Stakeholders’ Understanding of European Medicine Agency’s COVID-19 Vaccine Information Materials in EU and Regional Contexts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample
2.3. Survey
2.4. Data Analysis
2.5. Personal Data Protection Statement
3. Results
3.1. Survey Participant Profiling
3.2. Preferred Information Sources for Patients, Consumers, Healthcare Professionals, and Organizations at EU and Regional Levels
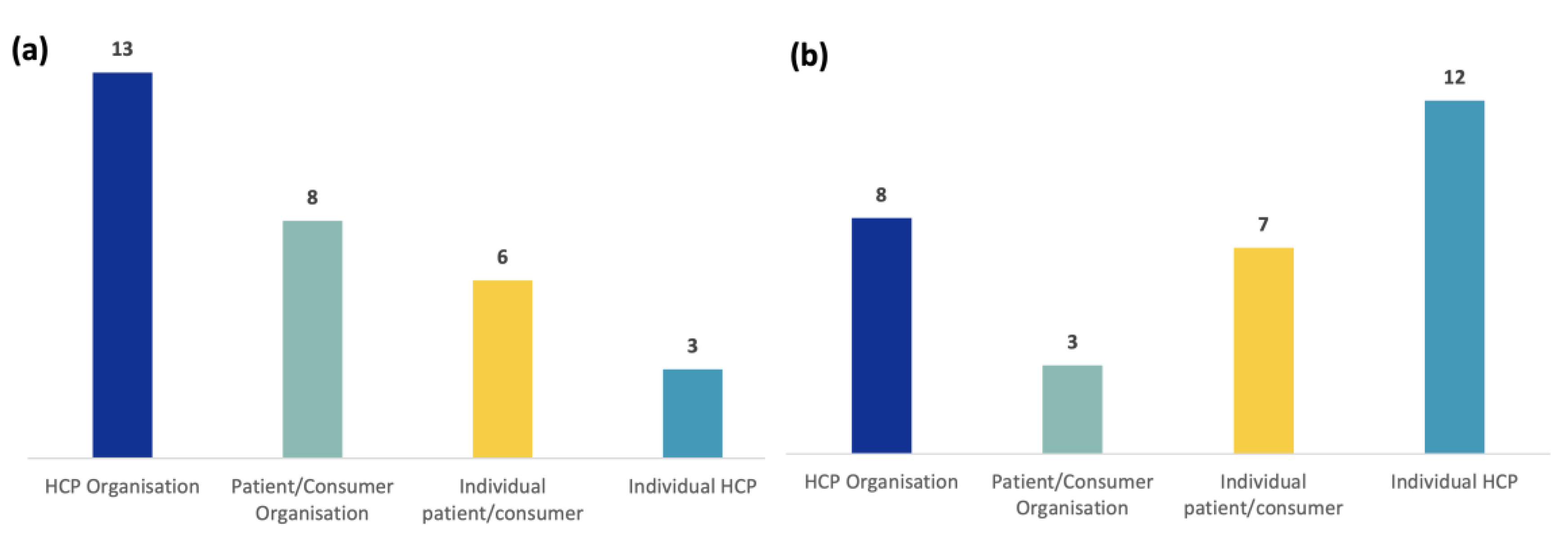
3.2.1. Individual Patients/Consumers and PCOs (Figure 3a)
3.2.2. Individual HCPs and HCPOs (Figure 3b)
3.3. The Understanding of Key Messages by Stakeholders at EU or Regional Levels
3.4. Participants’ Feedback on Usefulness, Language, and Level of Detail
3.4.1. Feedback on Tier 1
3.4.2. Feedback on Tier 2
3.5. Participants’ Feedback on Visuals to Convey Regulatory Principles or Processes
3.6. Participants’ Preferred Statement about COVID-19 Vaccine Safety
3.7. Feedback on Shareable/Audiovisual Materials, Translation into Official EU Languages, and Missing Information
3.8. Recommendations from the User-Testing Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- WHO. Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 16 March 2021).
