The Impact of Adjuvanted Influenza Vaccine on Disease Severity in the US: A Stochastic Model
Abstract
:1. Introduction
2. Methods
2.1. Model Input Parameters
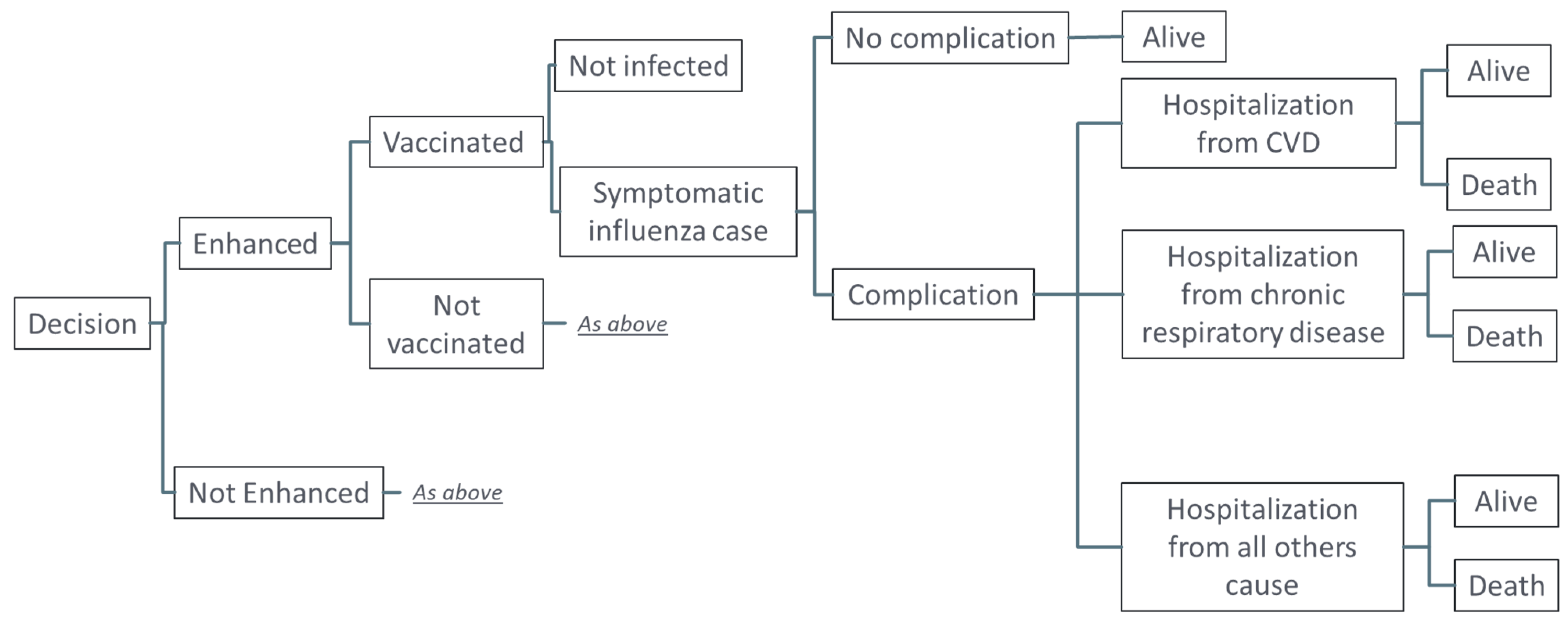
2.2. Stochastic Simulation
2.3. Outcomes Evaluated
2.4. Software
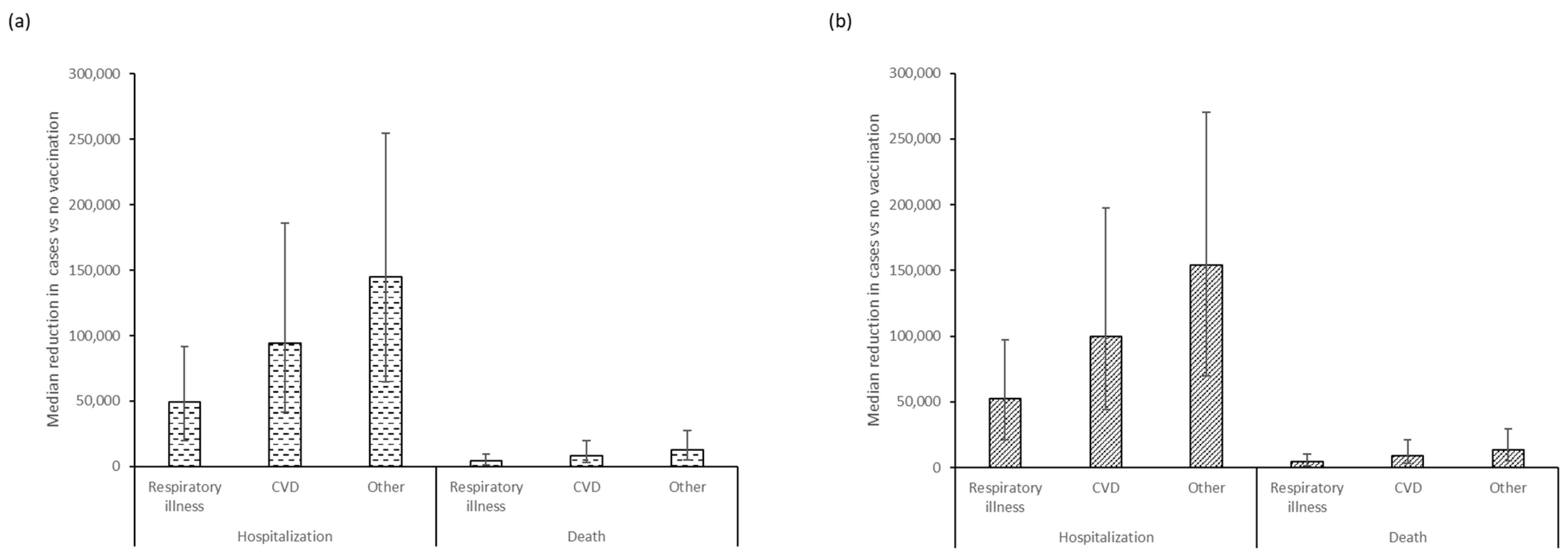
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC). Flu Vaccination Coverage, United States, 2021–2022 Influenza Season. Available online: https://www.cdc.gov/flu/fluvaxview/coverage-2022estimates.htm (accessed on 19 May 2023).
- Centers for Disease Prevention and Control. Disease Burden of Flu. Available online: https://www.cdc.gov/flu/about/burden/index.html (accessed on 7 March 2023).
- Centers for Disease Prevention Control. 2022–2023, U.S. Flu Season: Preliminary In-Season Burden Estimates. Available online: https://www.cdc.gov/flu/about/burden/preliminary-in-season-estimates.htm (accessed on 7 March 2023).
- Dugan, H.L.; Henry, C.; Wilson, P.C. Aging and influenza vaccine-induced immunity. Cell Immunol. 2020, 348, 103998. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Percent of US Adults 55 and over with Chronic Conditions. Available online: https://www.cdc.gov/nchs/health_policy/adult_chronic_conditions.htm (accessed on 19 May 2023).
- Centers for Disease Prevention and Control. Estimated Flu-Related Illnesses, Medical Visits, Hospitalizations, and Deaths in the United States—2019–2020 Flu Season. Available online: https://www.cdc.gov/flu/about/burden/2019-2020.html (accessed on 7 March 2023).
- Centers for Disease Prevention and Control. Estimated Flu-Related Illnesses, Medical Visits, Hospitalizations, and Deaths in the United States—2018–2019 Flu Season. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/flu/about/burden/2018-2019.html (accessed on 7 March 2023).
