Nanovaccines to Combat Aeromonas hydrophila Infections in Warm-Water Aquaculture: Opportunities and Challenges
Abstract
:1. Introduction
2. Minimizing Aeromonas Infection through Vaccination
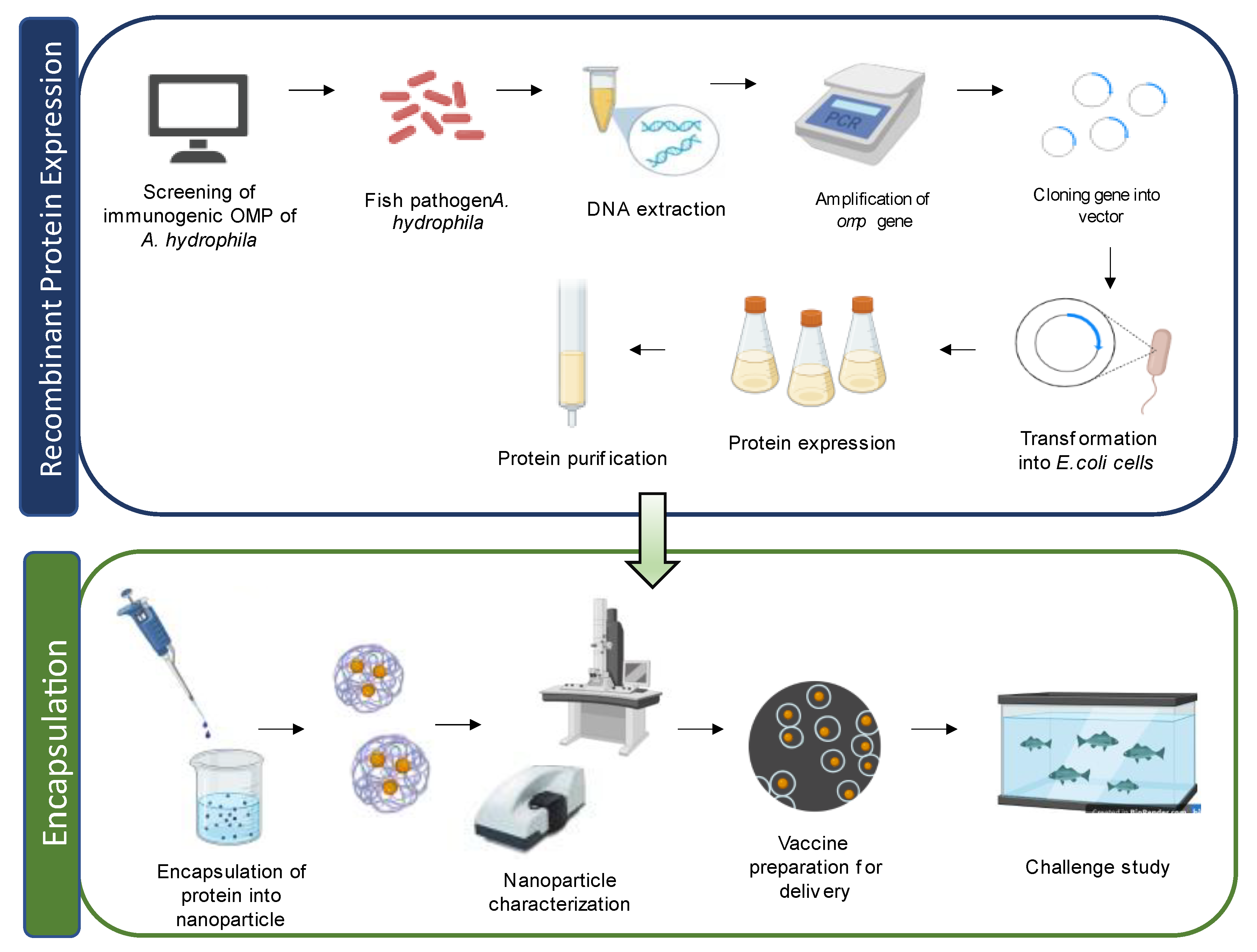
Cellular Components of A. hydrophila Used in Vaccine Development
3. Types of Nanoparticles Used in Fish Vaccine Preparation
3.1. Polymeric Nanoparticles
3.1.1. Synthetic Nanoparticles
3.1.2. Natural Polymers
3.2. Metal Nanoparticles
3.3. Liposomes
3.4. Emulsions
4. Highlights of the Fish Immune System
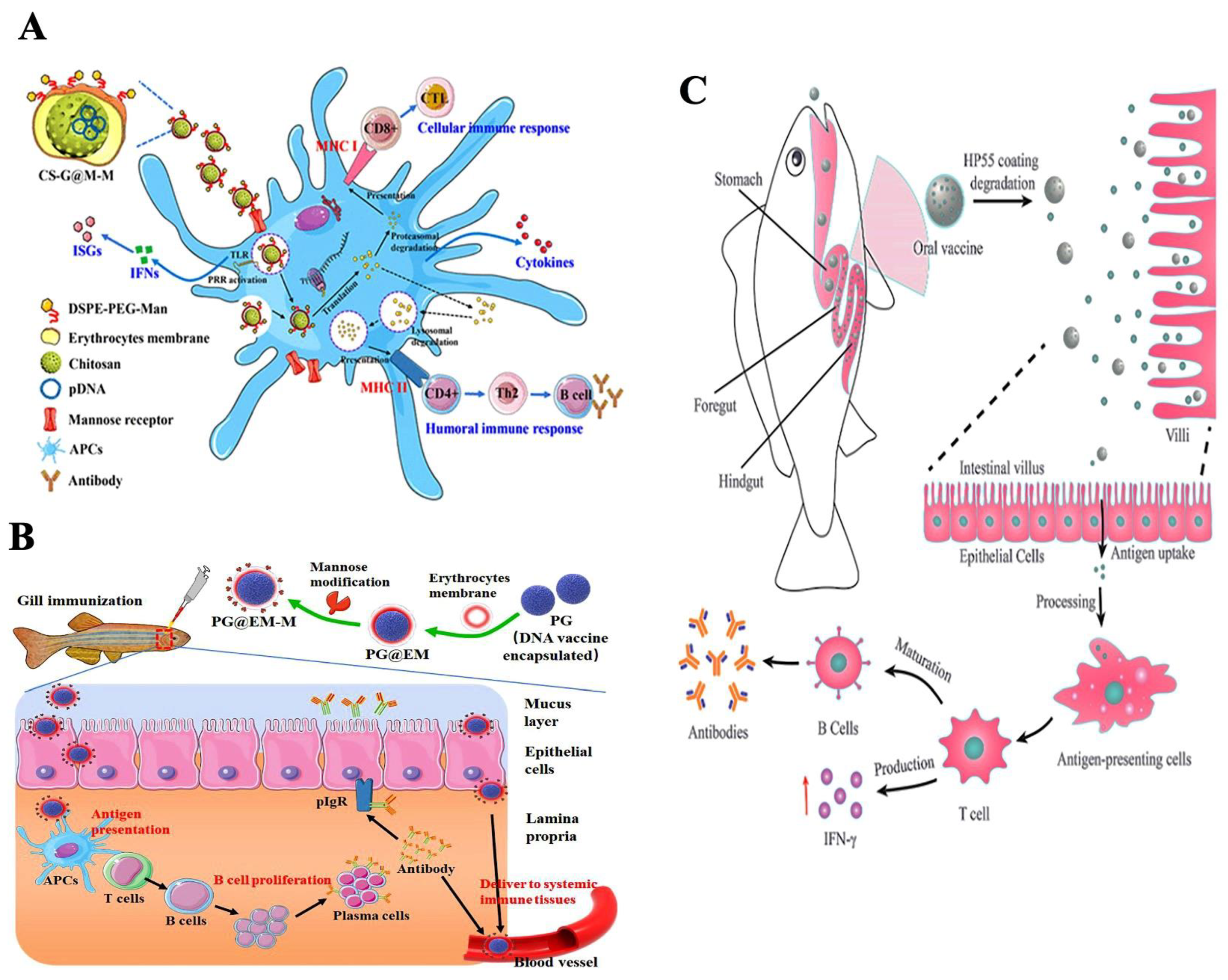
5. Nanoparticle-Mediated Antigen Delivery and Mode of Action
6. Routes of Vaccine Administration
6.1. Parenteral Vaccination
6.1.1. Intramuscular Route
6.1.2. Subcutaneous Route
6.2. Mucosal Vaccination
6.2.1. Oral Vaccination
6.2.2. Immersion Vaccination
6.2.3. Intradermal Vaccination
7. Nanovaccines against Aeromonas Infection in Fish
8. Immunological Memory in Teleost Fish
9. Advantages and Disadvantages of the Application of Nanovaccine
9.1. Advantages
9.2. Disadvantages
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baskaran, V. Nanotechnologies in Aquatic Disease Diagnosis and Drug Delivery. In Nanotechnological Approaches to the Advancement of Innovations in Aquaculture: Nanotechnology in the Life Sciences; Kirthi, A.V., Loganathan, K., Karunasagar, I., Eds.; Springer: Singapore, 2023; pp. 1–21. [Google Scholar]
- Nayak, S.K. Current prospects and challenges in fish vaccine development in India with special reference to Aeromonas hydrophila vaccine. Fish Shellfish Immunol. 2020, 100, 283–299. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture 2022: Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture. In Contributing to Food Security and Nutrition for All; FAO: Rome, Italy, 2016. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture: Towards Blue Transformation; FAO: Rome, Italy, 2017. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2020: Sustainability in Action 2020; FAO: Rome, Italy, 2020. [Google Scholar]
- Nayak, A.; Karunasagar, I.; Chakraborty, A.; Maiti, B. Potential application of bacteriocins for sustainable aquaculture. Rev. Aquac. 2022, 14, 1234–1248. [Google Scholar] [CrossRef]
- Maiti, B.; Dubey, S.; Munang’Andu, H.M.; Karunasagar, I.; Karunasagar, I.; Evensen, Ø. Application of outer membrane protein-based vaccines against major bacterial fish pathogens in India. Front. Immunol. 2020, 11, 1362. [Google Scholar] [CrossRef] [PubMed]
