Advancing Vaccine Strategies against Candida Infections: Exploring New Frontiers
Abstract
:1. Introduction
1.1. Brief Overview of Candidiasis and Its Global Burden
1.2. Challenges in Management of Candida Infections
1.3. Rationale for Developing Vaccines against Candidiasis
1.4. Description of Current Vaccine Candidates against Candidiasis
1.4.1. Live Attenuated Vaccines
1.4.2. Recombinant (Subunit) Vaccine
1.4.3. Conjugate Vaccines
1.4.4. Killed Whole Cell Vaccines
1.4.5. Oral Vaccines
1.4.6. Bacterial Ghost Vaccines
1.5. Preclinical and Clinical Data on Vaccine Efficacy and Safety
2. Mechanisms of Immune Protection
2.1. Discussion of the Various Immune Responses Elicited by Different Vaccine Types
2.2. The Role of Innate and Adaptive Immunity in Protection against Candidiasis
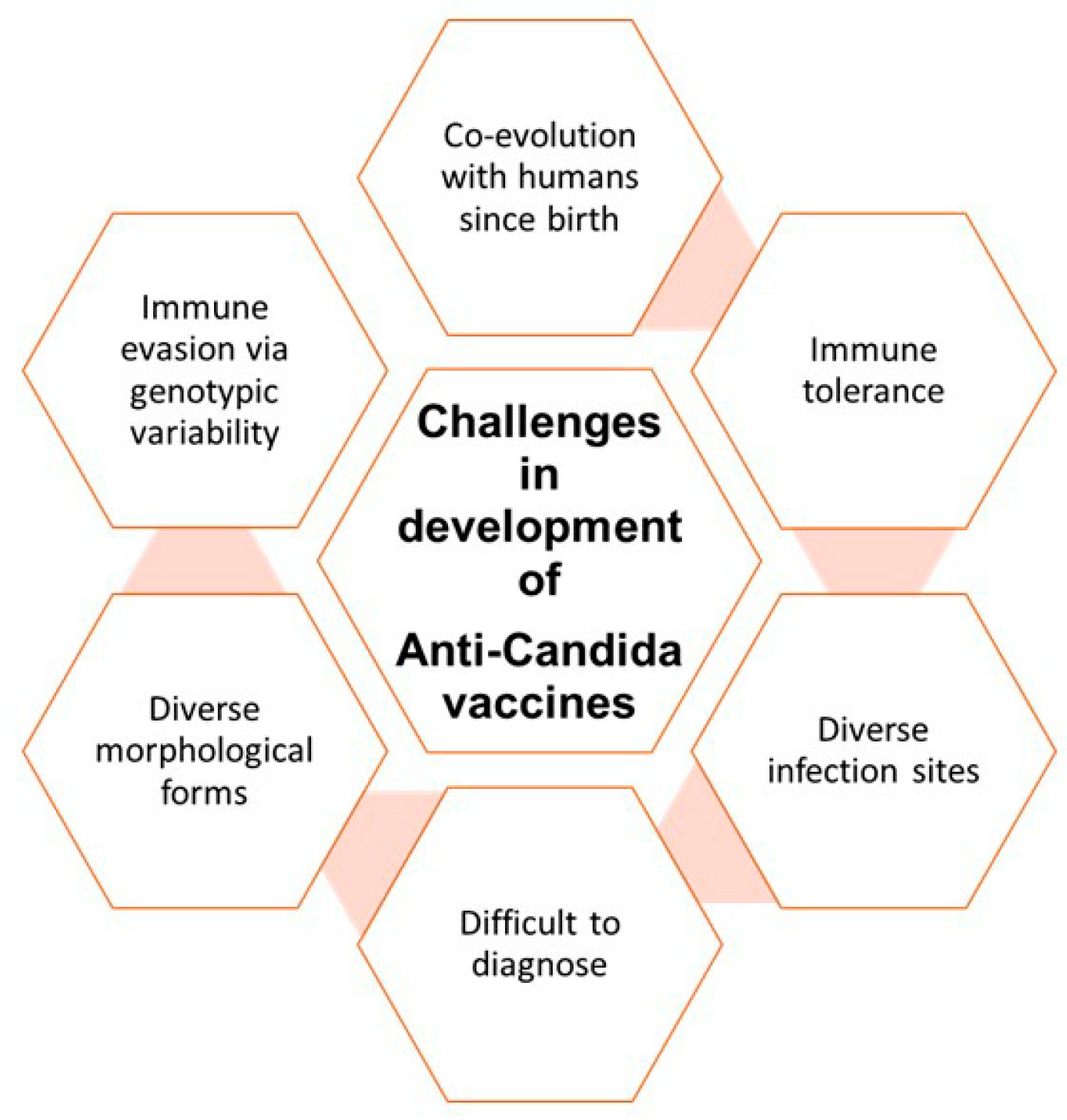
3. Challenges and Future Directions
3.1. Challenges in Developing Effective Vaccines against Candidiasis
3.2. Potential Directions for Future Research
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nature Rev. Dis. Primers 2018, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Centre for Disease Control and Prevention, CDC. Available online: https://www.cdc.gov/fungal/diseases/candidiasis/invasive/statistics.html (accessed on 24 August 2023).
