Factors Affecting SARS-CoV-2 IgG Production after Vaccination and/or Disease: A Large-Scale Seroprevalence Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Blood Sampling and Antibody Detection
2.3. Statistical Analysis
3. Results
3.1. Subject Demographics
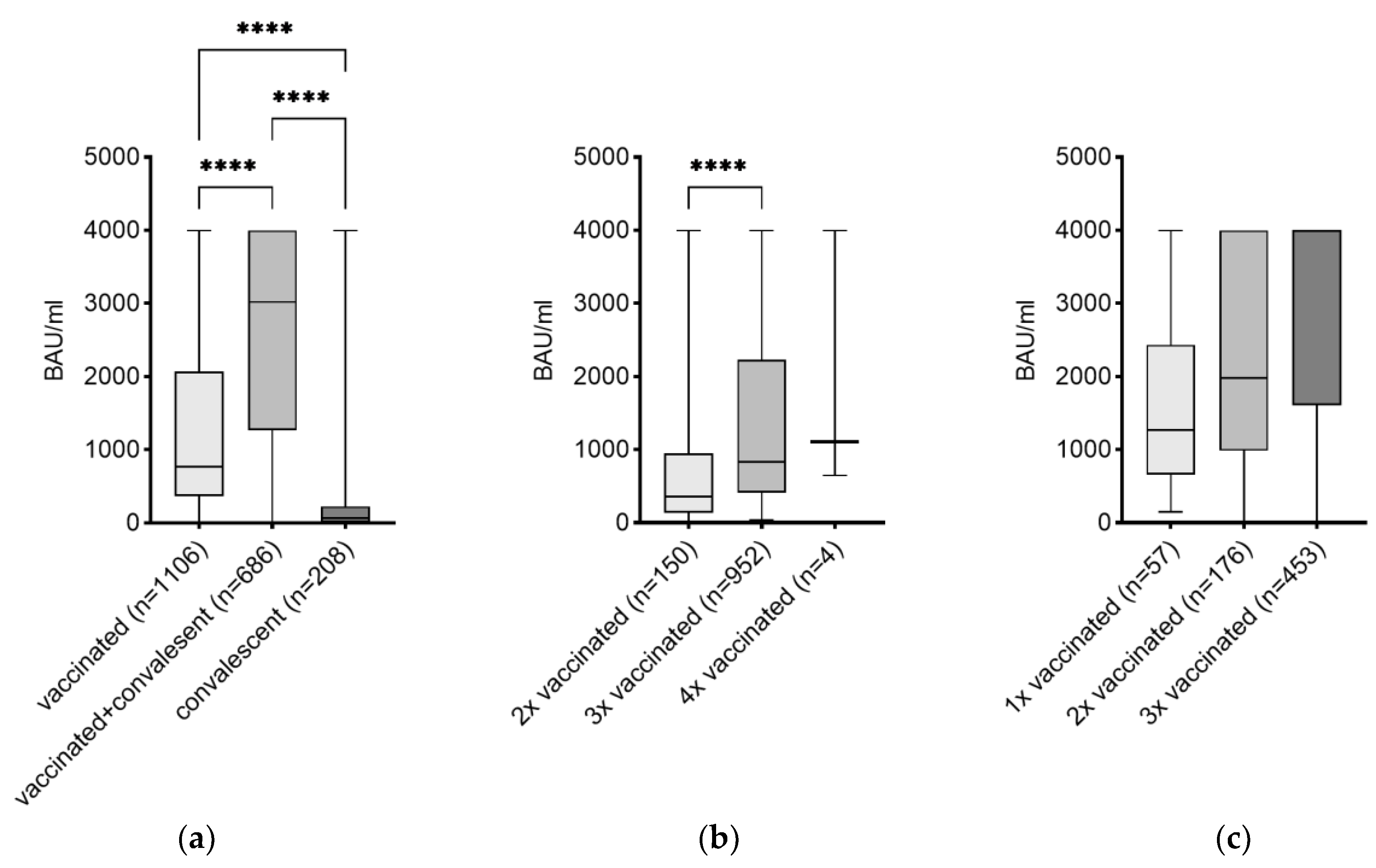
3.2. Effects of the Number of Vaccinations on the Level of IgG
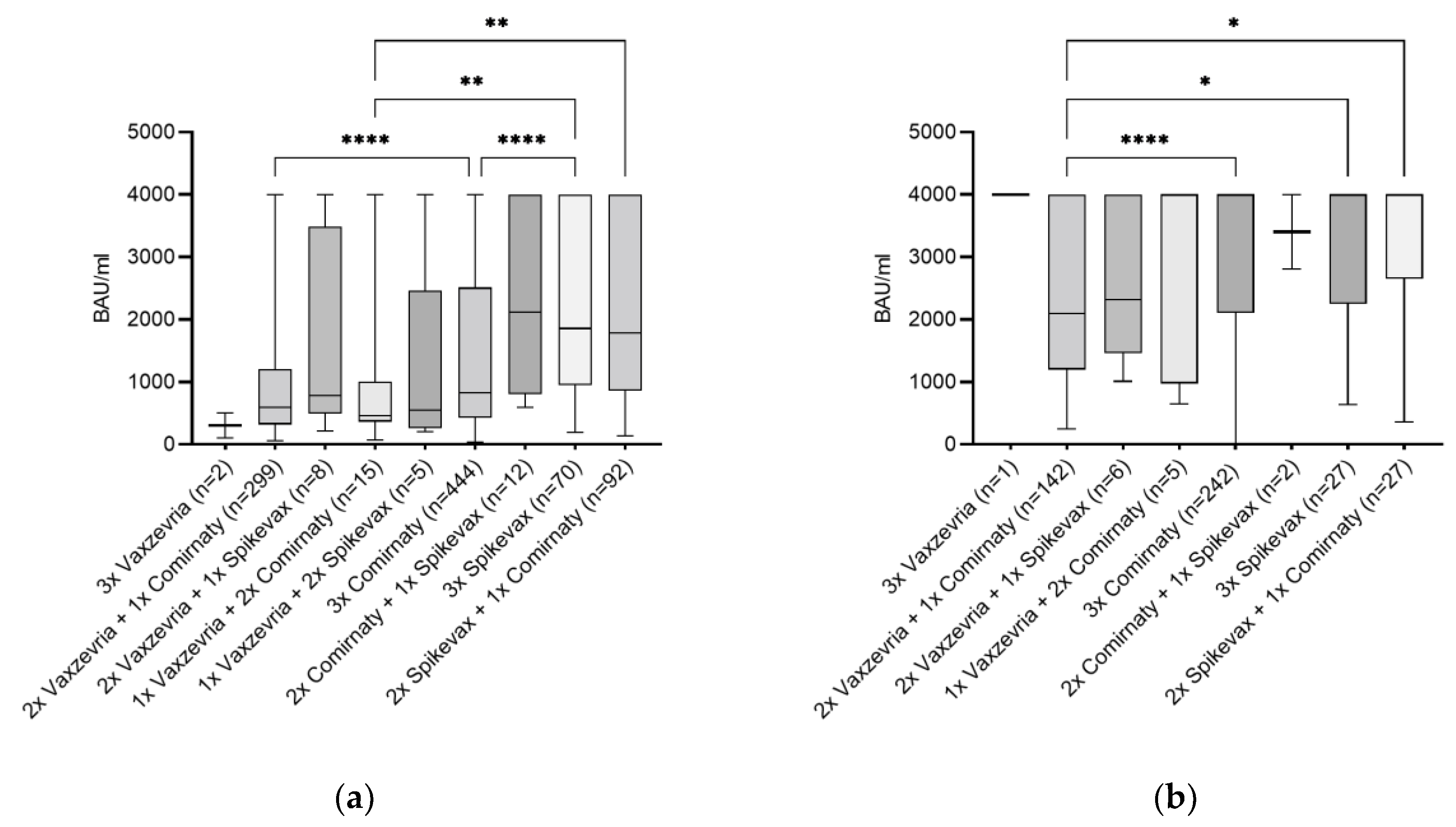
3.3. Vaccination Type and Its Combination as Influencing Factor
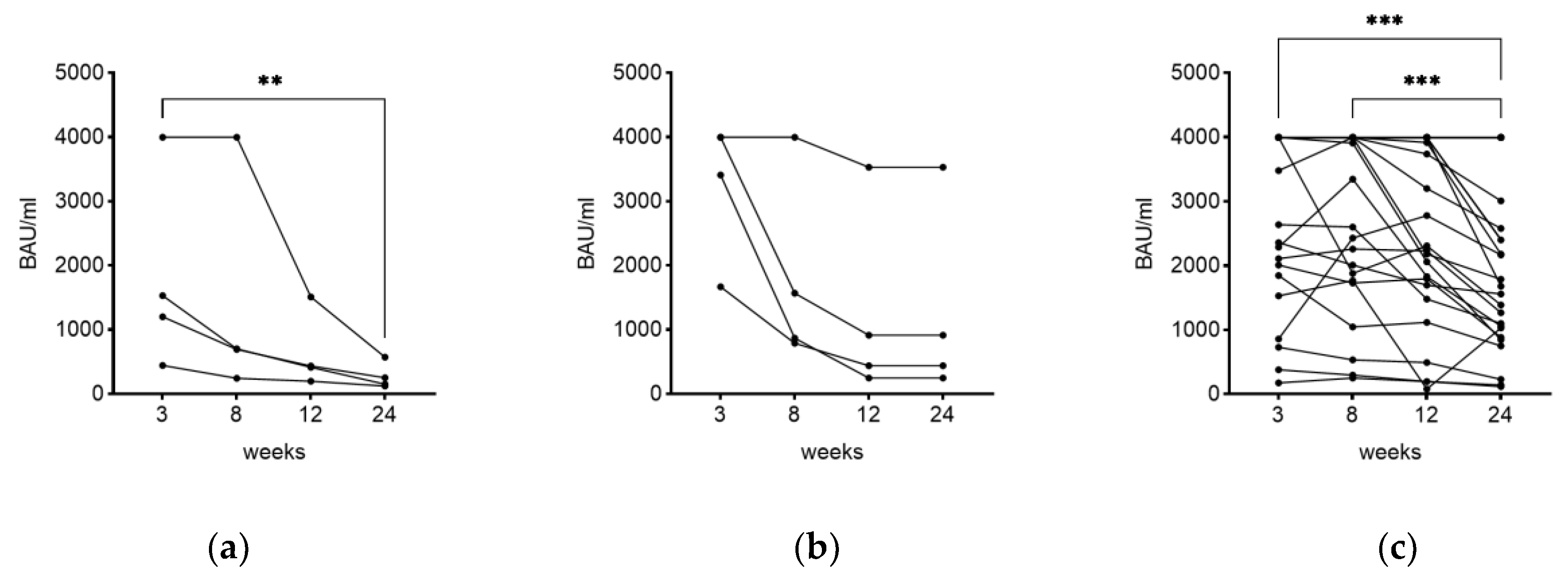
3.4. Time as Influencing Factor on IgG Level
3.5. Additional Influencing Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Frenck, R.W.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 COVID-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Bergeri, I.; Whelan, M.; Ware, H.; Subissi, L.; Nardone, A.; Lewis, H.C.; Li, Z.; Ma, X.; Valenciano, M.; Cheng, B.; et al. Global SARS-CoV-2 seroprevalence from January 2020 to April 2022: A systematic review and meta-analysis of standardized population-based studies. PLoS Med. 2022, 19, 1004107. [Google Scholar] [CrossRef] [PubMed]
- Bergeri, I.; Lewis, H.C.; Subissi, L.; Nardone, A.; Valenciano, M.; Cheng, B.; Glonti, K.; Williams, B.; Abejirinde, I.