- Pridgeon, J.W.; Klesius, P.H.; Mu, X.; Carter, D.; Fleming, K.; Xu, D.; Srivastava, K.; Reddy, G. Identification of unique DNA sequences present in highly virulent 2009 Alabama isolates of Aeromonas hydrophila. Vet. Microbiol. 2021, 152, 117–125. [Google Scholar] [CrossRef]
- Abdelhamed, H.; Nho, S.W.; Turaga, G.; Banes, M.M.; Karsi, A.; Lawrence, M.L. Protective efficacy of four recombinant fimbrial proteins of virulent Aeromonas hydrophila strain ML09-119 in channel catfish. Vet. Microbiol. 2016, 197, 8–14. [Google Scholar] [CrossRef]
- Rauta, P.R.; Nayak, B. Parenteral immunization of PLA/PLGA nanoparticle encapsulating outer membrane protein (Omp) from Aeromonas hydrophila: Evaluation of immunostimulatory action in Labeo rohita (rohu). Fish Shellfish Immunol. 2015, 44, 287–294. [Google Scholar] [CrossRef]
- Feng, J.; Lin, P.; Guo, S.; Jia, Y.; Wang, Y.; Zadlock, F.; Zhang, Z. Identification and characterization of a novel conserved 46 kD maltoporin of Aeromonas hydrophila as a versatile vaccine candidate in European eel (Anguilla anguilla). Fish Shellfish Immunol. 2017, 64, 93–103. [Google Scholar] [CrossRef]
- Liu, L.; Gong, Y.X.; Liu, G.L.; Zhu, B.; Wang, G.X. Protective immunity of grass carp immunized with DNA vaccine against Aeromonas hydrophila by using carbon nanotubes as a carrier molecule. Front. Immunol. 2016, 55, 516–522. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022: Towards Blue Transformation; FAO: Rome, Italy, 2018. [Google Scholar]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.; Middelberg, A.P. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef]
- Mamo, T.; Poland, G.A. Nanovaccinology: The next generation of vaccines meets 21st-century materials science and engineering. Vaccine 2012, 30, 6609–6611. [Google Scholar] [CrossRef]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of nanotechnology in cosmeceuticals: A review of recent advances. Int. J. Pharm. 2018, 2018, 3420204. [Google Scholar] [CrossRef]
- Vinay, T.N.; Tanmoy, G.C.; Anutosh, P.; Sanjay, K.G.; Biplab, S. Nanovaccines: A possible solution for mass vaccination in aquaculture. World Aquac. 2016, 33. Available online: https://www.was.org/Magazine/Vol/47/3 (accessed on 12 March 2023).