- Centers for Disease Prevention and Control. FluView Interactive: Laboratory-Confirmed Influenza Hospitalizations. Available online: https://gis.cdc.gov/GRASP/Fluview/FluHospRates.html (accessed on 13 March 2023).
- Macias, A.E.; McElhaney, J.E.; Chaves, S.S.; Nealon, J.; Nunes, M.C.; Samson, S.I.; Seet, B.T.; Weinke, T.; Yu, H. The disease burden of influenza beyond respiratory illness. Vaccine 2021, 39 (Suppl. 1), A6–A14. [Google Scholar] [CrossRef] [PubMed]
- Smeeth, L.; Thomas, S.L.; Hall, A.J.; Hubbard, R.; Farrington, P.; Vallance, P. Risk of myocardial infarction and stroke after acute infection or vaccination. N. Engl. J. Med. 2004, 351, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, R.; Zhao, H.; Pebody, R.; Hayward, A.; Warren-Gash, C. Laboratory-Confirmed Respiratory Infections as Predictors of Hospital Admission for Myocardial Infarction and Stroke: Time-Series Analysis of English Data for 2004–2015. Clin. Infect. Dis. 2018, 67, 8–17. [Google Scholar] [CrossRef]
- Czaja, C.A.; Miller, L.; Alden, N.; Wald, H.L.; Cummings, C.N.; Rolfes, M.A.; Anderson, E.J.; Bennett, N.M.; Billing, L.M.; Chai, S.J.; et al. Age-Related Differences in Hospitalization Rates, Clinical Presentation, and Outcomes Among Older Adults Hospitalized With Influenza—U.S. Influenza Hospitalization Surveillance Network (FluSurv-NET). Open Forum Infect. Dis. 2019, 6, ofz225. [Google Scholar] [CrossRef] [PubMed]
- Udell, J.A.; Zawi, R.; Bhatt, D.L.; Keshtkar-Jahromi, M.; Gaughran, F.; Phrommintikul, A.; Ciszewski, A.; Vakili, H.; Hoffman, E.B.; Farkouh, M.E.; et al. Association Between Influenza Vaccination and Cardiovascular Outcomes in High-Risk Patients: A Meta-analysis. JAMA 2013, 310, 1711–1720. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Mahimbo, A.; Moa, A.M.; Barnes, M. Influenza vaccine as a coronary intervention for prevention of myocardial infarction. Heart 2016, 102, 1953. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.R.; Bae, J.H.; Hwang, I.C.; Kim, K.K.; Suh, H.S.; Ko, K.D. Effect of Influenza Vaccination on Risk of Stroke: A Systematic Review and Meta-Analysis. Neuroepidemiology 2017, 48, 103–110. [Google Scholar] [CrossRef]
- Heo, J.Y.; Song, J.Y.; Noh, J.Y.; Choi, M.J.; Yoon, J.G.; Na Lee, S.; Cheong, H.J.; Kim, W.J. Effects of influenza immunization on pneumonia in the elderly. Hum. Vaccin. Immunother. 2018, 14, 744–749. [Google Scholar] [CrossRef]
- Centers for Disease Prevention and Control. Influenza Vaccination: A Summary for Clinicians. Available online: https://www.cdc.gov/flu/professionals/vaccination/vax-summary.htm (accessed on 7 March 2023).