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 16 March 2021).
- The Economist. The Pandemic’s True Death Toll. Available online: https://www.economist.com/graphic-detail/coronavirus-excess-deaths-estimates (accessed on 25 September 2023).
- European Commission. Facing the Impact of Post-COVID-19 Condition (long COVID) on Health Systems: Opinion of the Expert Panel on Effective Ways of Investing in Health (EXPH). Available online: https://health.ec.europa.eu/system/files/2022-12/031_longcovid_en.pdf (accessed on 20 December 2022).
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Sanmarchi, F.; Golinelli, D.; Lenzi, J.; Esposito, F.; Capodici, A.; Reno, C.; Gibertoni, D. Exploring the Gap Between Excess Mortality and COVID-19 Deaths in 67 Countries. JAMA Netw. Open 2021, 4, e2117359. [Google Scholar] [CrossRef] [PubMed]
- Antonini, M.; Eid, M.A.; Falkenbach, M.; Rosenbluth, S.T.; Prieto, P.A.; Brammli-Greenberg, S.; McMeekin, P.; Paolucci, F. An analysis of the COVID-19 vaccination campaigns in France, Israel, Italy and Spain and their impact on health and economic outcomes. Health Policy Technol. 2022, 11, 100594. [Google Scholar] [CrossRef] [PubMed]
- Lurie, N.; Saville, M.; Hatchett, R.; Halton, J. Developing COVID-19 Vaccines at Pandemic Speed. N. Engl. J. Med. 2020, 382, 1969–1973. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Council of the European Union. Council Recommendation on Strengthened Cooperation against Vaccine-Preventable Diseases. Available online: https://www.eumonitor.nl/9353000/1/j4nvgs5kjg27kof_j9vvik7m1c3gyxp/vktn9i1ifdzv/f=/14152_1_18_rev_1.pdf (accessed on 16 March 2021).
- Blastland, M.; Freeman, A.L.J.; van der Linden, S.; Marteau, T.M.; Spiegelhalter, D. Five rules for evidence communication. Nature 2020, 587, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Cavaleri, M.; Enzmann, H.; Straus, S.; Cooke, E. The European Medicines Agency’s EU conditional marketing authorisations for COVID-19 vaccines. Lancet 2021, 397, 355–357. [Google Scholar] [CrossRef]
- Sikora, D.; Rzymski, P. COVID-19 Vaccination and Rates of Infections, Hospitalizations, ICU Admissions, and Deaths in the European Economic Area during Autumn 2021 Wave of SARS-CoV-2. Vaccines 2022, 10, 437. [Google Scholar] [CrossRef]
- European Medicines Agency. COVID-19 Vaccines. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-vaccines (accessed on 16 March 2021).
- European Centre for Disease Prevention and Control. COVID-19 Vaccine Tracker. Available online: https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab (accessed on 16 March 2021).
- AlShurman, B.A.; Khan, A.F.; Mac, C.; Majeed, M.; Butt, Z.A. What Demographic, Social, and Contextual Factors Influence the Intention to Use COVID-19 Vaccines: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 9342. [Google Scholar] [CrossRef] [PubMed]
- ICMRA. ICMRA Statement on the Safety of COVID-19 Vaccines. Available online: https://icmra.info/drupal/strategicinitiatives/vaccines/safety_statement (accessed on 5 July 2023).
- Johnson, A.G.; Amin, A.B.; Ali, A.R.; Hoots, B.; Cadwell, B.L.; Arora, S.; Avoundjian, T.; Awofeso, A.O.; Barnes, J.; Bayoumi, N.S.; et al. COVID-19 Incidence and Death Rates Among Unvaccinated and Fully Vaccinated Adults with and Without Booster Doses During Periods of Delta and Omicron Variant Emergence—25 U.S. Jurisdictions, April 4-December 25, 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 132–138. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Public Stakeholder Meeting: Approval, Safety Monitoring and Impact of COVID-19 Vaccines in the EU. Available online: https://www.ema.europa.eu/en/events/public-stakeholder-meeting-approval-safety-monitoring-impact-covid-19-vaccines-eu (accessed on 26 March 2021).