- Maiti, B.; Harshitha, M.; Disha, S.; Badekila, A.K.; Kini, S.; Rai, P. Nanovaccine Nanotechnological approaches to the advancement of innovations in aquaculture. In Nanotechnology in the Life Sciences; Kirthi, A.V., Loganathan, K., Karunasagar, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2023; pp. 37–65. [Google Scholar]
- Kheirollahpour, M.; Mehrabi, M.; Dounighi, N.M.; Mohammadi, M.; Masoudi, A. Nanoparticles and vaccine development. Pharm. Nanotechnol. 2020, 8, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Munang’andu, H.M.; Salinas, I.; Tafalla, C.; Dalmo, R.A. Vaccines and Immunostimulants for finfish. Front. Immunol. 2020, 11, 573771. [Google Scholar] [CrossRef] [PubMed]
- Tafalla, C.; Bøgwald, J.; Dalmo, R.A.; Munang’andu, H.M.; Evensen, Ø. Adjuvants in fish vaccines. In Fish Vaccination; Gudding, R., Lillehaug, A., Evensen, Ø., Eds.; John Wiley & Sons, Ltd: Chichester, UK; pp. 68–84. [CrossRef]
- Khushiramani, R.; Girisha, S.K.; Karunasagar, I.; Karunasagar, I. Cloning and expression of an outer membrane protein ompTS of Aeromonas hydrophila and study of immunogenicity in fish. Protein Expr. Purif. 2007, 51, 303–307. [Google Scholar] [CrossRef]
- Khushiramani, R.; Girisha, S.K.; Karunasagar, I.; Karunasagar, I. Protective efficacy of recombinant OmpTS protein of Aeromonas hydrophila in Indian major carp. Vaccine 2007, 25, 1157–1158. [Google Scholar] [CrossRef]
- Sevaraj, V.; Sampath, K.; Sekar, V. Extraction and characterization of lipopolysaccharide from Aeromonas hydrophila and its effects on survival and hematology of the carp, Cyprinus carpio. Asian Fish. Sci. 2004, 17, 163–173. [Google Scholar]
- Nayak, S.K.; Swain, P.; Nanda, P.K.; Dash, S.; Shukla, S.; Meher, P.K.; Maiti, N.K. Effect of endotoxin on the immunity of Indian major carp, Labeo rohita. Fish Shellfish Immunol. 2008, 24, 394–399. [Google Scholar] [CrossRef]
- Munang’andu, H.M.; Mutoloki, S.; Evensen, Ø. Non-replicating vaccines. In Fish Vaccination; Gudding, R., Lillehaug, A., Evensen, Ø., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2014; pp. 22–32. [Google Scholar]
- Anbarasu, K.; Thangakrishnan, K.; Aruna, B.V.; Chandran, M.R. Assessment of immune response in freshwater catfish Mystus vittatus (Bloch) to different bacterins of Aeromonas hydrophila. J. Exp. Biol. 1998, 36, 990–995. [Google Scholar]
- Karunasagar, I.; Rosalind, G.; Karunasagar, I. Immunological response of the Indian major carps to Aeromonas hydrophila vaccine. J. Fish Dis. 1991, 14, 413–417. [Google Scholar] [CrossRef]
- Yamasaki, M.; Araki, K.; Maruyoshi, K.; Matsumoto, M.; Nakayasu, C.; Moritomo, T.; Yamamoto, A. Comparative analysis of adaptive immune response after vaccine trials using live attenuated and formalin-killed cells of Edwardsiella tarda in ginbuna crucian carp (Carassius auratus langsdorfii). Fish Shellfish Immunol. 2015, 45, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.K.; Shibasaki, Y.; Nakanishi, T. Immune responses to live and inactivated Nocardia seriolae and protective effect of recombinant interferon gamma (rIFN γ) against nocardiosis in ginbuna crucian carp, Carassius auratus langsdorfii. Fish Shellfish Immunol. 2014, 39, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wen, T.; Cao, P.; Shan, L.; Li, L. Alginate-chitosan coated layered double hydroxide nanocomposites for enhanced oral vaccine delivery. J Colloid Interface Sci. 2019, 556, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, H.; Xiao, N.; Liu, Y.; Li, J.; Li, L. Outer membrane protein U (OmpU) mediates adhesion of Vibrio mimicus to host cells via two novel N-terminal motifs. PLoS ONE. 2015, 10, e0119026. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Lai, Y.; Sun, J.; Chen, G.; Yan, A. Transcriptional regulation of the outer membrane porin gene ompW reveals its physiological role during the transition from the aerobic to the anaerobic lifestyle of Escherichia coli. Front. Microbiol. 2016, 7, 799. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pannuri, A.A.; Ni, D.; Zhou, H.; Cao, X.; Lu, X.; Romeo, T.; Huang, Y. Structural basis for translocation of a biofilm-supporting exopolysaccharide across the bacterial outer membrane. J. Biol. Chem. 2016, 291, 10046–10057. [Google Scholar] [CrossRef]
- Rojas, E.R.; Billings, G.; Odermatt, P.D.; Auer, G.K.; Zhu, L.; Miguel, A.; Miguel, A.; Chang, F.; Weibel, D.B.; Theriot, J.A.; et al. The outer membrane is an essential load-bearing element in Gram-negative bacteria. Nature 2018, 559, 617–621. [Google Scholar] [CrossRef]
- Acheson, J.F.; Derewenda, Z.S.; Zimmer, J. Architecture of the cellulose synthase outer membrane channel and its association with the periplasmic TPR domain. Structure 2019, 27, 1855–1861. [Google Scholar] [CrossRef]
- Van der Ley, P.; Struyve, M.; Tommassen, J. Topology of outer membrane pore protein PhoE of Escherichia coli. Identification of cell surface-exposed amino acids with the aid of monoclonal antibodies. J. Biol. Chem. 1986, 261, 12222–12225. [Google Scholar] [CrossRef]
- Cowan, S.W.; Schirmer, T.; Rummel, G.; Steiert, M.; Ghosh, R.; Pauptit, R.A.; Jansonius, J.N.; Rosenbusch, J.P. Crystal structures explain functional properties of two E. coli porins. Nature 1992, 358, 727–733. [Google Scholar]