- Ko, E.J.; Kang, S.M. Immunology and efficacy of MF59-adjuvanted vaccines. Hum. Vaccin. Immunother. 2018, 14, 3041–3045. [Google Scholar] [CrossRef]
- Izurieta, H.S.; Chillarige, Y.; Kelman, J.; Wei, Y.; Lu, Y.; Xu, W.; Lu, M.; Pratt, D.; Wernecke, M.; MaCurdy, T.; et al. Relative Effectiveness of Influenza Vaccines Among the United States Elderly, 2018–2019. J. Infect. Dis. 2020, 222, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Coleman, B.L.; Sanderson, R.; Haag, M.D.M.; McGovern, I. Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis. Influenza Other Respir. Viruses 2021, 15, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, B.; Weinke, T.; Wahle, K.; Kwetkat, A.; Beier, D.; Schmidt, K.; Schwarz, T. Importance and value of adjuvanted influenza vaccine in the care of older adults from a European perspective—A systematic review of recently published literature on real-world data. Vaccine 2022, 40, 2999–3008. [Google Scholar] [CrossRef]
- Ferdinands, J.M.; Thompson, M.G.; Blanton, L.; Spencer, S.; Grant, L.; Fry, A.M. Does influenza vaccination attenuate the severity of breakthrough infections? A narrative review and recommendations for further research. Vaccine 2021, 39, 3678–3695. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Puig-Barbera, J.; Ortiz, J.R.; Fischer, L.; O’Brien, D.; Bonafede, M.; Mansi, J.A.; Boikos, C. Relative Effectiveness of MF59 Adjuvanted Trivalent Influenza Vaccine vs Nonadjuvanted Vaccines During the 2019–2020 Influenza Season. Open Forum Infect. Dis. 2022, 9, ofac167. [Google Scholar] [CrossRef] [PubMed]
- Lapi, F.; Marconi, E.; Simonetti, M.; Baldo, V.; Rossi, A.; Sessa, A.; Cricelli, C. Adjuvanted versus nonadjuvanted influenza vaccines and risk of hospitalizations for pneumonia and cerebro/cardiovascular events in the elderly. Expert Rev. Vaccines 2019, 18, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Puig-Barberà, J.; Díez-Domingo, J.; Varea, Á.B.; Chavarri, G.S.; Rodrigo, J.A.L.; Hoyos, S.P.; Vidal, D.G. Effectiveness of MF59-adjuvanted subunit influenza vaccine in preventing hospitalisations for cardiovascular disease, cerebrovascular disease and pneumonia in the elderly. Vaccine 2007, 25, 7313–7321. [Google Scholar] [CrossRef]
- Cocchio, S.; Gallo, T.; Del Zotto, S.; Clagnan, E.; Iob, A.; Furlan, P.; Fonzo, M.; Bertoncello, C.; Baldo, V. Preventing the Risk of Hospitalization for Respiratory Complications of Influenza among the Elderly: Is There a Better Influenza Vaccination Strategy? A Retrospective Population Study. Vaccines 2020, 8, 344. [Google Scholar] [CrossRef]
- Mangen, M.J.J.; Rozenbaum, M.H.; Huijts, S.M.; van Werkhoven, C.H.; Postma, D.F.; Atwood, M.; van Deursen, A.M.M.; van der Ende, A.; Grobbee, D.E.; Sanders, E.A.M.; et al. Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands. Eur. Respir. J. 2015, 46, 1407–1416. [Google Scholar] [CrossRef]
- Centers for Disease Prevention and Control. Past Seasons Estimated Influenza Disease Burden. Available online: https://www.cdc.gov/flu/about/burden/past-seasons.html (accessed on 17 February 2023).
- Molinari, N.-A.M.; Ortega-Sanchez, I.R.; Messonnier, M.L.; Thompson, W.W.; Wortley, P.M.; Weintraub, E.; Bridges, C.B. The annual impact of seasonal influenza in the US: Measuring disease burden and costs. Vaccine 2007, 25, 5086–5096. [Google Scholar] [CrossRef]
- van Aalst, R.; Russo, E.M.; Neupane, N.; Mahmud, S.M.; Wilschut, J.; Samson, S.I.; Chit, A.; Postma, M.; Young-Xu, Y. Comparing the impact of high-dose versus standard dose influenza vaccines on hospitalization cost for cardiovascular and respiratory diseases: Economic assessment in the US Veteran population during 5 respiratory seasons using an instrumental variable method. Vaccine 2021, 39 (Suppl. 1), A51–A55. [Google Scholar]
- Mauskopf, J.; Klesse, M.; Lee, S.; Herrera-Taracena, G. The burden of influenza complications in different high-risk groups: A targeted literature review. J. Med. Econ. 2013, 16, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Talbot, H.K.; Trabue, C.H.; Gaglani, M.; McNeal, T.M.; Monto, A.S.; Martin, E.T.; Zimmerman, R.K.; Silveira, F.P.; Middleton, D.B.; et al. Influenza Vaccine Effectiveness Against Hospitalization in the United States, 2019–2020. J. Infect. Dis. 2021, 224, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Prevention and Control. Flu Vaccination Coverage, United States, 2019–2020 Influenza Season. Available online: https://www.cdc.gov/flu/fluvaxview/coverage-1920estimates.htm (accessed on 24 April 2023).