- Hui, L.; Hong, T.; Jiang, Y.; Whiteway, M.; Zhang, S. Candidiasis: From cutaneous to systemic, new perspectives of potential targets and therapeutic strategies. Adv. Drug Deliv. Rev. 2023, 199, 114960. [Google Scholar]
- Sharma, M.; Chakrabarti, A. Candidiasis and Other Emerging Yeasts. Curr. Fungal Infect. Rep. 2023, 17, 15–24. [Google Scholar] [CrossRef]
- Vazquez, J.A.; Sobel, J.D. Candidiasis. In Essentials of Clinical Mycology; Springer: New York, NY, USA, 2011; pp. 167–206. [Google Scholar]
- Akpan, A.; Morgan, R. Oral candidiasis. Postgrad. Med. J. 2002, 78, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.V.; Wang, R.; Specht, C.A.; Levitz, S.M. Vaccines for human fungal diseases: Close but still a long way to go. Npj Vaccines 2021, 6, 33. [Google Scholar] [CrossRef]
- Rodríguez Stewart, R.M.; Gold, J.A.W.; Chiller, T.; Sexton, D.J.; Lockhart, S.R. Will invasive fungal infections be The Last of Us? The importance of surveillance, public-health interventions, and antifungal stewardship. Expert Rev. Anti. Infect. Ther. 2023, 21, 787–790. [Google Scholar] [CrossRef]
- Soriano, A.; Honore, P.M.; Puerta-Alcalde, P.; Garcia-Vidal, C.; Pagotto, A.; Gonçalves-Bradley, D.C.; Verweij, P.E. Invasive candidiasis: Current clinical challenges and unmet needs in adult populations. J. Antimicrob. Chemother. 2023, 78, 1569–1585. [Google Scholar] [CrossRef]
- Sahu, S.R.; Bose, S.; Singh, M.; Kumari, P.; Dutta, A.; Utkalaja, B.G.; Patel, S.K.; Acharya, N. Vaccines against candidiasis: Status, challenges and emerging opportunity. Front. Cell. Infect. Microbiol. 2022, 12, 1002406. [Google Scholar] [CrossRef]
- Lamoth, F.; Kontoyiannis, D.P. The Candida auris alert: Facts and perspectives. J. Infect Dis. 2018, 217, 516–520. [Google Scholar] [CrossRef]
- WHO. Available online: https://iris.who.int/bitstream/handle/10665/363682/9789240060241-eng.pdf?sequence=1 (accessed on 30 September 2023).
- Lee, W.G.; Shin, J.H.; Uh, Y.; Kang, M.G.; Kim, S.H. First three reported cases of nosocomial fungemia caused by Candida auris. J. Clin. Microbiol. 2011, 49, 3139–3142. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Sood, P. On the emergence, spread and resistance of Candida auris: Host, pathogen and environmental tipping points. J. Med. Microbiol. 2021, 70, 001318. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Nguyen, M.H. Diagnosing Invasive Candidiasis. J. Clin. Microbiol. 2018, 56, e01909–e01917. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Wu, J.; Cheng, M.; Zhu, X.; Du, M.; Chen, C.; Liao, W.; Zhi, K.; Pan, W. Diagnosis of invasive fungal infections: Challenges and recent developments. J. Biomed. Sci. 2023, 30, 42. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Mahmood, M.S.; Ullah, M.A.; Araf, Y.; Rahaman, T.I.; Moin, A.T.; Hosen, M.J. COVID-19-Associated Candidiasis: Possible Patho-Mechanism, Predisposing Factors, and Prevention Strategies. Curr. Microbiol. 2022, 79, 127. [Google Scholar] [CrossRef]
- Riad, A.; Gad, A.; Hockova, B.; Klugar, M. Oral candidiasis in non-severe COVID-19 patients: Call for antibiotic stewardship. Oral Surg. 2020, 15, 465. [Google Scholar] [CrossRef]
- Riad, A.; Gomaa, E.; Hockova, B.; Klugar, M. Oral candidiasis of COVID-19 patients: Case report and review of evidence. J. Cosmet. Dermatol. 2021, 20, 1580–1584. [Google Scholar] [CrossRef]
- Kozlova, O.; Burygina, E.; Khostelidi, S.; Shadrivova, O.; Saturnov, A.; Gusev, D.; Rysev, A.; Zavrazhnov, A.; Vashukova, M.; Pichugina, G.; et al. Invasive Candidiasis in Adult Patients with COVID-19: Results of a Multicenter Study in St. Petersburg, Russia. J. Fungi 2023, 9, 927. [Google Scholar] [CrossRef]
- Levitz, S.M.; Golenbock, D.T. Beyond empiricism: Informing vaccine development through innate immunity research. Cell 2012, 148, 1284–1292. [Google Scholar] [CrossRef]
- Hervé, C.; Laupèze, B.; Del Giudice, G.; Didierlaurent, A.M.; Tavares Da Silva, F. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines 2019, 4, 39. [Google Scholar] [CrossRef]