- Rollauer, S.E.; Sooreshjani, M.A.; Noinaj, N.; Buchanan, S.K. Outer membrane protein biogenesis in Gram-negative bacteria. Philos. Trans. R. Soc. B Biol. 2015, 370, 20150023. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Dash, P.; Sahoo, P.K.; Garg, L.C.; Dixit, A. Modulation of immune response and protective efficacy of recombinant outer-membrane protein F (rOmpF) of Aeromonas hydrophila in Labeo rohita. Fish Shellfish Immunol. 2018, 80, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yang, Z.; Zang, M.; Liu, Y.; Lu, C. Identification of Omp38 by immunoproteomic analysis and evaluation as a potential vaccine antigen against Aeromonas hydrophila in Chinese breams. Fish Shellfish Immunol. 2013, 34, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.; Sahoo, P.K.; Gupta, P.K.; Garg, L.C.; Dixit, A. Immune responses and protective efficacy of recombinant outer membrane protein R (rOmpR)-based vaccine of Aeromonas hydrophila with a modified adjuvant formulation in rohu (Labeo rohita). Fish Shellfish Immunol. 2014, 39, 512–523. [Google Scholar] [CrossRef]
- Mires, D. The Open Access Israeli Journal of Aquaculture–Bamidgeh. Isr. J. Aquac.-Bamidgeh. 2005, 57, 81–89. [Google Scholar]
- Poobalane, S.; Thompson, K.D.; Ardó, L.; Verjan, N.; Han, H.J.; Jeney, G.; Hirono, I.; Aoki, T.; Adams, A. Production and efficacy of an Aeromonas hydrophila recombinant S-layer protein vaccine for fish. Vaccine 2010, 28, 3540–3547. [Google Scholar] [CrossRef]
- Ji, J.; Torrealba, D.; Ruyra, À.; Roher, N. Nano delivery systems as new tools for immunostimulant or vaccine administration: Targeting the fish immune system. Biology 2015, 4, 664–696. [Google Scholar] [CrossRef]
- Maiti, B.; Chakraborty, A.; Karunasagar, I. Biotechnological advances in the development of outer membrane protein-based vaccines for use in aquaculture. In Biotechnological Advances in Aquaculture Health Management; Gupta, S.K., Giri, S.S., Eds.; Nature Singapore Pte Ltd.: Singapore, 2021; pp. 43–59. [Google Scholar]
- Shaalan, M.; Saleh, M.; El-Mahdy, M.; El-Matbouli, M. Recent progress in applications of nanoparticles in fish medicine: A review. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 701–710. [Google Scholar] [CrossRef]
- Badekila, A.K.; Kini, S.; Jaiswal, A.K. Fabrication techniques of biomimetic scaffolds in three-dimensional cell culture: A review. J. Cell. Physiol. 2021, 236, 741–762. [Google Scholar] [CrossRef]
- Akagi, T.; Baba, M.; Akashi, M. Biodegradable Nanoparticles as Vaccine Adjuvants and Delivery Systems: Regulation of Immune Responses by Nanoparticle-Based Vaccine; Springer: Berlin/Heidelberg, Germany, 2012; pp. 31–64. [Google Scholar]
- Dawood, M.A.; Gewaily, M.S.; Soliman, A.A.; Shukry, M.; Amer, A.A.; Younis, E.M.; Fadl, S.E. Marine-derived chitosan nanoparticles improved the intestinal histo-morphometrical features in association with the health and immune response of grey mullet (Liza ramada). Mar. Drugs 2020, 18, 611. [Google Scholar] [CrossRef]
- Chua, B.Y.; Kobaisi, M.; Zeng, W.; Mainwaring, D.; Jackson, D.C. Chitosan microparticles and nanoparticles as biocompatible delivery vehicles for peptide and protein-based immunocontraceptive vaccines. Mol. Pharm. 2012, 9, 81–90. [Google Scholar] [CrossRef]
- Foged, C.; Brodin, B.; Frokjaer, S.; Sundblad, A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int. J. Pharm. 2005, 298, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, P.Q.; Guo, S.; Chen, G.; Zhao, Z.; Wang, G.X.; Zhu, B. Application of biomimetic cell-derived nanoparticles with mannose modification as a novel vaccine delivery platform against teleost fish viral disease. ACS Biomater. Sci. Eng. 2020, 6, 6770–6777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, C.; Xiao, F.; Liu, X.; Xie, A.; Chen, F.; Dong, P.; Lin, P.; Zheng, C.; Zhang, H.; et al. PH-controlled release of antigens using mesoporous silica nanoparticles delivery system for developing a fish oral vaccine. Front. Immunol. 2021, 12, 644396. [Google Scholar] [CrossRef] [PubMed]
- Giddam, A.K.; Zaman, M.; Skwarczynski, M.; Toth, I. Liposome-based delivery system for vaccine candidates: Constructing an effective formulation. Nanomedicine 2012, 7, 1877–1893. [Google Scholar] [CrossRef]
- Aguila, A.; Donachie, A.M.; Peyre, M.; McSharry, C.P.; Sesardic, D.; Mowat, A.M. Induction of protective and mucosal immunity against diphtheria by an immune-stimulating complex (ISCOMS) based vaccine. Vaccine 2006, 24, 5201–5210. [Google Scholar] [CrossRef]
- Kingsman, S.M.; Kingsman, A.J. Polyvalent recombinant antigens: A new vaccine strategy. Vaccine 1988, 6, 304–306. [Google Scholar] [CrossRef]
- Zeltins, A. Construction and characterization of virus-like particles: A review. Mol Biotechnol. 2013, 53, 92–107. [Google Scholar] [CrossRef]
- Pati, R.; Shevtsov, M. Sonawane, A Nanoparticle vaccine against infectious diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef]
- Fajardo, C.; Martinez-Rodriguez, G.; Blasco, J.; Mancera, J.M.; Thomas, B.; De Donato, M. Nanotechnology in aquaculture: Applications, perspectives and regulatory challenges. Aquac. Fish 2022, 7, 185–200. [Google Scholar] [CrossRef]
- Salinas, I. The Mucosal Immune System of Teleost Fish. Biology. 2015, 4, 525–539. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Q.; Huang, Z.; Ding, L.; Xu, Z. Immunoglobulins, mucosal immunity and vaccination in teleost fish. Front. Immunol. 2020, 11, 567941. [Google Scholar] [CrossRef]
- Wu, L.; Qin, Z.; Liu, H.; Lin, L.; Ye, J.; Li, J. Recent advances on phagocytic B cells in teleost fish. Front. Immunol. 2020, 11, 824. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Kong, L.; Yang, Y.; Bian, X.; Wu, S.; Li, B.; Ye, J. Effects of cell differentiation on the phagocytic activities of IgM+ B cells in a teleost fish. Front. Immunol. 2019, 10, 2225. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Evensen, Ø.; Munang’andu, H.M. De Novo Transcriptome Analysis Shows That SAV-3 Infection Upregulates Pattern Recognition Receptors of the Endosomal Toll-Like and RIG-I-Like Receptor Signaling Pathways in Macrophage/Dendritic Like TO-Cells. Viruses 2016, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Evensen, Ø.; Munang’andu, H.M. De novo assembly and transcriptome analysis of Atlantic salmon macrophage/dendritic-like TO cells following type I IFN treatment and Salmonid alphavirus subtype-3 infection. BMC Genom. 2015, 16, 96. [Google Scholar] [CrossRef] [PubMed]