- European Medicines Agency. COVID-19 Vaccines: Key Facts. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-key-facts (accessed on 16 March 2021).
- European Medicines Agency. COVID-19 Vaccines: Development, Evaluation, Approval and Monitoring. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-development-evaluation-approval-monitoring (accessed on 16 March 2021).
- European Medicines Agency. EMA Individual Experts’ Stakeholder Database: Patients and Consumers. Available online: https://www.ema.europa.eu/en/documents/other/ema-individual-experts-stakeholder-database-patients-consumers-frequently-asked-questions_en.pdf (accessed on 13 July 2023).
- DemoIstat Italian Demographic Data. 2021. Available online: https://demo.istat.it/app/?l=it&a=2021&i=POS (accessed on 16 March 2021).
- Italian Government. COVID-19 Vaccination Report. Available online: https://www.governo.it/it/cscovid19/report-vaccini/ (accessed on 16 March 2021).
- European Commission. EUSurvey. Available online: https://ec.europa.eu/eusurvey/home/about (accessed on 16 March 2021).
- European Medicines Agency. COVID-19, Communication Perception Survey. 2022. Available online: https://www.ema.europa.eu/en/documents/presentation/presentation-ema-communication-perception-survey-2022-c-gadd-ema_en.pdf (accessed on 11 October 2023).
- European Medicines Agency. COVID-19 Vaccines Safety Updates. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised (accessed on 16 March 2021).
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.L.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.; Lutrick, K.; et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers—Eight U.S. Locations, December 2020-March 2021. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Gesualdo, F.; Parisi, L.; Croci, I.; Comunello, F.; Parente, A.; Russo, L.; Campagna, I.; Lanfranchi, B.; Rota, M.C.; Filia, A.; et al. How the Italian Twitter Conversation on Vaccines Changed During the First Phase of the Pandemic: A Mixed-Method Analysis. Front. Public Health 2022, 10, 824465. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, B.; Madsen, B.E. Prioritizing health care workers and first responders for access to the COVID19 vaccine is not unethical, but both fair and effective—An ethical analysis. Scand. J. Trauma. Resusc. Emerg. Med. 2021, 29, 77. [Google Scholar] [CrossRef]
- Languages on This Website|European Medicines Agency (Europa.Eu). Available online: https://www.ema.europa.eu/en/about-us/about-website/languages-website (accessed on 16 March 2021).
- Innovative Medicines Initiative. ADVANCE: Accelerated Development of Vaccine Benefit-Risk Collaboration in Europe. Available online: https://www.imi.europa.eu/projects-results/project-factsheets/advance (accessed on 16 March 2021).
- The European Council and the Council of the European Union. Employment, Social Policy, Health and Consumer Affairs Council Configuration (EPSCO). Available online: https://www.consilium.europa.eu/en/council-eu/configurations/epsco/ (accessed on 16 March 2021).
- The European Council and the Council of the European Union. Employment, Social Policy, Health and Consumer Affairs Council (Health), 9 December 2022. Available online: https://www.consilium.europa.eu/en/meetings/epsco/2022/12/09/ (accessed on 10 December 2022).