- Inácio, M.M.; Moreira, A.L.E.; Cruz-Leite, V.R.M.; Mattos, K.; Silva, L.O.S.; Venturini, J.; Ruiz, O.H.; Ribeiro-Dias, F.; Weber, S.S.; Soares, C.M.A.; et al. Fungal Vaccine Development: State of the Art and Perspectives Using Immunoinformatics. J. Fungi 2023, 9, 633. [Google Scholar] [CrossRef]
- Scriven, J.E.; Tenforde, M.W.; Levitz, S.M.; Jarvis, J.N. Modulating host immune responses to fight invasive fungal infections. Curr. Opin. Microbiol. 2017, 40, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.S.; White, C.J.; Ibrahim, A.S.; Filler, S.G.; Fu, Y.; Yeaman, M.R.; Edwards, J.E., Jr.; Hennessey, J.P., Jr. NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine 2012, 30, 7594–7600. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.J.; Ibrahim, A.S.; Avenissian, V.; Filler, S.G.; Myers, C.L.; Fu, Y.; Edwards, J.E., Jr. The anti-Candida albicans vaccine composed of the recombinant n terminus of Als1p reduces fungal burden and improves survival in both immunocompetent and immunocompromised mice. Infect. Immun. 2005, 73, 6191–6193. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.J.; Ibrahim, A.S.; Avanesian, V.; Fu, Y.; Myers, C.; Phan, Q.T.; Filler, S.G.; Yeaman, M.R.; Edwards, J.E., Jr. Efficacy of the anti-Candida rAls3p-n or rAls1p-n vaccines against disseminated and mucosal candidiasis. J. Infect. Dis. 2006, 194, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef]
- Benitez, L.L.; Carver, P.L. Adverse effects associated with long-term administration of azole antifungal agents. Drugs 2019, 79, 833–853. [Google Scholar] [CrossRef]
- Tso, G.H.W.; Reales-Calderon, J.A.; Pavelka, N. The Elusive Anti-Candida Vaccine: Lessons from the Past and Opportunities for the Future. Front. Immunol. 2018, 9, 897. [Google Scholar] [CrossRef]
- World Health Organisation. Available online: https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1 (accessed on 24 August 2023).
- Kashte, S.; Gulbake, A.; El-Amin Iii, S.F.; Gupta, A. COVID-19 vaccines: Rapid development, implications, challenges and future prospects. Hum. Cell 2021, 34, 711–733. [Google Scholar] [CrossRef]
- De Bernardis, F.; Amacker, M.; Arancia, S.; Sandini, S.; Gremion, C.; Zurbriggen, R.; Moser, C.; Cassone, A. A virosomal vaccine against Candida vaginitis: Immunogenicity, efficacy and safety profile in animal models. Vaccine 2012, 30, 4490–4498. [Google Scholar] [CrossRef]
- Cutler, J.E.; Deepe, G.S., Jr.; Klein, B.S. Advances in combating fungal diseases: Vaccines on the threshold. Nat. Rev. Microbiol. 2007, 5, 13–28. [Google Scholar] [CrossRef]
- Leibovitch, E.C.; Jacobson, S. Vaccinations for Neuroinfectious Disease: A Global Health Priority. Neurotherapeutics 2016, 13, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Nami, S.; Mohammadi, R.; Vakili, M.; Khezripour, K.; Mirzaei, H.; Morovati, H. Fungal vaccines, mechanism of actions and immunology: A comprehensive review. Biomed Pharmacother. 2019, 109, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Saville, S.P.; Lazzell, A.L.; Chaturvedi, A.K.; Monteagudo, C.; Lopez-Ribot, J.L. Efficacy of a genetically engineered Candida albicans tet-NRG1 strain as an experimental live attenuated vaccine against hematogenously disseminated candidiasis. Clin. Vaccine Immunol. 2009, 16, 430–432. [Google Scholar] [CrossRef]
- Fernández-Arenas, E.; Molero, G.; Nombela, C.; Diez-Orejas, R.; Gil, C. Low virulent strains of Candida albicans: Unravelling the antigens for a future vaccine. Proteomics 2004, 4, 3007–3020. [Google Scholar] [CrossRef] [PubMed]
- Bistoni, F.; Vecchiarelli, A.; Cenci, E.; Puccetti, P.; Marconi, P.; Cassone, A. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect. Immun. 1986, 51, 668–674. [Google Scholar] [CrossRef]
- Shen, H.; Yu, Y.; Chen, S.M.; Sun, J.J.; Fang, W.; Guo, S.Y.; Hou, W.T.; Qiu, X.R.; Zhang, Y.; Chen, Y.L.; et al. Dectin-1 facilitates IL-18 production for the generation of protective antibodies against Candida albicans. Front. Microbiol. 2020, 11, 1648. [Google Scholar] [CrossRef]