-O.O.; Simniceanu, A.; et al. Early epidemiological investigations: World Health Organization UNITY protocols provide a standardized and timely international investigation framework during the COVID-19 pandemic. Influenza Other Respir. Viruses 2022, 16, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Saade, E.; Noguez, J.H.; Schmotzer, C. SARS-CoV-2 Seroprevalence Among First Responders in Northeastern Ohio, 2020. Public Health Rep. 2023, 138, 140–148. [Google Scholar] [CrossRef]
- Solastie, A.; Nieminen, T.; Ekström, N.; Nohynek, H.; Lehtonen, L.; Palmu, A.A.; Melin, M. Changes in SARS-CoV-2 seroprevalence and population immunity in Finland, 2020–2022. Emerg. Microbes Infect. 2023, 12, 2222849. [Google Scholar] [CrossRef]
- Ali, A.; Waqar, M.; Akram, A.; Rafique, S.; Rehman, G.; Idrees, M.; Halim, S.A.; Waqas, M.; Uddin, J.; Gojayev, A.; et al. Seroprevalence of SARS-CoV-2: Insights into the epidemiology of the pandemic. J. Infect. Public Health 2023, 16, 1256–1261. [Google Scholar] [CrossRef]
- Aldridge, R.W.; Yavlinsky, A.; Nguyen, V.; Eyre, M.T.; Shrotri, M.; Navaratnam, A.M.D.; Beale, S.; Braithwaite, I.; Byrne, T.; Kovar, J.; et al. SARS-CoV-2 antibodies and breakthrough infections in the Virus Watch cohort. Nat. Commun. 2022, 13, 4869. [Google Scholar] [CrossRef] [PubMed]
