7, 100) | ||||||
| Buddy Creech et al. [34] | mRNA-1273 vaccine (mRNA vaccine) | 57 | 6–11 | 50 μg | 320 | 1610.2 (1456.6, 1780.0) | 1.2 (1.1, 1.4) | 313/316 (97.3, 99.8) | 0.1 (−1.9, 2.1) | Yes |
| 18–25 | 100 μg | 295 | 1299.9 (1170.6, 1443.4) | 292/295 (97.1, 99.8) | ||||||
| Frenck et al. [35] | BNT162b2 Vaccine (mRNA vaccine) | A month after dose 2 | 12–15 | 30 µg | 190 | 1239.5 (1095.5, 1402.5) | 1.76 (1.47, 2.10) | NA | NA | Yes |
| 16–25 | NA | 170 | 705.1 (621.4, 800.2) | NA | ||||||
| Liu et al. [37] | MVC-COV1901 (subunit vaccine) | 57 | 12–17 | 0.5 mL | 334 | 648.47 (608.62, 690.93) | 1.16 (1.04, 1.29) | 334/334 (98.90, 100.00) | −0.0% (0.00, 0.00) | Yes |
| 20–30 | NA | 210 | 559.54 (512.05, 611.34) | 210/210 (98.26, 100.00) | ||||||
| Walter et al. [39] | BNT162b2 vaccine (mRNA vaccine) | A month after dose 2 | 5–11 | 10 μg | 264 | 1197.6 (1106.1, 1296.6) | 1.04 (0.93, 1.18) | NA | NA | Yes |
| 16–25 | 30 μg | 253 | 1146.5 (1045.5, 1257.2) | NA |
| Vaccine Type | Time | No. of Studies | RR (95% CI) | I² | p-Value |
|---|---|---|---|---|---|
| Inactivated vaccine | 28 days after dose 1 | 2 | 245.69 [34.93, 1727.83] | 67 | <0.05 |
| 28 days after dose 2 | 3 | 363.09 [73.82, 1785.92] | 0 | <0.05 | |
| 28 days after dose 3 | 1 | 392.95 [24.66, 6260.89] | Not applicable | <0.05 | |
| Subunit vaccine | 28 days after dose 2 | 1 | 31.29 [6.48, 151.07] | Not applicable | <0.05 |
| Adenovirus vaccine | 28 days after dose 1 | 1 | 14.67 [4.88, 44.04] | Not applicable | <0.05 |
| 28 days after dose 2 | 1 | 24.50 [6.30, 95.28] | Not applicable | <0.05 |
| No. of Studies | RR (95% CI) | I² | p-Value | |
|---|---|---|---|---|
| 28 days after Dose 1 | 1 | 99.48 [6.31, 1569.12] | Not applicable | <0.05 |
| 28 days after Dose 2 | 1 | 101.50 [6.44, 1600.76] | Not applicable | <0.05 |
| No. of Studies | RR (95% CI) | I² | p-Value | |
|---|---|---|---|---|
| Neutralizing antibody 28 days after Dose 2 | ||||
| 3–5 years old | 3 | 125.90 [25.72, 616.35] | 0 | <0.05 |
| 6–11/12 years old | 3 | 122.82 [25.05, 602.16] | 0 | <0.05 |
| 12/13–17 years old | 3 | 117.87 [24.04, 577.88] | 0 | <0.05 |
| Neutralizing antibody 28 days after Dose 3 | ||||
| 3–5 years old | 1 | 163.67 [10.32, 2594.58] | Not applicable | <0.05 |
| 6–12 years old | 1 | 120.30 [7.61, 1901.57] | Not applicable | <0.05 |
| 13–17 years old | 1 | 112.80 [7.13, 1783.53] | Not applicable | <0.05 |
| No. of Studies | RR (95% CI) | I² | p-Value | |
|---|---|---|---|---|
| COVID-19 after the vaccination | ||||
| After dose 1 to before dose 2 | 2 | 0.16 [0.08, 0.32] | 0 | <0.05 |
| Within 7 days after dose 2 | 1 | 0.09 [0.01, 1.64] | Not applicable | >0.05 |
| 7 days after dose 2 | 2 | 0.08 [0.03, 0.24] | 0 | <0.05 |
| 14 days after dose 2 | 3 | 0.30 [0.09, 0.97] | 62 | <0.05 |
| COVID-19 after dose 2 | ||||
| mRNA-1273 vaccine | 3 | 0.30 [0.09, 0.97] | 62 | <0.05 |
| BNT162b2 COVID-19 Vaccine | 2 | 0.08 [0.03, 0.24] | 0 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Chen, L.; Shi, Y. Safety, Efficacy, and Immunogenicity of Varying Types of COVID-19 Vaccines in Children Younger Than 18 Years: An Update of Systematic Review and Meta-Analysis. Vaccines 2023, 11, 87. https://doi.org/10.3390/vaccines11010087
Tian Y, Chen L, Shi Y. Safety, Efficacy, and Immunogenicity of Varying Types of COVID-19 Vaccines in Children Younger Than 18 Years: An Update of Systematic Review and Meta-Analysis. Vaccines. 2023; 11(1):87. https://doi.org/10.3390/vaccines11010087
Chicago/Turabian StyleTian, Yan, Long Chen, and Yuan Shi. 2023. "Safety, Efficacy, and Immunogenicity of Varying Types of COVID-19 Vaccines in Children Younger Than 18 Years: An Update of Systematic Review and Meta-Analysis" Vaccines 11, no. 1: 87. https://doi.org/10.3390/vaccines11010087
APA StyleTian, Y., Chen, L., & Shi, Y. (2023). Safety, Efficacy, and Immunogenicity of Varying Types of COVID-19 Vaccines in Children Younger Than 18 Years: An Update of Systematic Review and Meta-Analysis. Vaccines, 11(1), 87. https://doi.org/10.3390/vaccines11010087