- Rauta, P.R. Immunological Evaluation of Biodegradable Particle-Based Nanoparticles Encapsulating OMP Antigen as Potential Vaccine Candidate. Ph.D. Thesis, Department of Life Science, National Institute of Technology, Rourkela, India, 2016. [Google Scholar]
- Shen, H.; Ackerman, A.L.; Cody, V.; Giodini, A.; Hinson, E.R.; Cresswell, P.; Edelson, R.L.; Saltzman, W.M.; Hanlon, D.J. Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology 2006, 117, 78–88. [Google Scholar] [CrossRef]
- Ballesteros, N.A.; Castro, R.; Abos, B.; Rodríguez Saint-Jean, S.S.; Pérez-Prieto, S.I.; Tafalla, C. The pyloric caeca area is a major site for IgM+ and IgT+ B cell recruitment in response to oral vaccination in rainbow trout. PLoS ONE 2013, 8, e66118. [Google Scholar] [CrossRef]
- Najafi-Hajivar, S.; Zakeri-Milani, P.; Mohammadi, H.; Niazi, M.; Soleymani-Goloujeh, M.; Baradaran, B.; Valizadeh, H. Overview on experimental models of interactions between nanoparticles and the immune system. Biomed. Pharmacother. 2016, 83, 1365–1378. [Google Scholar] [CrossRef]
- Fifis, T.; Mottram, P.; Bogdanoska, V.; Hanley, J.; Plebanski, M. Short peptide sequences containing MHC class I and/or class II epitopes linked to nano-beads induce strong immunity and inhibition of growth of antigen-specific tumour challenge in mice. Vaccine 2004, 23, 258–266. [Google Scholar] [CrossRef]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef]
- Mody, K.T.; Popat, A.; Mahony, D.; Cavallaro, A.S.; Yu, C.; Mitter, N. Mesoporous silica nanoparticles as antigen carriers and adjuvants for vaccine delivery. Nanoscale 2013, 5, 5167–5179. [Google Scholar] [CrossRef]
- Wendorf, J.; Singh, M.; Chesko, J. A practical approach to the use of nanoparticles for vaccine delivery. J Pharm. Sci. 2006, 95, 2738–2750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Guo, S.; Chen, G.; Zhao, Z.; Wang, G.X.; Zhu, B. Mucosal delivery of mannose functionalized biomimetic nanoparticles via the branchial route induces robust mucosal and systemic immune responses against fish viral disease. Aquaculture 2022, 546, 737329. [Google Scholar] [CrossRef]
- Adams, A.; Aoki, T.; Berthe, C.; Grisez, L.; Karunasagar, I. Recent technological advancements on aquatic animal health and their contributions toward reducing disease risks review. In Diseases in Asian Aquaculture VI. Fish Health Section; Bondad-Reantaso, M.G., Mohan, C.V., Crumlish, Margaret, Subasinghe, R.P., Eds.; Asian Fisheries Society: Manila, Philippines, 2012; pp. 71–88. [Google Scholar]
- Palm, R.C., Jr.; Landolt, M.L.; Busch, R.A. Route of vaccine administration: Effects on the specific humoral response in rainbow trout Oncorhynchus mykiss. Dis. Aquat. Org. 1998, 33, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Yanong, R.P.; Erlacher-Reid, C. Biosecurity in aquaculture, part 1: An overview. SRAC Publ. 2012, 4707, 522. [Google Scholar]
- Raghuvanshi, R.S.; Katare, Y.K.; Lalwani, K.; Ali, M.M.; Singh, O.; Panda, A.K. Improved immune response from biodegradable polymer particles entrapping tetanus toxoid by use of different immunization protocol and adjuvants. Int. J. Pharm. 2002, 245, 109–121. [Google Scholar] [CrossRef]
- He, L.; Wu, L.; Lin, P.; Zhai, S.; Guo, S.; Xiao, Y.; Wan, Q. First expression and immunogenicity study of a novel trivalent outer membrane protein (OmpII-UA) from Aeromonas hydrophila, Vibrio vulnificus and Edwardsiella anguillarum. Aquaculture 2020, 519, 734932. [Google Scholar] [CrossRef]
- Guo, S.; He, L.; Wu, L.; Xiao, Y.; Zhai, S.; Yan, Q. Immunization of a novel bivalent outer membrane protein simultaneously resisting Aeromonas hydrophila, Edwardsiella anguillarum and Vibrio vulnificus infection in European eels (Anguilla anguilla). Fish Shellfish Immunol. 2020, 97, 46–57. [Google Scholar] [CrossRef]
- Guo, Z.; Lin, Y.; Wang, X.; Fu, Y.; Lin, W.; Lin, X. The protective efficacy of four iron related recombinant proteins and their single-walled carbon nanotube encapsulated counterparts against Aeromonas hydrophila infection in zebrafish. Fish Shellfish Immunol. 2018, 82, 50–59. [Google Scholar] [CrossRef]
- Han, B.; Xu, K.; Liu, Z.; Ge, W.; Shao, S.; Li, P.; Yan, N.; Li, X.; Zhang, Z. Oral yeast-based DNA vaccine confers effective protection from Aeromonas hydrophila infection on Carassius auratus. Fish Shellfish Immunol. 2019, 84, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamed, H.; Ibrahim, I.; Nho, S.W.; Banes, M.M.; Wills, R.W.; Karsi, A.; Lawrence, M.L. Evaluation of three recombinant outer membrane proteins, OmpA1, Tdr, and TbpA, as potential vaccine antigens against virulent Aeromonas hydrophila infection in channel catfish (Ictalurus punctatus). Fish Shellfish Immunol. 2017, 66, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.; Yadav, S.K.; Garg, L.C.; Dixit, A.; Sahoo, P.K. Post-challenge immune geneexpression profiling in rohu, Labeo rohita vaccinated with modified adjuvant-based Aeromonas hydrophila outer membrane protein R formulation. Vet. Arh. 2017, 87, 607–622. [Google Scholar] [CrossRef]