- Nguyen, V.H.; Mould-Quevedo, J.F. Estimating the Impact of Influenza Vaccination on Acute and ICU Hospital Bed Usage in an Influenza Season under Endemic COVID-19 in the US. Vaccines 2022, 10, 1908. [Google Scholar] [CrossRef]
- McElhaney, J.E. The unmet need in the elderly: Designing new influenza vaccines for older adults. Vaccine 2005, 23 (Suppl. 1), S10–S25. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, M.J.; Mulder, P.G.; Beyer, W.E.; Van Strik, R.; Masurel, N. Impact of influenza on mortality in relation to age and underlying disease, 1967–1989. Int. J. Epidemiol. 1993, 22, 334–340. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Klompas, M. Understanding Breakthrough Infections Following mRNA SARS-CoV-2 Vaccination. JAMA 2021, 326, 2018–2020. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.; Romero, A.; Soldevila, N.; Torner, N.; Jané, M.; Martínez, A.; A Caylà, J.; Rius, C.; Domínguez, A. Influenza vaccine effectiveness in reducing severe outcomes over six influenza seasons, a case-case analysis, Spain, 2010/11 to 2015/16. Eur. Surveill. 2018, 23, 1700732. [Google Scholar] [CrossRef]
- Thompson, M.G.; Pierse, N.; Huang, Q.S.; Prasad, N.; Duque, J.; Newbern, E.C.; Baker, M.G.; Turner, N.; McArthur, C. Influenza vaccine effectiveness in preventing influenza-associated intensive care admissions and attenuating severe disease among adults in New Zealand 2012–2015. Vaccine 2018, 36, 5916–5925. [Google Scholar] [CrossRef]
- Johansen, N.D.; Modin, D.; Nealon, J.; Samson, S.; Salamand, C.; Larsen, C.S.; Claggett, B.L.; Solomon, S.D.; Landray, M.J.; Gislason, G.H.; et al. Feasibility of randomizing Danish citizens aged 65–79 years to high-dose quadrivalent influenza vaccine vs. standard-dose quadrivalent influenza vaccine in a pragmatic registry-based setting: Rationale and design of the DANFLU-1 Trial. Pilot Feasibility Stud. 2022, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; York, I.A.; Monto, A.S.; Thompson, M.G.; Fry, A.M. Immune-mediated attenuation of influenza illness after infection: Opportunities and challenges. Lancet Microbe 2021, 2, e715–e725. [Google Scholar] [CrossRef] [PubMed]
- Keilich, S.R.; Bartley, J.M.; Haynes, L. Diminished immune responses with aging predispose older adults to common and uncommon influenza complications. Cell Immunol. 2019, 345, 103992. [Google Scholar] [CrossRef] [PubMed]
- Yedlapati, S.H.; Khan, S.U.; Talluri, S.; Lone, A.N.; Khan, M.S.; Navar, A.M.; Gulati, M.; Johnson, H.; Baum, S.; Michos, E.D. Effects of Influenza Vaccine on Mortality and Cardiovascular Outcomes in Patients With Cardiovascular Disease: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e019636. [Google Scholar] [CrossRef]
- Kalil, A.C.; Thomas, P.G. Influenza virus-related critical illness: Pathophysiology and epidemiology. Crit. Care 2019, 23, 258. [Google Scholar] [CrossRef]