- Austriaco, N. Yeast oral vaccines against infectious diseases. Front. Microbiol. 2023, 14, 1150412. [Google Scholar] [CrossRef]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, S.; Nanda, S.; Zafar, M.A.; Malik, J.A.; Arshi, M.U.; Lamba, T.; Agrewala, J.N. Fiction and Facts about BCG Imparting Trained Immunity against COVID-19. Vaccines 2022, 10, 1006. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef]
- Arts, R.J.; Moorlag, S.J.; Novakovic, B.; Li, Y.; Wang, S.Y.; Oosting, M.; Kumar, V.; Xavier, R.J.; Wijmenga, C.; Joosten, L.A.; et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 2018, 23, 89–100. [Google Scholar] [CrossRef]
- Lancaster, K.Z.; Pfeiffer, J.K. Mechanisms controlling virulence thresholds of mixed viral populations. J. Virol. 2011, 85, 9778–9788. [Google Scholar] [CrossRef] [PubMed]
- Pappagianis, D. Evaluation of the protective efficacy of the killed Coccidioides immitis spherule vaccine in humans. The Valley Fever Vaccine Study Group. Am. Rev. Respir. Dis. 1993, 148, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Pirofski, L.A.; Casadevall, A. Use of licensed vaccines for active immunization of the immunocompromised host. Clin. Microbiol. Rev. 1998, 11, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Piccione, D.; Mirabelli, S.; Minto, N.; Bouklas, T. Difficult but not impossible: In search of an anti-Candida vaccine. Curr. Trop. Med. Rep. 2019, 15, 42–49. [Google Scholar] [CrossRef]
- Wang, X.J.; Sui, X.; Yan, L.; Wang, Y.; Cao, Y.B.; Jiang, Y.Y. Vaccines in the treatment of invasive candidiasis. Virulence 2015, 6, 309–315. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Spellberg, B.J.; Avanesian, V.; Fu, Y.; Edwards, J.E., Jr. The anti-candida vaccine based on the recombinant n-terminal domain of Als1p is broadly active against disseminated candidiasis. Infect. Immun. 2006, 74, 3039–3304. [Google Scholar] [CrossRef]
- Alqarihi, A.; Singh, S.; Edwards, J.E., Jr.; Ibrahim, A.S.; Uppuluri, P. NDV-3A vaccination prevents C. albicans colonization of jugular vein catheters in mice. Sci Rep. 2019, 9, 6194. [Google Scholar] [CrossRef]
- Vilanova, M.; Teixeira, L.; Caramalho, I.; Torrado, E.; Marques, A.; Madureira, P.; Ribeiro, A.; Ferreira, P.; Gama, M.; Demengeot, J. Protection against systemic candidiasis in mice immunized with secreted aspartic proteinase 2. Immunology 2004, 111, 334–342. [Google Scholar] [CrossRef]
- De Bernardis, F.; Boccanera, M.; Adriani, D.; Girolamo, A.; Cassone, A. Intravaginal and intranasal immunizations are equally effective in inducing vaginal antibodies and conferring protection against vaginal candidiasis. Infect. Immun. 2002, 70, 2725–2729. [Google Scholar] [CrossRef]
- Akhtar, N.; Magdaleno, J.S.L.; Ranjan, S.; Wani, A.K.; Grewal, R.K.; Oliva, R.; Shaikh, A.R.; Cavallo, L.; Chawla, M. Secreted Aspartyl Proteinases Targeted Multi-Epitope Vaccine Design for Candida dubliniensis Using Immunoinformatics. Vaccines 2023, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, S.; Aoki, W.; Nomura, T.; Karasaki, M.; Sewaki, T.; Ueda, M. Evaluation of Mdh1 protein as an antigenic candidate for a vaccine against candidiasis. Biocontrol Sci. 2014, 19, 51–55. [Google Scholar] [CrossRef]
- Matthews, R.C.; Rigg, G.; Hodgetts, S.; Carter, T.; Chapman, C.; Gregory, C.; Illidge, C.; Burnie, J. Preclinical assessment of the efficacy of mycograb, a human recombinant antibody against fungal HSP90. Antimicrob. Agents Chemother. 2003, 47, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Ibrahim, A.S.; French, S.W.; Edwards, J.E., Jr.; Fu, Y. Active and passive immunization with rHyr1p-N protects mice against hematogenously disseminated candidiasis. PLoS ONE 2011, 6, e25909. [Google Scholar] [CrossRef] [PubMed]
- Wüthrich, M.; Dobson, H.E.; Ledesma Taira, C.; Okaa, U.J.; Dos Santos Dias, L.; Isidoro-Ayza, M.; Petrovsky, N.; Klein, B.S. Combination Adjuvants Enhance Recombinant Protein Vaccine Protection against Fungal Infection. mBio 2021, 21, e0201821. [Google Scholar] [CrossRef] [PubMed]