- Bencharif, S.-T. Council Calls for Vaccine Hesitancy Task Force; Politico: Arlington County, VA, USA, 2022. [Google Scholar]
- Kerr, J.R.; Schneider, C.R.; Recchia, G.; Dryhurst, S.; Sahlin, U.; Dufouil, C.; Arwidson, P.; Freeman, A.L.; van der Linden, S. Correlates of intended COVID-19 vaccine acceptance across time and countries: Results from a series of cross-sectional surveys. BMJ Open 2021, 11, e048025. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.R.; Freeman, A.L.J.; Marteau, T.M.; van der Linden, S. Effect of Information about COVID-19 Vaccine Effectiveness and Side Effects on Behavioural Intentions: Two Online Experiments. Vaccines 2021, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Peretti-Watel, P.; Seror, V.; Cortaredona, S.; Launay, O.; Raude, J.; Verger, P.; Fressard, L.; Beck, F.; Legleye, S.; L’Haridon, O.; et al. A future vaccination campaign against COVID-19 at risk of vaccine hesitancy and politicisation. Lancet Infect. Dis. 2020, 20, 769–770. [Google Scholar] [CrossRef] [PubMed]
- Roozenbeek, J.; Schneider, C.R.; Dryhurst, S.; Kerr, J.; Freeman, A.L.J.; Recchia, G.; van der Bles, A.M.; van der Linden, S. Susceptibility to misinformation about COVID-19 around the world. R. Soc. Open Sci. 2020, 7, 201199. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Infodemic. Available online: https://www.who.int/health-topics/infodemic (accessed on 16 March 2021).
- World Health Organization. Immunizing the Public against Misinformation. Available online: https://www.who.int/news-room/feature-stories/detail/immunizing-the-public-against-misinformation (accessed on 16 March 2021).
- Centers for Disease Control and Prevention. How to Address COVID-19 Vaccine Misinformation. Available online: https://www.cdc.gov/vaccines/covid-19/health-departments/addressing-vaccine-misinformation.html (accessed on 5 November 2021).
- Valenti, A.; Mirabile, M.; Cannone, E.; Boccuni, F.; Dionisi, P.; Fortuna, G.; Gagliardi, D.; Vizzaccaro, R.; Iavicoli, S. The Impact of COVID-19 Pandemics on the Development of Health Risk Communication: Challenges and Opportunities. Int. J. Environ. Res. Public Health 2022, 20, 645. [Google Scholar] [CrossRef]
- Cavaleri, M.; Sweeney, F.; Gonzalez-Quevedo, R.; Carr, M. Shaping EU medicines regulation in the post COVID-19 era. Lancet Reg. Health Eur. 2021, 9, 100192. [Google Scholar] [CrossRef]
- European Medicines Agency. Public Stakeholder Meeting: Development and Authorisation of Safe and Effective COVID-19 Vaccines in the EU. Available online: https://www.ema.europa.eu/en/events/public-stakeholder-meeting-development-authorisation-safe-effective-covid-19-vaccines-eu (accessed on 16 March 2021).
- European Medicines Agency. Public Stakeholder Meeting on the Approval and Roll-Out of COVID-19 Vaccines in the EU. Available online: https://www.ema.europa.eu/en/events/public-stakeholder-meeting-approval-roll-out-covid-19-vaccines-eu (accessed on 16 March 2021).
- European Medicines Agency. COVID-19: Latest Updates (Archive). Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/covid-19-latest-updates (accessed on 16 March 2021).
- European Medicines Agency. Safety of COVID-19 Vaccines. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/safety-covid-19-vaccines (accessed on 23 June 2023).
- European Medicines Agency. Annex to Vaxzevria Art.5.3—Visual Risk Contextualization. Available online: https://www.ema.europa.eu/en/documents/chmp-annex/annex-vaxzevria-art53-visual-risk-contextualisation_en.pdf (accessed on 24 April 2021).
- Giese, H.; Neth, H.; Gaissmaier, W. Determinants of information diffusion in online communication on vaccination: The benefits of visual displays. Vaccine 2021, 39, 6407–6413. [Google Scholar] [CrossRef]
- Edwards, P.A. Reconceptualizing literacy. Read. Today 2010, 27, 22. [Google Scholar]
- The European Council and the Council of the European Union. Infographic—How mRNA Vaccines Protect You against COVID-19. Available online: https://www.consilium.europa.eu/en/infographics/covid-19-mrna-vaccine/ (accessed on 3 October 2022).