- Amanatidou, E.; Gkiouliava, A.; Pella, E.; Serafidi, M.; Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Dalamaga, M. Breakthrough infections after COVID-19 vaccination: Insights, perspectives and challenges. Metabol. Open 2022, 14, 100180. [Google Scholar] [CrossRef] [PubMed]
- Stærke, N.B.; Reekie, J.; Nielsen, H.; Benfield, T.; Wiese, L.; Knudsen, L.S.; Iversen, M.B.; Iversen, K.; Fogh, K.; Bodilsen, J.; et al. Levels of SARS-CoV-2 antibodies among fully vaccinated individuals with Delta or Omicron variant breakthrough infections. Nat. Commun. 2022, 13, 4466. [Google Scholar] [CrossRef] [PubMed]
- Addo, I.Y.; Dadzie, F.A.; Okeke, S.R.; Boadi, C.; Boadu, E.F. Duration of immunity following full vaccination against SARS-CoV-2: A systematic review. Arch. Public Health 2022, 80, 200. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Wu, Z.; Zhong, S.; Li, M.; Shu, Y. Factors influencing the immunogenicity of influenza vaccines. Hum. Vaccin. Immunother. 2021, 17, 2706–2718. [Google Scholar] [CrossRef] [PubMed]
- Khafagy, A.; AlJahdaly, I.; Goweda, R. Hepatitis B Vaccine: Assessment of Immunologic Response, Coverage Rate, and Factors Influencing Seroreactivity. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef]
- Pérez-Alós, L.; Armenteros, J.J.A.; Madsen, J.R.; Hansen, C.B.; Jarlhelt, I.; Hamm, S.R.; Heftdal, L.D.; Pries-Heje, M.M.; Møller, D.L.; Fogh, K.; et al. Modeling of waning immunity after SARS-CoV-2 vaccination and influencing factors. Nat. Commun. 2022, 13, 1614. [Google Scholar] [CrossRef]