- Adamo, R.; Tontini, M.; Brogioni, G.; Romano, M.R.; Costantini, G.; Danieli, E.; Proietti, D.; Berti, F.; Costantino, P. Synthesis of laminarin fragments and evaluation of a β-(1, 3) glucan hexasaccaride-CRM197 conjugate as vaccine Candidate against Candida albicans. J. Carbohydr. Chem. 2011, 30, 249–280. [Google Scholar] [CrossRef]
- Clemons, K.V.; Danielson, M.E.; Michel, K.S.; Liu, M.; Ottoson, N.C.; Leonardo, S.M.; Martinez, M.; Chen, V.; Antonysamy, M.A.; Stevens, D.A. Whole Glucan Particles as a Vaccine against Murine Aspergillosis. J. Med. Microbiol. 2014, 63, 1750–1759. [Google Scholar] [CrossRef]
- Xin, H.; Dziadek, S.; Bundle, D.R.; Cutler, J.E. Synthetic glycopeptide vaccines combining beta-mannan and peptide epitopes induce protection against candidiasis. Proc. Natl. Acad. Sci. USA 2008, 105, 13526–13531. [Google Scholar] [CrossRef]
- Xin, H.; Cartmell, J.; Bailey, J.J.; Dziadek, S.; Bundle, D.R.; Cutler, J.E. Self-adjuvanting glycopeptide conjugate vaccine against disseminated candidiasis. PLoS ONE 2012, 7, e35106. [Google Scholar] [CrossRef]
- Astronomo, R.D.; Burton, D.R. Carbohydrate vaccines: Developing sweet solutions to sticky situations? Nat. Rev. Drug Discov. 2010, 9, 308–324. [Google Scholar] [CrossRef]
- Peri, F. Clustered carbohydrates in synthetic vaccines. Chem. Soc. Rev. 2013, 42, 4543–4556. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Glee, P.; Adams, A.; Mohiuddin, F.; Eberle, K. Design of a mimotope-peptide based double epitope vaccine against disseminated candidiasis. Vaccine 2019, 37, 2430–2438. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Freytag, L.; Cheng, E.; Mayeux, P.; Domer, J.E.; Clements, J.D. Effectiveness of a vaccine composed of heat-killed candida albicans and a novel mucosal adjuvant, LT(R192G), against systemic candidiasis. Infect. Immun. 1999, 67, 826–833. [Google Scholar] [CrossRef]
- Liu, M.; Clemons, K.V.; Johansen, M.E.; Martinez, M.; Chen, V.; Stevens, D.A. Saccharomyces as a vaccine against systemic candidiasis. Immunol. Investig. 2012, 41, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.A.; Clemons, K.V.; Liu, M. Developing a vaccine against-aspergillosis. Med. Mycol. 2011, 49 (Suppl. 1), S170–S176. [Google Scholar] [CrossRef]
- Martinez, M.; Clemons, K.V.; Stevens, D.A. Heat-Killed Yeast as a Pan-Fungal Vaccine. Methods Mol. Biol. 2017, 1625, 23–30. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, P. Yeast-based vaccines: New perspective in vaccine development and application. FEMS Yeast Res. 2019, 19, foz007. [Google Scholar] [CrossRef]
- Martin-Cruz, L.; Sevilla-Ortega, C.; Benito-Villalvilla, C.; Diez-Rivero, C.M.; Sanchez-Ramón, S.; Subiza, J.L.; Palomares, O. A combination of polybacterial MV140 and Candida albicans V132 as a potential novel trained immunity-based vaccine for genitourinary tract infections. Front. Immunol. 2020, 11, 612269. [Google Scholar] [CrossRef]
- Shibasaki, S.; Aoki, W.; Nomura, T.; Miyoshi, A.; Tafuku, S.; Sewaki, T.; Ueda, M. An oral vaccine against candidiasis generated by a yeast molecular display system. Pathog. Dis. 2013, 69, 262–268. [Google Scholar] [CrossRef]
- Shibasaki, S.; Karasaki, M.; Tafuku, S.; Aoki, W.; Sewaki, T.; Ueda, M. Oral Immunization Against Candidiasis Using Lactobacillus casei Displaying Enolase 1 from Candida albicans. Sci. Pharm. 2014, 82, 697–708. [Google Scholar] [CrossRef]
- Aoki, W.; Ueda, T.; Tatsukami, Y.; Kitahara, N.; Morisaka, H.; Kuroda, K.; Ueda, M. Time-course proteomic profile of Candida albicans during adaptation to a fetal serum. Pathog. Dis. 2013, 67, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ji, H.; Kong, X.; Lei, P.; Yang, Q.; Wu, W.; Jin, L.; Sun, D. Bacterial Ghosts-Based Vaccine and Drug Delivery Systems. Pharmaceutics 2021, 13, 1892. [Google Scholar] [CrossRef] [PubMed]
- Abdelnaby, M.A.; Shoueir, K.R.; Ghazy, A.A.; Abdelhamid, S.M.; El Kemary, M.A.; Mahmoud, H.E.; Baraka, K.; Abozahra, R.R. Synthesis and evaluation of metallic nanoparticles-based vaccines against Candida albicans infections. J. Drug Deliv. Sci. Technol. 2022, 68, 102862. [Google Scholar] [CrossRef]
