Different Safety Pattern of an Inactivated SARS-CoV-2 Vaccine (CoronaVac®) According to Age Group in a Pediatric Population from 3 to 17 Years Old, in an Open-Label Study in Chile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedures
2.3. Outcomes
2.4. Statistical Analyses
3. Results
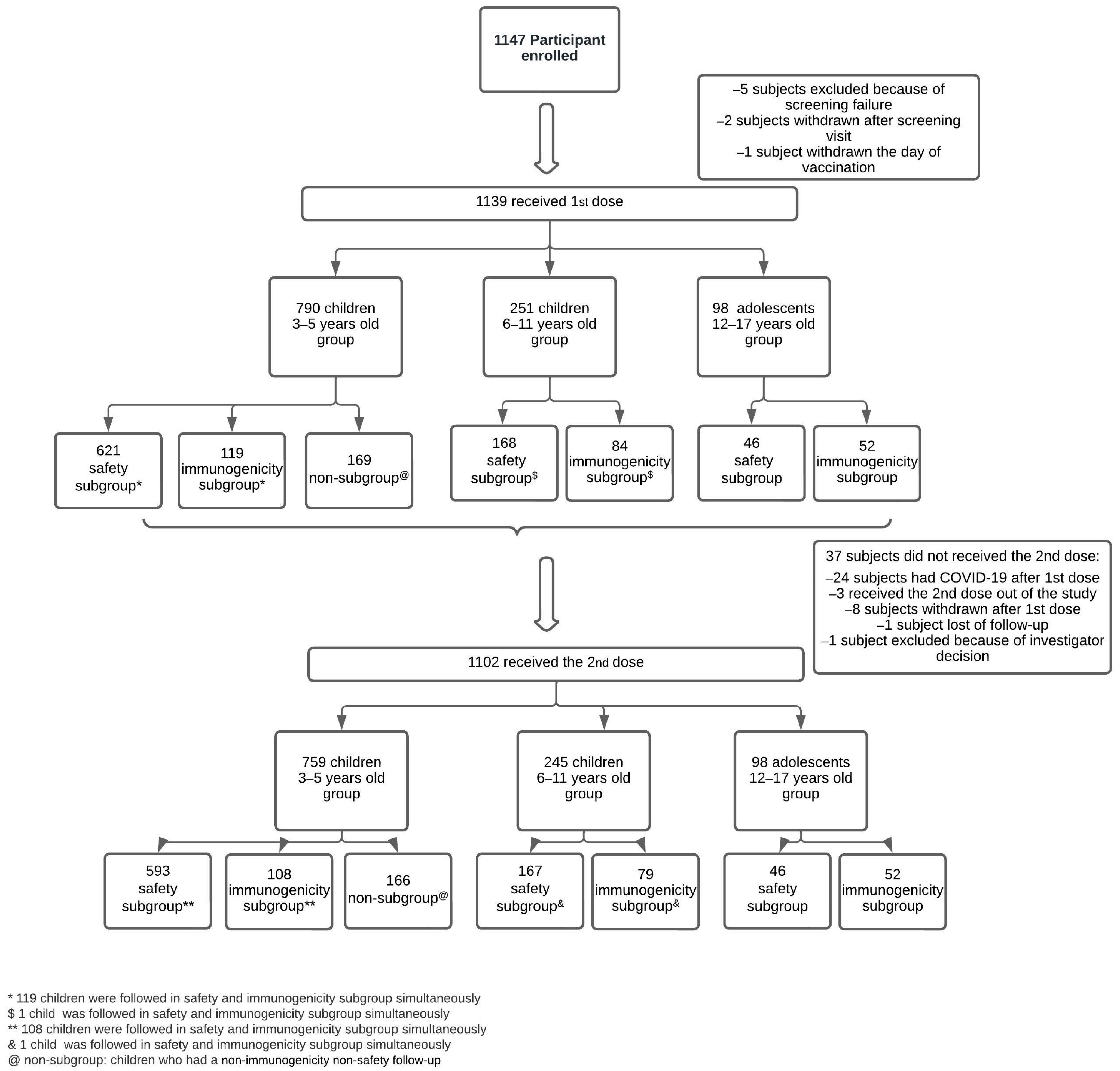
3.1. Study Population
3.2. Safety
3.3. Breakthrough COVID-19 Cases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Weekly Epidemiological Update on COVID-19. 3 August 2022. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---3-august-2022 (accessed on 20 August 2022).