- Fu, X.; Lin, Q.; Liu, L.; Liang, H.; Huang, Z.; Li, N. Display of ISKNV orf086 protein on the surface of Aeromonas hydrophila and its immunogenicity in Chinese perch (Siniperca chuatsi). Fish Shellfish Immunol. 2016, 56, 286–293. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, C.; Zhao, Y.; Kong, X.; Pei, C.; Li, L.; Nie, G.; Li, X. Immune effects of the vaccine of live attenuated Aeromonas hydrophila screened by rifampicin on common carp (Cyprinus carpio L). Vaccine. 2016, 34, 3087–3092. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.X.; Zhu, B.; Liu, G.L.; Liu, L.; Ling, F.; Wang, G.X.; Xu, X.G. Single-walled carbon nanotubes as delivery vehicles enhance the immunoprotective effects of a recombinant vaccine against Aeromonas hydrophila. Fish Shellfish Immunol. 2015, 42, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Avadhani, K.; Mutalik, S.; Sivadasan, S.M.; Maiti, B.; Paul, J.; Girisha, S.K.; Venugopal, M.N.; Mutoloki, S.; Evensen, Ø.; et al. Aeromonas hydrophila OmpW PLGA nanoparticle oral vaccine shows a dose-dependent protective immunity in rohu (Labeo rohita). Vaccines 2016, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Khushiramani, R.M.; Maiti, B.; Shekar, M.; Girisha, S.K.; Akash, N.; Deepanjali, A.; Karunasagar, I.; Karunasagar, I. Recombinant Aeromonas hydrophila outer membrane protein 48 (Omp48) induces a protective immune response against Aeromonas hydrophila and Edwardsiella tarda. Int. J. Microbiol. Res. 2012, 163, 286–291. [Google Scholar] [CrossRef]
- Maiti, B.; Shetty, M.; Shekar, M.; Karunasagar, I.; Karunasagar, I. Evaluation of two outer membrane proteins, Aha1 and OmpW of Aeromonas hydrophila as vaccine candidate for common carp. Vet. Immunol. Immunopathol. 2012, 149, 298–301. [Google Scholar] [CrossRef]
- Dehghani, S.; Akhlaghi, M.; Dehghani, M. Efficacy of formalin-killed, heat-killed and lipopolysaccharide vaccines against motile aeromonads infection in rainbow trout (Oncorhynchus mykiss). Vet. World. 2012, 9, 409–415. [Google Scholar]
- Behera, T.; Nanda, P.K.; Mohanty, C.; Mohapatra, D.; Swain, P.; Das, B.K.; Routray, P.; Mishra, B.K.; Sahoo, S.K. Parenteral immunization of fish, Labeo rohita with Poly D, L-lactide-co-glycolic acid (PLGA) encapsulated antigen microparticles promotes innate and adaptive immune responses. Fish Shellfish Immunol. 2010, 28, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Vivas, J.; Riaño, J.; Carracedo, B.; Razquin, B.E.; López-Fierro, P.; Naharro, G.; Villena, A.J. The auxotrophic aroA mutant of Aeromonas hydrophila as a live attenuated vaccine against A. salmonicida infections in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2004, 16, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.M.; Ge, R.; Sin, Y.M. Cloning, characterization and expression of Aeromonas hydrophila major adhesin. Fish Shellfish Immunol. 2004, 16, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Kawai, K. Outer membrane proteins of Aeromonas hydrophila induce protective immunity in goldfish. Fish Shellfish Immunol. 2000, 10, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Aravena, A.; Sandino, A.M.; Spencer, E. Nanoparticles and microparticles of polymers and polysaccharides to administer fish vaccines. Biol. Res. 2013, 46, 407–419. [Google Scholar] [CrossRef]
- Leleux, J.; Roy, K. Micro and nanoparticle-based delivery systems for vaccine immunotherapy: An immunological and materials perspective. Adv. Healthc. Mater. 2013, 2, 72–94. [Google Scholar] [CrossRef]
- Combadière, B.; Mahé, B. Particle-based vaccines for transcutaneous vaccination. Comp. Immunol. Microbiol. Infect. Dis. 2008, 31, 293–315. [Google Scholar] [CrossRef]
- Mitchell, H. Choosing a furunculosis vaccine: Points to consider. Bull. Aquac. Assoc. Can. 1995, 95, 30–33. [Google Scholar]
- Horne, M.T. Technical aspects of the administration of vaccines. Biologicals 1997, 90, 79–89. [Google Scholar]
- Tissot, A.C.; Maurer, P.; Nussberger, J.; Sabat, R.; Pfister, T.; Ignatenko, S.; Volk, H.D.; Stocker, H.; Müller, P.; Jennings, G.T.; et al. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: A double-blind, randomised, placebo-controlled phase IIa study. Lancet 2008, 371, 821–827. [Google Scholar] [CrossRef]
- Correia-Pinto, J.F.; Csaba, N.; Alonso, M.J. Vaccine delivery carriers: Insights and future perspectives. Int. J. Pharm. 2013, 440, 27–38. [Google Scholar] [CrossRef]
- Caruthers, S.D.; Wickline, S.A.; Lanza, G.M. Nanotechnological applications in medicine. Curr. Opin. 2007, 18, 26–30. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and prospects. FASEB J. 2005, 19, 311–333. [Google Scholar] [CrossRef] [PubMed]
- Treuel, L.; Jiang, X.; Nienhaus, G.U. New views on cellular uptake and trafficking of manufactured nanoparticles. J. R. Soc. Interface 2013, 10, 20120939. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Mumper, R.J. Microparticles and nanoparticles as delivery systems for DNA vaccines. J. Crit. Rev. 2003, 20, 103–137. [Google Scholar] [CrossRef]
- Mishra, D.; Mishra, H.; Mishra, P.K.; Nahar, M.; Dubey, V.; Jain, N.K. Evaluation of solid lipid nanoparticles as carriers for delivery of hepatitis B surface antigen for vaccination using the subcutaneous route. J. Pharm. Pharm. Sci. 2010, 13, 495–509. [Google Scholar] [CrossRef]
- Prabhu, P.; Patravale, V. Potential of nanocarriers in antigen delivery: The path to successful vaccine delivery. Nanocarriers 2014, 1, 10–45. [Google Scholar]
- Quentel, C.; Vigneulle, M. Antigen uptake, and immune responses after oral vaccination. Biologicals 1997, 90, 69–78. [Google Scholar]
- Ballesteros, N.A.; Saint-Jean, S.R.; Perez-Prieto, S.I. Food pellets as an effective delivery method for a DNA vaccine against infectious pancreatic necrosis virus in rainbow trout (Oncorhynchus mykiss, Walbaum). Fish Shellfish Immunol. 2014, 37, 220–228. [Google Scholar] [CrossRef]