- Peroumal, D.; Sahu, S.R.; Kumari, P.; Utkalaja, B.; Acharya, N. Commensal fungi candida albicans modulates dietary high-fat induced alterations in metabolism, immunity, and gut microbiota. bioRxiv 2022. [Google Scholar] [CrossRef]
- Akhtar, N.; Singh, A.; Upadhyay, A.K.; Mannan, M.A. Design of a multi-epitope vaccine against the pathogenic fungi Candida tropicalis using an in silico approach. J. Genet. Eng. Biotechnol. 2022, 20, 140. [Google Scholar] [CrossRef] [PubMed]
- Tarang, S.; Kesherwani, V.; LaTendresse, B.; Lindgren, L.; Rocha-Sanchez, S.M.; Weston, M.D. In silico design of a multivalent vaccine against Candida albicans. Sci. Rep. 2020, 10, 1066. [Google Scholar] [CrossRef]
- Rayens, E.; Rabacal, W.; Willems, H.M.E.; Kirton, G.M.; Barber, J.P.; Mousa, J.J.; Celia-Sanchez, B.N.; Momany, M.; Norris, K.A. Immunogenicity and protective efficacy of a pan-fungal vaccine in preclinical models of aspergillosis, candidiasis, and pneumocystosis. Proc. Natl. Acad. Sci. USA Nexus 2022, 1, pgac248. [Google Scholar] [CrossRef]
- Sobel, J.D. Recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 2016, 214, 15–21. [Google Scholar] [CrossRef]
- Ferwerda, G.; Netea, M.G.; Joosten, L.A.; van der Meer, J.W.M.; Romani, L.; Kullberg, B.J. The role of Toll-like receptors and C-type lectins for vaccination against Candida albicans. Vaccine 2010, 28, 614–622. [Google Scholar] [CrossRef]
- Sharma, J.; Mudalagiriyappa, S.; Nanjappa, S.G. T cell responses to control fungal infection in an immunological memory lens. Front. Immunol. 2022, 13, 905867. [Google Scholar] [CrossRef]
- Portuondo, D.L.F.; Ferreira, L.S.; Urbaczek, A.C.; Batista-Duharte, A.; Carlos, I.Z. Adjuvants and delivery systems for antifungal vaccines: Current state and future developments. Med. Mycol. 2015, 53, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, M.R.; Aggarwal, B.B.; Rinderknecht, E.R.N.S.T.; Svedersky, L.P.; Finkle, B.S.; Palladino, M.A., Jr. Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J. Immunol. 1985, 135, 2069–2073. [Google Scholar] [CrossRef] [PubMed]
- Mengesha, B.G.; Conti, H.R. The role of IL-17 in protection against mucosal Candida infections. J. Fungi 2017, 3, 52. [Google Scholar] [CrossRef] [PubMed]
- Fidel, P.L., Jr. Immunity to Candida. Oral Dis. 2002, 8, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Hohl, T.M.; Rivera, A.; Pamer, E.G. Immunity to fungi. Curr. Opin. Immunol. 2006, 18, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Cassone, A.; De Bernardis, F.; Torososantucci, A. An outline of the role of anti-Candida antibodies within the context of passive immunization and protection from candidiasis. Curr. Mol. Med. 2005, 5, 377–382. [Google Scholar] [CrossRef]
- Nathan, C.F.; Murray, H.W.; Wiebe, M.E.; Rubin, B.Y. Identification of interferon-γ as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 1983, 158, 670–689. [Google Scholar] [CrossRef]
- Eyerich, S.; Wagener, J.; Wenzel, V.; Scarponi, C.; Pennino, D.; Albanesi, C.; Schaller, M.; Behrendt, H.; Ring, J.; Schmidt-Weber, C.B.; et al. IL-22 and TNF-α represent a key cytokine combination for epidermal integrity during infection with Candida albicans. Eur. J. Immunol. 2011, 41, 1894–1901. [Google Scholar] [CrossRef]
- Tavares, D.; Ferreira, P.; Arala-Chaves, M. Increased resistance to systemic candidiasis in athymic or interleukin-10-depleted mice. J. Infect. Dis. 2000, 182, 266–273. [Google Scholar] [CrossRef]
- Mencacci, A.; Del Sero, G.; Cenci, E.; d’Ostiani, C.F.; Bacci, A.; Montagnoli, C.; Kopf, M.; Romani, L. Endogenous interleukin 4 is required for development of protective CD4+ T helper type 1 cell responses to Candida albicans. J. Exp. Med. 1998, 187, 307–317. [Google Scholar] [CrossRef]