- WHO Coronavirus (COVID-19) Dashboard. 28 December 2020. Available online: https://covid19.who.int/?mapFilter=cases (accessed on 25 August 2023).
- Booth, A.; Reed, A.B.; Ponzo, S.; Yassaee, A.; Aral, M.; Plans, D.; Labrique, A.; Mohan, D. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS ONE 2021, 16, e0247461. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Jakhmola, S.; Indari, O.; Jha, H.C.; Chen, Z.S.; Tripathi, V.; Perez de la Lastra, J.M. Potential Therapeutic Targets and Vaccine Development for SARS-CoV-2/COVID-19 Pandemic Management: A Review on the Recent Update. Front. Immunol. 2021, 12, 658519. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, N.C.; Lopez-Cortes, A.; Gonzalez, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. NPJ Vaccines 2021, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Tanriover, M.D.; Doganay, H.L.; Akova, M.; Guner, H.R.; Azap, A.; Akhan, S.; Kose, S.; Erdinc, F.S.; Akalin, E.H.; Tabak, O.F.; et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021, 398, 213–222. [Google Scholar] [CrossRef]
- Chen, L.; Cai, X.; Zhao, T.; Han, B.; Xie, M.; Cui, J.; Zhang, J.; Wang, C.; Liu, B.; Lu, Q.; et al. Safety of Global SARS-CoV-2 Vaccines, a Meta-Analysis. Vaccines 2022, 10, 596. [Google Scholar] [CrossRef]
- Wu, Q.; Dudley, M.Z.; Chen, X.; Bai, X.; Dong, K.; Zhuang, T.; Salmon, D.; Yu, H. Evaluation of the safety profile of COVID-19 vaccines: A rapid review. BMC Med. 2021, 19, 173. [Google Scholar] [CrossRef]
- Hause, A.M.; Baggs, J.; Marquez, P.; Myers, T.R.; Gee, J.; Su, J.R.; Zhang, B.; Thompson, D.; Shimabukuro, T.T.; Shay, D.K. COVID-19 Vaccine Safety in Children Aged 5–11 Years—United States, November 3–December 19, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1755–1760. [Google Scholar] [CrossRef]
- Schleiss, M.R.; John, C.C.; Permar, S.R. Children are the key to the Endgame: A case for routine pediatric COVID vaccination. Vaccine 2021, 39, 5333–5336. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Kalantari, Y.; Shokri, S.; Fallahpour, M.; Nafissi, N.; Goodarzi, A.; Valizadeh, R. Immunologic response, Efficacy, and Safety of Vaccines against COVID-19 Infection in Healthy and immunosuppressed Children and Adolescents Aged 2–21 years old: A Systematic Review and Meta-analysis. J. Clin. Virol. 2022, 153, 105196. [Google Scholar] [CrossRef] [PubMed]
- Frenck, R.W., Jr.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 COVID-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Song, Y.; Li, C.; Yang, W.; Ma, Q.; Jiang, Z.; Li, M.; Lian, X.; Jiao, W.; Wang, L.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: A double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Munoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 COVID-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Jin, P.; Zhu, T.; Wang, W.; Ye, H.; Pan, H.; Hou, L.; Li, J.; Wang, X.; Wu, S.; et al. Safety and immunogenicity of a recombinant adenovirus type-5-vectored COVID-19 vaccine with a homologous prime-boost regimen in healthy participants aged 6 years and above: A randomised, double-blind, placebo-controlled, phase 2b trial. Clin. Infect. Dis. 2021, 75, e783–e791. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drugs Administration. COVID-19 Vaccines Authorized for Emergency Use or FDA-Approved. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines (accessed on 12 August 2022).