- The European Council and the Council of the European Union. Infographic—Viral Vector Vaccines against COVID-19: How They Work. Available online: https://www.consilium.europa.eu/en/infographics/covid-19-viral-vector-vaccines (accessed on 3 October 2022).
- The European Council and the Council of the European Union. Infographic—How Protein-Based Vaccines Work against COVID-19. Available online: https://www.consilium.europa.eu/en/infographics/covid-19-protein-based-vaccine (accessed on 3 October 2022).
- European Medicines Agency. First Regular Press Briefing on COVID-19. Available online: https://www.ema.europa.eu/en/events/first-ema-regular-press-briefing-covid-19 (accessed on 16 May 2021).
- YouTube. European Medicines Agency’s Channel. Available online: https://www.youtube.com/channel/UC7eQwP125fAtwgOhKguOJNg (accessed on 16 March 2021).
- European Medicines Agency. Transparency: Exceptional Measures for COVID-19 Medicines. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/transparency-exceptional-measures-covid-19-medicines (accessed on 16 March 2021).
- Abed, I.; Gonzalez-Quevedo, R.; Mura, M.; Dias, M.; da Rocha Dias, S.; García Burgos, J. Commentary on the European Medicines Agency’s extended mandate: Protecting public health in times of crisis and improving availability of medicines and medical devices. Br. J. Clin. Pharmacol. 2023, 89, 5–10. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Multilingualism on the EMA Website and in External Communications. Available online: https://www.ema.europa.eu/en/documents/other/multilingualism-ema-website-external-communications_en.pdf (accessed on 11 October 2023).





| EU Level | Regional Level | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Correct Responses by Individual Patients | Correct Responses by Individual HCPs | Correct Responses by PCOs | Correct Responses by HCP Organizations | Average Percentage across all EU Stakeholders | Correct Responses by Individual Patients | Correct Responses by Individual HCPs | Correct Responses by PCOs | Correct Responses by HCP Organizations | Average Percentage across All Regional Stakeholders | |
| Tier 1 key messages on: | ||||||||||
| Safety requirements 1 | 6 (100%) | 2 (67%) | 8 (100%) | 13 (100%) | 91.75% | 7 (100%) | 12 (100%) | 3 (100%) | 8 (100%) | 100% |
| Approval 2 | 6 (100%) | 3 (100%) | 8 (100%) | 13 (100%) | 100% | 7 (100%) | 12 (100%) | 3 (100%) | 8 (100%) | 100% |
| Safe and effective vaccines 3 | 1 (17%) | 0 (0%) | 3 (38%) | 7 (54%) | 27.3% | 0 (0%) | 3 (25%) | 2 (67%) | 4 (50%) | 35.5% |
| Tier 2 key messages on: | ||||||||||
| Safety monitoring 4 | 2 (100%) | 2 (100%) | 7 (88%) | 10 (91%) | 94.8% | 4 (80%) | 8 (73%) | N.A. | 6 (86%) | 79.7% |
| Rolling review 5 | 2 (100%) | 2 (100%) | 8 (100%) | 11 (100%) | 100% | 5 (100%) | 11 (100%) | N.A. | 7 (100%) | 100% |
| Development 6 | 2 (100%) | 1 (50%) | 6 (75%) | 10 (91%) | 79% | 4 (80%) | 7 (64%) | N.A. | 7 (100%) | 81.3% |
| Visuals (Tier 2) | Level | Did You Think They Were Clear and Easy to Understand? | ||||
|---|---|---|---|---|---|---|
| Yes | No/Don’t Know | If Answered Yes, Which Figure Do You Prefer? | ||||
| Resource pooling visuals |  Resources COVID-19 development mobilises more extensive resources, simultaneously |  Resources COVID-19 development mobilises more extensive resources, simultaneously | ||||
| EU | 15 (65%) | 8 (35%) | 5 (31%) | 11 (69%) | ||
| Regional | 6 (26%) | 17 (74%) | 0 (0%) | 8 (100%) | ||
| Positive benefit–risk balance visuals |  (a) (a) |  (b) (b) |  (c) (c) | |||