- Reusch, J.; Wagenhäuser, I.; Gabel, A.; Eggestein, A.; Höhn, A.; Lâm, T.-T.; Frey, A.; Schubert-Unkmeir, A.; Dölken, L.; Frantz, S.; et al. Influencing factors of anti-SARS-CoV-2-spike-IgG antibody titers in healthcare workers: A cross-section study. J. Med. Virol. 2023, 95, e28300. [Google Scholar] [CrossRef]
- Visalli, G.; Laganà, A.; Lo Giudice, D.; Calimeri, S.; Caccamo, D.; Trainito, A.; Di Pietro, A.; Facciolà, A. Towards a Future of Personalized Vaccinology: Study on Individual Variables Influencing the Antibody Response to the COVID-19 Vaccine. Vaccines 2023, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Foddis, R.; Marino, R.; Silvestri, R.; Fallahi, P.; Perretta, S.; Garaffa, C.; Morganti, R.; Corsi, M.; Mennucci, J.; Porciatti, F.; et al. Evaluation of the Anti-Spike (RDB) IgG Titer among Workers Employed at the University of Pisa Vaccinated with Different Types of SARS-CoV-2 Vaccines. Vaccines 2022, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- WHO. Coronavirus Disease (COVID-19) Technical Guidance: The Unity Studies: Early Investigation Protocols. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/early-investigations (accessed on 18 September 2023).