- European Medicines Agency. COVID-19 Vaccines: Authorised. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised (accessed on 12 August 2022).
- Abarca, K.; Iturriaga, C.; Urzua, M.; Le Corre, N.; Pineda, A.; Fernandez, C.; Dominguez, A.; Gonzalez, P.A.; Bueno, S.M.; Donato, P.; et al. Safety and Non-Inferiority Evaluation of Two Immunization Schedules with an Inactivated SARS-CoV-2 Vaccine in Adults: A Randomized Clinical Trial. Vaccines 2022, 10, 1082. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, Y.; Xu, M.; Chen, Z.; Yang, W.; Jiang, Z.; Li, M.; Jin, H.; Cui, G.; Chen, P.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 803–812. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- World Health Organization. Interim Recommendations for Use of the Inactivated COVID-19 Vaccine, CoronaVac, Developed by Sinovac; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Klok, F.A.; Pai, M.; Huisman, M.V.; Makris, M. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2022, 9, e73–e80. [Google Scholar] [CrossRef]
- Ling, R.R.; Ramanathan, K.; Tan, F.L.; Tai, B.C.; Somani, J.; Fisher, D.; MacLaren, G. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: A systematic review and meta-analysis. Lancet Respir. Med. 2022, 10, 679–688. [Google Scholar] [CrossRef]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.A.; Melo-Gonzalez, F.; Gutierrez-Vera, C.; Schultz, B.M.; Berrios-Rojas, R.V.; Rivera-Perez, D.; Pina-Iturbe, A.; Hoppe-Elsholz, G.; Duarte, L.F.; Vazquez, Y.; et al. Inactivated Vaccine-Induced SARS-CoV-2 Variant-Specific Immunity in Children. mBio 2022, 13, e0131122. [Google Scholar] [CrossRef] [PubMed]
- WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964 and Amended by the: 64th WMA General Assembly, Fortaleza, Brazil, October 2013. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 23 January 2022).
- National Medical Products Administration. Guidelines for Adverse Event Classification Standards for Clinical Trials of Preventive Vaccines. Available online: http://english.nmpa.gov.cn/2019-12/31/c_448385.htm (accessed on 22 August 2022).
- World Health Organization; Uppsala Monitoring Center. The Use of the WHO-UMC System for Standardised Case Causality Assessment; The Uppsala Monitoring Centre: Uppsala, Sweden, 2011; Available online: https://www.who-umc.org/media/164200/who-umc-causality-assessment_new-logo.pdf (accessed on 23 January 2022).
- Yang, H.; Li, Z.; Zhang, R.; Guo, S.; Wang, B.; Fang, X.; Zhang, D.; Zhang, X.; Tong, Y.; Wang, Q.; et al. Safety of primary immunization using inactivated SARS-CoV-2 vaccine (CoronaVac®) among population aged 3 years and older in a large-scale use: A multi-center open-label study in China. Vaccine 2023, 41, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Kang, L.Y.; Liu, J.; Liu, M. Immunogenicity, effectiveness, and safety of COVID-19 vaccines among children and adolescents aged 2–18 years: An updated systematic review and meta-analysis. World J. Pediatr. 2023, 1–14. [Google Scholar] [CrossRef]