| EU | 20 (87%) | 3 (13%) | 12 (60%) | 2 (10%) | 6 (30%) | |
| Regional | 17 (74%) | 6 (26%) | 15 (83%) | 0 (0%) | 3 (17%) | |
| EU Level (n = 23) | Regional Level (n = 23) | |||||||
|---|---|---|---|---|---|---|---|---|
| Individual Patient and Consumer Preferences | Individual HCPs’ Preferences | PCOs’ Preferences | HCPOs’ Preferences | Individual Patient and Consumer Preferences | Individual HCPs’ Preferences | PCOs’ Preferences | HCPOs’ Preferences | |
| First safety statement proposal 1 | 2 (100%) | 0 (0%) | 4 (50%) | 9 (82%) | 2 (40%) | 4 (36%) | N.A. | 4 (57%) |
| Second safety statement proposal 2 | 0 (0%) | 2 (100%) | 4 (50%) | 2 (18%) | 3 (60%) | 7 (64%) | N.A. | 3 (43%) |
| Improvement Identified by Stakeholder | Recommendation for theEMA |
| Replace visual on positive benefit–risk balance | Replace the visual in all the EMA’s materials with the one preferred by stakeholders |
| Improve key messages and more friendly language | Update text and section headings |
| Prepare more succinct materials | * Make available on the EMA’s website the recordings and presentations of the EMA’s public stakeholder meetings, which summarize all information on COVID-19 vaccines |
| Produce more information on vaccine safety | Prepare a dedicated webpage on COVID-19 vaccine safety: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/covid-19-medicines/safety-covid-19-vaccines (accessed on 11 October 2023) Make available on the EMA’s website dedicated safety updates for each approved COVID-19 vaccine as needed, in addition to all information on safety routinely published for each vaccine (safety communications and summaries of scientific assessments) |
| Produce more friendly (audio) visual materials | In addition to (*), produce infographics, social media campaigns |
| Keep information updated | Keep the EMA’s webpages on COVID-19 vaccines regularly updated |
| Use of preferred general safety statement | Use in EMA materials the safety statement preferred by EU HCP organizations |
| Translations into official EU languages | Explore translating key information on COVID-19 vaccines into official EU languages |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, I.; Van Tricht, M.; Bonaccorso, N.; Sciortino, M.; Garcia Burgos, J.; Costantino, C.; Gonzalez-Quevedo, R. Stakeholders’ Understanding of European Medicine Agency’s COVID-19 Vaccine Information Materials in EU and Regional Contexts. Vaccines 2023, 11, 1616. https://doi.org/10.3390/vaccines11101616
Castro I, Van Tricht M, Bonaccorso N, Sciortino M, Garcia Burgos J, Costantino C, Gonzalez-Quevedo R. Stakeholders’ Understanding of European Medicine Agency’s COVID-19 Vaccine Information Materials in EU and Regional Contexts. Vaccines. 2023; 11(10):1616. https://doi.org/10.3390/vaccines11101616
Chicago/Turabian StyleCastro, Indiana, Marie Van Tricht, Nicole Bonaccorso, Martina Sciortino, Juan Garcia Burgos, Claudio Costantino, and Rosa Gonzalez-Quevedo. 2023. "Stakeholders’ Understanding of European Medicine Agency’s COVID-19 Vaccine Information Materials in EU and Regional Contexts" Vaccines 11, no. 10: 1616. https://doi.org/10.3390/vaccines11101616
APA StyleCastro, I., Van Tricht, M., Bonaccorso, N., Sciortino, M., Garcia Burgos, J., Costantino, C., & Gonzalez-Quevedo, R. (2023). Stakeholders’ Understanding of European Medicine Agency’s COVID-19 Vaccine Information Materials in EU and Regional Contexts. Vaccines, 11(10), 1616. https://doi.org/10.3390/vaccines11101616