- Yan, X.; Chen, G.; Jin, Z.; Zhang, Z.; Zhang, B.; He, J.; Yin, S.; Huang, J.; Fan, M.; Li, Z.; et al. Anti-SARS-CoV-2 IgG levels in relation to disease severity of COVID-19. J. Med. Virol. 2022, 94, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Young, A. T cells in SARS-CoV-2 infection and vaccination. Ther. Adv. Vaccines Immunother. 2022, 10, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lang-Meli, J.; Luxenburger, H.; Wild, K.; Karl, V.; Oberhardt, V.; Salimi Alizei, E.; Graeser, A.; Reinscheid, M.; Roehlen, N.; Reeg, D.B.; et al. SARS-CoV-2-specific T-cell epitope repertoire in convalescent and mRNA-vaccinated individuals. Nat. Microbiol. 2022, 7, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after COVID-19 Vaccination and Previous Infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raddad, L.J.; Chemaitelly, H.; Ayoub, H.H.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Tang, P.; Hasan, M.R.; Coyle, P.; Al Kanaani, Z.; et al. Association of Prior SARS-CoV-2 Infection with Risk of Breakthrough Infection Following mRNA Vaccination in Qatar. JAMA 2021, 326, 1930–1939. [Google Scholar] [CrossRef]
- Kung, Y.-A.; Huang, S.-Y.; Huang, C.-G.; Liu, K.-T.; Huang, P.-N.; Yu, K.-Y.; Yang, S.-L.; Chen, C.-P.; Cheng, C.-Y.; Lee, I.-K.; et al. Factors influencing neutralizing antibody titers elicited by coronavirus disease 2019 vaccines. Microbes Infect. 2023, 25, 105044. [Google Scholar] [CrossRef]
- Sauré, D.; O’Ryan, M.; Torres, J.P.; Zuñiga, M.; Soto-Rifo, R.; Valiente-Echeverría, F.; Gaete-Argel, A.; Neira, I.; Saavedra, V.; Acevedo, M.L.; et al. COVID-19 lateral flow IgG seropositivity and serum neutralising antibody responses after primary and booster vaccinations in Chile: A cross-sectional study. Lancet Microbe 2023, 4, e149–e158. [Google Scholar] [CrossRef]
- Doke, P.; Gothankar, J.S.; Doke, P.P.; Kulkarni, M.M.; Khalate, K.K.; Shrivastava, S.; Patil, J.R.; Arankalle, V.A. Time dependent decline of neutralizing antibody titers in COVID-19 patients from Pune, India and evidence of reinfection. Microbes Infect. 2022, 24, 104979. [Google Scholar] [CrossRef]
- Barin, B.; Kasap, U.; Selçuk, F.; Volkan, E.; Uluçkan, Ö. Comparison of SARS-CoV-2 anti-spike receptor binding domain IgG antibody responses after CoronaVac, BNT162b2, ChAdOx1 COVID-19 vaccines, and a single booster dose: A prospective, longitudinal population-based study. Lancet Microbe 2022, 3, e274–e283. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Virgilio, E.; Trevisan, C.; Abbatecola, A.; Malara, A.; Palmieri, A.; Fedele, G.; Stefanelli, P.; Leone, P.; Schiavoni, I.; Maggi, S.; et al. Diabetes Affects Antibody Response to SARS-CoV-2 Vaccination in Older Residents of Long-term Care Facilities: Data from the GeroCovid Vax Study. Diabetes Care 2022, 45, 2935–2942. [Google Scholar] [CrossRef]
- Ali, H.; Alterki, A.; Sindhu, S.; Alahmad, B.; Hammad, M.; Al-Sabah, S.; Alghounaim, M.; Jamal, M.H.; Aldei, A.; Mairza, M.J.; et al. Robust Antibody Levels in Both Diabetic and Non-Diabetic Individuals After BNT162b2 mRNA COVID-19 Vaccination. Front. Immunol. 2021, 12, 752233. [Google Scholar] [CrossRef]
- Kidd, B.A.; Wroblewska, A.; Boland, M.R.; Agudo, J.; Merad, M.; Tatonetti, N.P.; Brown, B.D.; Dudley, J.T. Mapping the effects of drugs on the immune system. Nat. Biotechnol. 2016, 34, 47–54. [Google Scholar] [CrossRef]