- Creech, C.B.; Anderson, E.; Berthaud, V.; Yildirim, I.; Atz, A.M.; Melendez Baez, I.; Finkelstein, D.; Pickrell, P.; Kirstein, J.; Yut, C.; et al. Evaluation of mRNA-1273 COVID-19 Vaccine in Children 6 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 2011–2023. [Google Scholar] [CrossRef]
- Wood, N.; Lopez, L.K.; Glover, C.; Leeb, A.; Cashman, P.; Deng, L.; Macartney, K. Short term adverse event profile of COVID-19 mRNA vaccines in children aged 5–15 years in Australia. Lancet Reg. Health West. Pac. 2023, 31, 100684. [Google Scholar] [CrossRef]
- Anderson, E.J.; Creech, C.B.; Berthaud, V.; Piramzadian, A.; Johnson, K.A.; Zervos, M.; Garner, F.; Griffin, C.; Palanpurwala, K.; Turner, M.; et al. Evaluation of mRNA-1273 Vaccine in Children 6 Months to 5 Years of Age. N. Engl. J. Med. 2022, 387, 1673–1687. [Google Scholar] [CrossRef] [PubMed]
- Hause, A.M.; Shay, D.K.; Klein, N.P.; Abara, W.E.; Baggs, J.; Cortese, M.M.; Fireman, B.; Gee, J.; Glanz, J.M.; Goddard, K.; et al. Safety of COVID-19 Vaccination in United States Children Ages 5 to 11 Years. Pediatrics 2022, 150, e2022057313. [Google Scholar] [CrossRef]
- Watanabe, A.; Kani, R.; Iwagami, M.; Takagi, H.; Yasuhara, J.; Kuno, T. Assessment of Efficacy and Safety of mRNA COVID-19 Vaccines in Children Aged 5 to 11 Years: A Systematic Review and Meta-analysis. JAMA Pediatr. 2023, 177, 384–394. [Google Scholar] [CrossRef]
- Witberg, G.; Magen, O.; Hoss, S.; Talmor-Barkan, Y.; Richter, I.; Wiessman, M.; Aviv, Y.; Grinberg, T.; Shiyovich, A.; Schamroth-Pravda, N.; et al. Myocarditis after BNT162b2 Vaccination in Israeli Adolescents. N. Engl. J. Med. 2022, 387, 1816–1817. [Google Scholar] [CrossRef]
- Instituto de Salud Pública, Chile. ESAVI Vacunas SARS-CoV-2. Available online: https://vigilancia.ispch.gob.cl/app/esavi (accessed on 20 October 2022).
- Ministerio de Salud, Chile. Informe Epidemiológico Semana 31 Año 2022. Available online: https://www.minsal.cl/wp-content/uploads/2022/08/Informe_Epidemiolo%CC%81gico-197.pdf (accessed on 20 October 2022).
- Departamento de Epidemiología, Minsal. Situación Epidemiológica Síndrome Inflamatorio Multisistémico Chile, Abril 2022. Available online: http://epi.minsal.cl/wp-content/uploads/2022/04/BOLETIN-SIM-07042022.pdf (accessed on 13 September 2023).
- Buonsenso, D.; Perramon, A.; Catala, M.; Torres, J.P.; Camacho-Moreno, G.; Rojas-Solano, M.; Ulloa-Gutierrez, R.; Camacho-Badilla, K.; Perez-Corrales, C.; Cotugno, N.; et al. Multisystem Inflammatory Syndrome in Children in Western Countries? Decreasing Incidence as the Pandemic Progresses?: An Observational Multicenter International Cross-sectional Study. Pediatr. Infect. Dis. J. 2022, 41, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Jara, A.; Undurraga, E.A.; Flores, J.C.; Zubizarreta, J.R.; González, C.; Pizarro, A.; Ortuño-Borroto, D.; Acevedo, J.; Leo, K.; Paredes, F.; et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Children and Adolescents: A Large-Scale Observational Study. Lancet Reg. Health Am. 2023, 21, 100487. [Google Scholar] [CrossRef] [PubMed]