- Sellers, S.A.; Hagan, R.S.; Hayden, F.G.; Fischer Ii, W.A. The hidden burden of influenza: A review of the extra-pulmonary complications of influenza infection. Influenza Other Respir. Viruses 2017, 11, 372–393. [Google Scholar] [CrossRef]
- Bekkat-Berkani, R.; Wilkinson, T.; Buchy, P.; Dos Santos, G.; Stefanidis, D.; Devaster, J.-M.; Meyer, N. Seasonal influenza vaccination in patients with COPD: A systematic literature review. BMC Pulm. Med. 2017, 17, 79. [Google Scholar] [CrossRef]
- Behrouzi, B.; Bhatt, D.L.; Cannon, C.P.; Vardeny, O.; Lee, D.S.; Solomon, S.D.; Udell, J.A. Association of Influenza Vaccination With Cardiovascular Risk: A Meta-analysis. JAMA Netw. Open 2022, 5, e228873. [Google Scholar] [CrossRef]
- Vardeny, O.; Kim, K.; Udell, J.A.; Joseph, J.; Desai, A.S.; Farkouh, M.E.; Hegde, S.M.; Hernandez, A.F.; McGeer, A.; Talbot, H.K.; et al. Effect of High-Dose Trivalent vs. Standard-Dose Quadrivalent Influenza Vaccine on Mortality or Cardiopulmonary Hospitalization in Patients With High-risk Cardiovascular Disease: A Randomized Clinical Trial. JAMA 2021, 325, 39–49. [Google Scholar] [CrossRef]
- Centers for Disease Prevention and Control. 2021–2022 Estimated Flu Illnesses, Medical Visits, Hospitalizations, and Deaths Prevented by Flu Vaccination. Available online: https://www.cdc.gov/flu/about/burden-averted/2021-2022.htm#table1 (accessed on 25 April 2023).
| Variables | Lower Bound | Upper Bound | Distribution | Reference |
|---|---|---|---|---|
| Incidence | 4% | 10% | Uniform | [28] |
| Complications from CVD | 17% | 50% | Beta | [30] |
| Complications from respiratory illness | 9% | 26% | Beta | [30] |
| Probability of death given CVD | 5% | 14% | Beta | [31] |
| Probability of death given respiratory illness | 5% | 14% | Beta | [31] |
| Probability of death given all other diseases | 5% | 14% | Beta | [31] |
| rVE CVD aQIV | 5% | 21% | Normal | [23] |
| VE CVD QIVe | 32% | 48% | Normal | [32] |
| rVE respiratory illness aQIV | 5% | 21% | Normal | [23] |
| VE respiratory illness QIVe | 30% | 50% | Normal | [32] |
| rVE other aQIV | 5% | 21% | Normal | [23] |
| VE other QIVe | 32% | 48% | Normal | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelton, S.I.; Mould-Quevedo, J.F.; Nguyen, V.H. The Impact of Adjuvanted Influenza Vaccine on Disease Severity in the US: A Stochastic Model. Vaccines 2023, 11, 1525. https://doi.org/10.3390/vaccines11101525
Pelton SI, Mould-Quevedo JF, Nguyen VH. The Impact of Adjuvanted Influenza Vaccine on Disease Severity in the US: A Stochastic Model. Vaccines. 2023; 11(10):1525. https://doi.org/10.3390/vaccines11101525
Chicago/Turabian StylePelton, Stephen I., Joaquin F. Mould-Quevedo, and Van Hung Nguyen. 2023. "The Impact of Adjuvanted Influenza Vaccine on Disease Severity in the US: A Stochastic Model" Vaccines 11, no. 10: 1525. https://doi.org/10.3390/vaccines11101525
APA StylePelton, S. I., Mould-Quevedo, J. F., & Nguyen, V. H. (2023). The Impact of Adjuvanted Influenza Vaccine on Disease Severity in the US: A Stochastic Model. Vaccines, 11(10), 1525. https://doi.org/10.3390/vaccines11101525