- Szałach, Ł.P.; Lisowska, K.A.; Cubała, W.J. The Influence of Antidepressants on the Immune System. Arch. Immunol. Ther. Exp. 2019, 67, 143–151. [Google Scholar] [CrossRef] [PubMed]

| n = 2000 | Mean/SD or n-Number | IgG BAU/mL Median (IQR) | Correlation Coefficient (r/p-Value) | p-Value (IgG Titer) 1 |
|---|---|---|---|---|
| Age in years | 42.6 (12.6) | 1070 (382–3573) | −0.033 (0.141) | 0.156 |
| 20–40 | 885 | 1190 (430–3530) | NA 2 | NA |
| 41–60 | 986 | 962 (345·3–3415) | NA | NA |
| >60 | 129 | 1050 (358.5–4000) | NA | NA |
| Sex assigned at birth | NA | NA | NA | 0.018 (female vs. male) |
| Female | 1321 | 1000 (355–3210) | NA | NA |
| Male | 679 | 1170 (446–4000) | NA | NA |
| SARS-CoV-2 infections | NA | NA | NA | 0.002 (1 inf. 3 vs. 2 inf.) |
| 1 infection | 849 | 1900 (484–4000) | NA | NA |
| 2 infections | 45 | 843 (276–1590) | NA | NA |
| 3 infections | 1 | 446 | NA | NA |
| Symptoms severity | NA | NA | NA | 0.020 |
| None | 64 | 965 (250–3115) | NA | NA |
| Mild | 451 | 1820 (383–4000) | NA | 0.083 (compared to none) |
| Moderate | 349 | 1910 (629–4000) | NA | 0.013 (compared to none) |
| Severe | 32 | 1790 (487–3823) | NA | NA |
| Hospitalization | 0 | NA | NA | NA |
| Breathing difficulties | 47 | 1910 (1160–4000) | NA | NA |
| Chronic diseases | NA | NA | NA | 0.134 |
| Morbus Crohn | 3 | 4000 (769–4000) | NA | NA |
| Colitis ulcerosa | 4 | 1157 (249–2143) | NA | NA |
| High blood pressure | 108 | 924 (348–4000) | NA | NA |
| Psoriasis arthritis | 4 | 4000 (1228–4000) | NA | NA |
| Rheumatoide arthritis | 11 | 581 (130–986) | NA | NA |
| Lupus erythematodes | 1 | 4000 | NA | NA |
| Diabetes mellitus type 1 | 4 | 1090 (426–3280) | NA | NA |
| Diabetes mellitus type 2 | 7 | 526 (419–1700) | NA | NA |
| Kidney disease | 1 | 1390 | NA | NA |
| Liver disease | 3 | 176 (2.59–363) | NA | NA |
| Thyroid disease | 176 | 1065 (338–4000) | NA | NA |
| Other/multiple | 234 | 1115 (507–4000) | NA | NA |
| BMI | NA | NA | 0.039 (0.084) | 0.614 |
| Underweight (<18.5) | 52 | 860 (335–2580) | NA | NA |
| Normal (18.5 <=> 24.9) | 1151 | 1050 (375–3210) | NA | NA |
| Overweight (25.0 <=> 29.9) | 559 | 1080 (383–4000) | NA | NA |
| Obese (>30.0) | 238 | 1155 (418–4000) | NA | NA |
| Ongoing medication | 599 | 1080 (381–3920) | NA | 0.405 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karl, T.; Schuster, A.; Stangassinger, L.M.; Stiboller, T.; Cadamuro, J.; Oostingh, G.J. Factors Affecting SARS-CoV-2 IgG Production after Vaccination and/or Disease: A Large-Scale Seroprevalence Study. Vaccines 2023, 11, 1615. https://doi.org/10.3390/vaccines11101615
Karl T, Schuster A, Stangassinger LM, Stiboller T, Cadamuro J, Oostingh GJ. Factors Affecting SARS-CoV-2 IgG Production after Vaccination and/or Disease: A Large-Scale Seroprevalence Study. Vaccines. 2023; 11(10):1615. https://doi.org/10.3390/vaccines11101615
Chicago/Turabian StyleKarl, Tanja, Anja Schuster, Lea Maria Stangassinger, Tanja Stiboller, Janne Cadamuro, and Gertie Janneke Oostingh. 2023. "Factors Affecting SARS-CoV-2 IgG Production after Vaccination and/or Disease: A Large-Scale Seroprevalence Study" Vaccines 11, no. 10: 1615. https://doi.org/10.3390/vaccines11101615
APA StyleKarl, T., Schuster, A., Stangassinger, L. M., Stiboller, T., Cadamuro, J., & Oostingh, G. J. (2023). Factors Affecting SARS-CoV-2 IgG Production after Vaccination and/or Disease: A Large-Scale Seroprevalence Study. Vaccines, 11(10), 1615. https://doi.org/10.3390/vaccines11101615