- Mutoloki, S.; Munang’andu, H.M.; Evensen, Ø. Oral vaccination of fish–antigen preparations, uptake, and immune induction. Front. Immunol. 2015, 6, 519. [Google Scholar] [CrossRef] [PubMed]
- Plummer, E.M.; Manchester, M. Viral nanoparticles and virus-like particles: Platforms for contemporary vaccine design. Wiley Interdiscip. Rev. Nanomed. Nanotechnol. Biol. Med. 2011, 3, 174–196. [Google Scholar] [CrossRef]
- Tatner, M.F.; Horne, M.T. Factors influencing the uptake of 14C-labelled Vibrio anguillarum vaccine in direct immersion experiments with rainbow trout, Salmo gairdneri Richardson. J. Fish Biol. 1983, 22, 585–591. [Google Scholar] [CrossRef]
- Anderson, D.P.; Roberson, B.S.; Dixon, O.W. Plaque-forming cells and humoral antibody in rainbow trout (Salmo gairdneri) induced by immersion in a Yersinia ruckeri O-antigen preparation. Fish Res. Board. Can. 1979, 36, 636–639. [Google Scholar] [CrossRef]
- Nakanishi, T.; Kiryu, I.; Ototake, M. Development of a new vaccine delivery method for fish: Percutaneous administration by immersion with the application of a multiple puncture instrument. Vaccine 2002, 20, 3764–3769. [Google Scholar] [CrossRef] [PubMed]
- Navot, N.; Kimmel, E.; Avtalion, R.R. Enhancement of antigen uptake and antibody production in goldfish (Carassius auratus) following bath immunization and ultrasound treatment. Vaccine 2004, 22, 2660–2666. [Google Scholar] [CrossRef]
- Bal, S.M.; Slütter, B.; van Riet, E.; Kruithof, A.C.; Ding, Z.; Kersten, G.F.; Jiskoot, W.; Bouwstra, J.A. Efficient induction of immune responses through intradermal vaccination with N-trimethyl chitosan containing antigen formulations. J. Control. Release 2010, 142, 374–383. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Vaseeharan, B.; Malaikozhundan, B.; Gobi, N.; Ravichandran, S.; Karthi, S.; Ashokkumar, B.; Sivakumar, N. A novel antimicrobial therapy for the control of Aeromonas hydrophila infection in aquaculture using marine polysaccharide coated gold nanoparticle. Microb. Pathog. 2017, 110, 140–151. [Google Scholar] [CrossRef]
- Angulo, C.; Tello-Olea, M.; Reyes-Becerril, M.; Monreal-Escalante, E.; Hernández-Adame, L.; Angulo, M.; Mazon-Suastegui, J.M. Developing oral nanovaccines for fish: A modern trend to fight infectious diseases. Rev Aquac. 2021, 3, 1172–1192. [Google Scholar] [CrossRef]
- Nagaraju, V.T. Nanovaccines in aquaculture. Arch. Nanomed. 2019, 2, 153–158. [Google Scholar]
- Dong, C.F.; Lin, T.L.; Gong, H.; Ou-Yang, S.D.; Yang, S. Major outer membrane protein (momp) of Aeromonas hydrophila induced protective immunity to European eels (Anguilla anguilla). Acta Hydrobiol. Sin. 2005, 29, 285–290. [Google Scholar]
- Behera, T.; Swain, P. Antigen adsorbed calcium phosphate nanoparticles stimulate both innate and adaptive immune response in fish, Labeo rohita. Cell. Immunol. 2011, 271, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Munang’andu, H.M.; Evensen, Ø. Correlates of protective immunity for fish vaccines. Fish Shellfish Immunol. 2019, 85, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Immunologic correlates of protection induced by vaccination. J. Pediatr. Infect. Dis. 2001, 20, 63–75. [Google Scholar] [CrossRef]
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Bricknell, I.R.; King, J.A.; Bowden, T.J.; Ellis, A.E. Duration of protective antibodies, and the correlation with protection in Atlantic salmon (Salmo salar L), following vaccination with an Aeromonas salmonicida vaccine containing iron-regulated outer membrane proteins and secretory polysaccharide. Fish Shellfish Immunol. 1999, 9, 139–151. [Google Scholar] [CrossRef]
- Pasnik, D.J.; Evans, J.J.; Klesius, P.H. Duration of protective antibodies and correlation with survival in Nile tilapia Oreochromis niloticus following Streptococcus agalactiae vaccination. Dis. Aquat. Org. 2005, 66, 129–134. [Google Scholar] [CrossRef]
- Munang’andu, H.M.; Fredriksen, B.N.; Mutoloki, S.; Dalmo, R.A.; Evensen, Ø. Antigen dose and humoral immune response correspond with protection for inactivated infectious pancreatic necrosis virus vaccines in Atlantic salmon (Salmo salar L.). Vet. Res. 2013, 44, 1–16. [Google Scholar] [CrossRef]
- Huang, H.Y.; Chen, Y.C.; Wang, P.C.; Tsai, M.A.; Yeh, S.C.; Liang, H.J.; Chen, S.C. Efficacy of a formalin-inactivated vaccine against Streptococcus iniae infection in the farmed grouper Epinephelus coioides by intraperitoneal immunization. Vaccine 2014, 32, 7014–7020. [Google Scholar] [CrossRef]
- Tobar, I.; Arancibia, S.; Torres, C.; Vera, V.; Soto, P.; Carrasco, C.; Tobar, J.A. Successive oral immunizations against Piscirickettsia salmonis and infectious salmon anemia virus are required to maintain a long-term protection in farmed salmonids. Front. Immunol. 2015, 6, 244. [Google Scholar] [CrossRef]
- Ma, C.; Ye, J.; Kaattari, S.L. Differential compartmentalization of memory B cells versus plasma cells in salmonid fish. Eur. J. Immunol. 2013, 43, 360–370. [Google Scholar] [CrossRef]
- Kaattari, S.; Bromage, E.; Kaattari, I. Analysis of long-lived plasma cell production and regulation: Implications for vaccine design for aquaculture. Aquaculture 2005, 246, 1–9. [Google Scholar] [CrossRef]
- Karunasagar, I.; Karunasagar, I. Effect of thymectomy on the humoral immune response of Labeo rohita against Aeromonas hydrophila vaccine. J. Aquacult. Trop. 1996, 11, 79–82. [Google Scholar]
- Miller, N.W.; Sizemore, R.C.; Clem, L.W. Phylogeny of lymphocyte heterogeneity: The cellular requirements for in vitro antibody responses of channel catfish leukocytes. J. Immunol. Res. 1985, 134, 2884–2888. [Google Scholar] [CrossRef]