- Jara, A.; Undurraga, E.A.; Zubizarreta, J.R.; Gonzalez, C.; Acevedo, J.; Pizarro, A.; Vergara, V.; Soto-Marchant, M.; Gilabert, R.; Flores, J.C.; et al. Effectiveness of CoronaVac in children 3 to 5 years during the SARS-CoV-2 Omicron outbreak in Chile. Nat. Med. 2022, 28, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Viner, R.M.; Ward, J.L.; Hudson, L.D.; Ashe, M.; Patel, S.V.; Hargreaves, D.; Whittaker, E. Systematic review of reviews of symptoms and signs of COVID-19 in children and adolescents. Arch. Dis. Child. 2021, 106, 802–807. [Google Scholar] [CrossRef]



| 3–5 Years Old Group n = 790 | 6–11 Years Old Group n = 251 | 12–17 Years Old Group n = 98 | |
|---|---|---|---|
| Age years, mean (SD) | 4.5 (0.8) | 8.7 (1.8) | 13.6 (1.5) |
| Gender female, n (%) | 392 (49.6) | 117(46.6) | 43 (43.9) |
| Ethnicity | |||
| Hispanic, n | 713 | 233 | 94 |
| Chilean native, n | 17 | 11 | 3 |
| White, n | 22 | 4 | 0 |
| Others, n | 38 | 3 | 1 |
| With co-morbidities | |||
| Allergic rhinitis, n | 150 | 31 | 8 |
| Asthma, n | 51 | 8 | 5 |
| Atopic dermatitis, n | 46 | 6 | 1 |
| Obesity, n | 3 | 6 | 5 |
| ENT a, n | 5 | 2 | 0 |
| Mental health b, n | 15 | 11 | 3 |
| Drugs allergy, n | 5 | 1 | 1 |
| Other allergies, n | 18 | 1 | 4 |
| Metabolic–endocrine disorders, n | 8 | 7 | 5 |
| Chromosomopathies c, n | 4 | 1 | 0 |
| Others, n | 77 | 21 | 5 |
| Baseline SARS-CoV-2 positive status, n (%) d | 22 (2.8) | 15 (6) | 3 (3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Corre, N.; Abarca, K.; Astudillo, P.; Potin, M.; López, S.; Goldsack, M.; Valenzuela, V.; Schilling, A.; Gaete, V.; Rubio, L.; et al. Different Safety Pattern of an Inactivated SARS-CoV-2 Vaccine (CoronaVac®) According to Age Group in a Pediatric Population from 3 to 17 Years Old, in an Open-Label Study in Chile. Vaccines 2023, 11, 1526. https://doi.org/10.3390/vaccines11101526
Le Corre N, Abarca K, Astudillo P, Potin M, López S, Goldsack M, Valenzuela V, Schilling A, Gaete V, Rubio L, et al. Different Safety Pattern of an Inactivated SARS-CoV-2 Vaccine (CoronaVac®) According to Age Group in a Pediatric Population from 3 to 17 Years Old, in an Open-Label Study in Chile. Vaccines. 2023; 11(10):1526. https://doi.org/10.3390/vaccines11101526
Chicago/Turabian StyleLe Corre, Nicole, Katia Abarca, Patricio Astudillo, Marcela Potin, Sofía López, Macarena Goldsack, Vania Valenzuela, Andrea Schilling, Victoria Gaete, Lilian Rubio, and et al. 2023. "Different Safety Pattern of an Inactivated SARS-CoV-2 Vaccine (CoronaVac®) According to Age Group in a Pediatric Population from 3 to 17 Years Old, in an Open-Label Study in Chile" Vaccines 11, no. 10: 1526. https://doi.org/10.3390/vaccines11101526
APA StyleLe Corre, N., Abarca, K., Astudillo, P., Potin, M., López, S., Goldsack, M., Valenzuela, V., Schilling, A., Gaete, V., Rubio, L., Calvo, M., Twele, L., González, M., Fuentes, D., Gutiérrez, V., Reyes, F., Tapia, L. I., Villena, R., Retamal-Díaz, A., ... Kalergis, A. M. (2023). Different Safety Pattern of an Inactivated SARS-CoV-2 Vaccine (CoronaVac®) According to Age Group in a Pediatric Population from 3 to 17 Years Old, in an Open-Label Study in Chile. Vaccines, 11(10), 1526. https://doi.org/10.3390/vaccines11101526