- Tsoni, S.V.; Kerrigan, A.M.; Marakalala, M.J.; Srinivasan, N.; Duffield, M.; Taylor, P.R.; Botto, M.; Steele, C.; Brown, G.D. Complement C3 plays an essential role in the control of opportunistic fungal infections. Infect. Immun. 2009, 77, 3679–3685. [Google Scholar] [CrossRef] [PubMed]
- Mullick, A.; Elias, M.; Picard, S.; Bourget, L.; Jovcevski, O.; Gauthier, S.; Tuite, A.; Harakidas, P.; Bihun, C.; Massie, B.; et al. Dysregulated inflammatory response to Candida albicans in a C5-deficient mouse strain. Infect. Immun. 2004, 72, 5868–5876. [Google Scholar] [CrossRef] [PubMed]
- Rudkin, F.M.; Raziunaite, I.; Workman, H.; Essono, S.; Belmonte, R.; MacCallum, D.M.; Johnson, E.M.; Silva, L.M.; Palma, A.S.; Feizi, T.; et al. Single human B cell-derived monoclonal anti-Candida antibodies enhance phagocytosis and protect against disseminated candidiasis. Nat. Commun. 2018, 9, 5288. [Google Scholar] [CrossRef] [PubMed]
- Sandini, S.; La Valle, R.; Deaglio, S.; Malavasi, F.; Cassone, A.; De Bernardis, F. A highly immunogenic recombinant and truncated protein of the secreted aspartic proteases family (rSap2t) of Candida albicans as a mucosal anti-candidal vaccine. FEMS Immunol. Med. Microbiol. 2011, 62, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Torosantucci, A.; Chiani, P.; Bromuro, C.; De Bernardis, F.; Palma, A.S.; Liu, Y.; Mignogna, G.; Maras, B.; Colone, M.; Stringaro, A.; et al. Protection by anti-β-glucan antibodies is associated with restricted β-1,3 glucan binding specificity and inhibition of fungal growth and adherence. PLoS ONE 2009, 4, e5392. [Google Scholar] [CrossRef]
- Traeder, C.; Kowoll, S.; Araste´h, K. Candida infection in HIV positive patients 1985–2007. Mycoses 2008, 51 (Suppl. 2), 58–61. [Google Scholar] [CrossRef]
- Cassone, A.; Cauda, R. Candida and candidiasis in HIV-infected patients: Where commensalism, opportunistic behavior and frank pathogenicity lose their borders. Aids 2012, 26, 1457–1472. [Google Scholar] [CrossRef]
- Hirakawa, M.P.; Martinez, D.A.; Sakthikumar, S.; Anderson, M.Z.; Berlin, A.; Gujja, S.; Zeng, Q.; Zisson, E.; Wang, J.M.; Greenberg, J.M.; et al. Genetic and phenotypic intra-species variation in Candida albicans. Genome Res. 2015, 25, 413–425. [Google Scholar] [CrossRef]
- Heilmann, C.J.; Sorgo, A.G.; Siliakus, A.R.; Dekker, H.L.; Brul, S.; De Koster, C.G.; de Koning, L.J.; Klis, F.M. Hyphal induction in the human fungal pathogen Candida albicans reveals a characteristic wall protein profile. Microbiology 2011, 157, 2297–2307. [Google Scholar] [CrossRef]
- Höfs, S.; Mogavero, S.; Hube, B. Interaction of Candida albicans with host cells: Virulence factors, host defense, escape strategies, and the microbiota. J. Microbiol. 2016, 54, 149–169. [Google Scholar] [CrossRef]
- Gaziano, R.; Sabbatini, S.; Monari, C. The Interplay between Candida albicans, Vaginal Mucosa, Host Immunity and Resident Microbiota in Health and Disease: An Overview and Future Perspectives. Microorganisms 2023, 11, 1211. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.B.; Johnson, A.D. White-opaque switching in Candida albicans. Curr. Opin. Microbiol. 2009, 12, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Selmecki, A.; Forche, A.; Berman, J. Genomic plasticity of the human fungal pathogen Candida albicans. Eukaryot. Cell 2010, 9, 991–1008. [Google Scholar] [CrossRef] [PubMed]
- Cassone, A. Development of vaccines for Candida albicans: Fighting a skilled transformer. Nat. Rev. Microbiol. 2013, 11, 884–891. [Google Scholar] [CrossRef]
- Romani, L.; Zelante, T.; De Luca, A.; Iannitti, R.G.; Moretti, S.; Bartoli, A.; Aversa, F.; Puccetti, P. Microbiota control of a tryptophan-AhR pathway in disease tolerance to fungi. Eur. J. Immunol. 2014, 44, 3192–3200. [Google Scholar] [CrossRef]
- Duggan, S.; Leonhardt, I.; Hunniger, K.; Kurzai, O. Host response to Candida albicans bloodstream infection and sepsis. Virulence 2015, 6, 316–326. [Google Scholar]
- Carpino, N.; Naseem, S.; Frank, D.M.; Konopka, J.B. Modulating host signaling pathways to promote resistance to infection by Candida albicans. Front. Cell. Infect. Microbiol. 2017, 7, 481. [Google Scholar] [CrossRef]