- Avtalion, R.R.; Wishkovsky, A.; Katz, D. Regulatory effects of temperature on specific suppression and enhancement of the humoral response in fish. In Phylogeny of Immunological Memory; Manning, M.J., Ed.; Elsevier/North-Holland Biomedical Press: Amsterdam, The Netherlands, 1980; pp. 113–121. [Google Scholar]
- Stosik, M.; Tokarz-Deptuła, B.; Deptuła, W. Immunological memory in teleost fish. Fish Shellfish Immunol. 2021, 115, 95–103. [Google Scholar] [CrossRef]
- Magadan, S.; Jouneau, L.; Puelma Touzel, M.; Marillet, S.; Chara, W.; Six, A.; Quillet, E.; Mora, T.; Walczak, A.M.; Boudinot, P. Origin of public memory B cell clones in fish after antiviral vaccination. Front. Immunol. 2018, 9, 2115. [Google Scholar] [CrossRef]
- Dadar, M.; Dhama, K.; Vakharia, V.N.; Hoseinifar, S.H.; Karthik, K.; Tiwari, R.; Khandia, R.; Munjal, A.; Salgado-Miranda, C.; Joshi, S.K. Advances in aquaculture vaccines against fish pathogens: Global status and current trends. Rev. Fish. Sci. Aquac. 2017, 25, 184–217. [Google Scholar] [CrossRef]
- Saroja, C.H.; Lakshmi, P.K.; Bhaskaran, S. Recent trends in vaccine delivery systems: A review. Int. J. Pharm. Investig. 2011, 1, 64. [Google Scholar] [PubMed]
- Jovanović, B.; Palić, D. Immunotoxicology of non-functionalized engineered nanoparticles in aquatic organisms with special emphasis on fish—Review of current knowledge, gap identification, and call for further research. Aquat Toxicol. 2012, 118, 141–151. [Google Scholar] [CrossRef]


| Antigenic Entity | Model Fish Species | Country | Administration Route | Reported Efficiency in RPS | Reference |
|---|---|---|---|---|---|
| OmpII-U-A | Eel | China | IP | 66.7 | [81] |
| OMP | European eel | China | IP | 83.4 | [82] |
| Iron-related recombinant protein | Zebrafish | China | IP | 66.67 | [83] |
| Oral yeast-based DNA vaccine (OmpG) | Goldfish | China | IP | 46.7 | [84] |
| OmpF | Rohu | India | IP | - | [41] |
| Tdr | Catfish | USA | IP | 95.59 | [85] |
| Tbpa | Catfish | USA | IP | 47.89 | [85] |
| OmpR | Rohu | India | IP | - | [86] |
| Maltoporin | European eel | China | IP | 75 | [12] |
| ISKNC ORF 086 protein + OmpA | Chinese perch | China | IP | 73.35 | [87] |
| Live-attenuated vaccine | Carp | China | IP | 83.7 | [88] |
| DNA vaccine + carbon nanotubes (aerA gene) | Grass carp | China | IM | - | [89] |
| OmpW + PLGA | Rohu | India | Oral | 79.9 | [90] |
| OMP fimA | Catfish | USA | IP | 59.83 | [10] |
| Fim | Catfish | USA | IP | 95.41 | [10] |
| MrfG | Catfish | USA | IP | 85.72 | [10] |
| Fimomp | Catfish | USA | IP | 75.01 | [10] |
| OMP + PLGA | Rohu | India | IP | - | [11] |
| OmpR | Rohu | India | IP | 52 | [43] |
| Omp38 | Chines breams | China | IP | 57 | [42] |
| Omp48 | Rohu | India | IM | 69 | [91] |
| OMP Aha1 | Common carp | India | IP | 52 | [92] |
| OmpW | Common carp | India | IP | 71 | [93] |
| FK, Vaccine | Rainbow trout | Iran | IP | 67 | [94] |
| HK | Rainbow trout | Iran | IP | 84 | [94] |
| LPS | Rainbow trout | Iran | IP | 34 | [94] |
| LPS | Grass carp | China | IP | 83.35 | [44] |
| OMP | Grass carp | China | IP | 72.2 | [44] |
| FK | Grass carp | China | IP | 55.6 | [44] |
| S-layer vaccine | Common carp | Japan | IP | - | [45] |
| OMP + PLGA | Rohu | India | IP | - | [95] |
| OmpTS | Rohu | India | IP | - | [24] |
| OMP | Goldfish | India | IP | 50 | [24] |
| Aero A live vaccine | Rainbow trout | Spain | IP | - | [96] |
| LPS | Carp | India | IP | - | [25] |
| Major adhesion (Aha1) | Carp | Singapore | IP | 87.5 | [97] |
| OMP | Goldfish | Japan | IP | - | [98] |
| Different Types of Nanoparticles | Advantage | Disadvantage | References |
|---|---|---|---|
| (1) Polymeric nanoparticle (a) Synthetic nanoparticle (b) Natural nanoparticle |
|
| [15,18,19,20,54,122,123] |
| (2) Inorganic nanoparticle |
|
| [19,123] |
| (3) Virus-like particle |
|
| [18,123] |
| (4) ISCOMs |
|
| [18,123] |
| (5) Emulsion |
|
| [18] |
| Antigenic Entity | Pathogen | Adjuvant/Carrier System | Fish Model | Route of Administration | RPS | Reference |
|---|---|---|---|---|---|---|
| Recombinant protein | A. hydrophila | SWCNT | Danio rerio | IP | 94 | [83] |
| DNA vaccine(aerA) | A. hydrophila | SWCNT | Ctenopharyngodon idella | IM | 83 | [13] |
| rOmpW | A. hydrophila | PLGA | Labeo rohita | Oral | 79.9 | [90] |
| rOmp | A. hydrophila | PLGA PLA | Labeo rohita | IP | 80 75 | [11] |
| MOMP | A. hydrophila | ISCOMs | Anguillia anguillia | IP | 80 | [124] |
| S-layer protein | A. hydrophila | Calcium phosphate | Labeo rohita | IP | 100 | [125] |
| aerA | A. hydrophila | OCMCS-hyaluronic acid | Cyprinous carpio | Oral | ND | [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harshitha, M.; Nayak, A.; Disha, S.; Akshath, U.S.; Dubey, S.; Munang’andu, H.M.; Chakraborty, A.; Karunasagar, I.; Maiti, B. Nanovaccines to Combat Aeromonas hydrophila Infections in Warm-Water Aquaculture: Opportunities and Challenges. Vaccines 2023, 11, 1555. https://doi.org/10.3390/vaccines11101555
Harshitha M, Nayak A, Disha S, Akshath US, Dubey S, Munang’andu HM, Chakraborty A, Karunasagar I, Maiti B. Nanovaccines to Combat Aeromonas hydrophila Infections in Warm-Water Aquaculture: Opportunities and Challenges. Vaccines. 2023; 11(10):1555. https://doi.org/10.3390/vaccines11101555
Chicago/Turabian StyleHarshitha, Mave, Ashwath Nayak, Somanath Disha, Uchangi Satyaprasad Akshath, Saurabh Dubey, Hetron Mweemba Munang’andu, Anirban Chakraborty, Indrani Karunasagar, and Biswajit Maiti. 2023. "Nanovaccines to Combat Aeromonas hydrophila Infections in Warm-Water Aquaculture: Opportunities and Challenges" Vaccines 11, no. 10: 1555. https://doi.org/10.3390/vaccines11101555