- Geddes-McAlister, J.; Shapiro, R.S. New pathogens, new tricks: Emerging, drug-resistant fungal pathogens and future prospects for antifungal therapeutics: Drug-resistant fungal pathogens. Ann. N. Y. Acad. Sci. 2019, 1435, 57–78. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.; van der Meer, J.W.; Mhlanga, M.M.; Mulder, W.J.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Quintin, J.; Saeed, S.; Martens, J.H.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.J.; Wijmenga, C.; et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef]
- Leentjens, J.; Quintin, J.; Gerretsen, J.; Kox, M.; Pickkers, P.; Netea, M.G. The effects of orally administered Beta-glucan on innate immune responses in humans, a randomized open-label intervention pilot-study. PLoS ONE 2014, 9, e108794. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ramón, S.; Conejero, L.; Netea, M.G.; Sancho, D.; Palomares, Ó.; Subiza, J.L. Trained Immunity-Based Vaccines: A New Paradigm for the Development of Broad-Spectrum Anti-infectious Formulations. Front. Immunol. 2018, 9, 2936. [Google Scholar] [CrossRef] [PubMed]
- Moragues, M.D.; Rementeria, A.; Sevilla, M.J.; Eraso, E.; Quindos, G. Candida antigens and immune responses: Implications for a vaccine. Expert Rev. Vaccines 2014, 13, 1001–1012. [Google Scholar] [CrossRef]
- Masuoka, J. Surface glycans of Candida albicans and other pathogenic fungi: Physiological roles, clinical uses, and experimental challenges. Clin. Microbiol. Rev. 2004, 17, 281–310. [Google Scholar] [CrossRef]
- Kaur, H.; Chakrabarti, A. Strategies to reduce mortality in adult and neonatal candidemia in developing countries. J. Fungi 2017, 3, 41. [Google Scholar] [CrossRef]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal drug resistance: Molecular mechanisms in Candida albicans and beyond. Chem. Rev. 2021, 121, 3390–3411. [Google Scholar] [CrossRef]
- Han, Y.; Rhew, K.Y. Ginsenoside Rd induces protective anti-Candida albicans antibody through immunological adjuvant activity. Int. Immunopharmacol. 2013, 17, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Yurina, V.; Adianingsih, O.R. Predicting epitopes for vaccine development using bioinformatics tools. Ther. Adv. Vaccines Immunother. 2022, 10, 25151355221100218. [Google Scholar] [CrossRef]


| Vaccine Category | Description | Clinical Trial Phase | Preclinical and Clinical Data on Vaccine Efficacy and Safety | Reference |
|---|---|---|---|---|
| Live Attenuated | Genetically modified C. albicans tet-NRG1 strain engineered for hyphal growth control; PCA-2, CNC13, RML2U, and tet-NRG1 strains effective against infections; Saccharomyces cerevisiae as a safe carrier for vaccines. | Preclinical | Genetically modified C. albicans effectively protect against candidiasis in mouse models. Saccharomyces cerevisiae as a vaccine carrier demonstrates safety and immune response. | [30,50] |
| Recombinant (Subunit) | Utilization of recombinant proteins (Als proteins, Sap-2, and NDV-3); PEV7 containing modified aspartyl proteinase-2; strong antibody responses and protection in animal models. | Phase I/II | Phase I/II trials show stimulation of strong antibody and specific T cell responses. PEV7 and NDV-3 demonstrate enhanced efficacy in animal models. | [10,33,36] |
| Conjugate | Fusion of potent antigens with polysaccharides; targeting shared polysaccharide epitopes (β-glucans; mannans); protection against candidiasis and aspergillosis. | Preclinical/Phase I/II | Preclinical studies demonstrate protection against fungal infections. Phase I/II trials show immunostimulatory effects and potential for pan-fungal vaccine. | [30,50,63] |
| Killed Whole-Cell | Whole-cell killed vaccine approach (heat-killed C. albicans); protection against systemic candidiasis and other fungal infections. | Preclinical/Phase I/II | Preclinical studies show protection against systemic candidiasis. Phase I/II trials indicate potential for broad-spectrum fungal protection. | [10,68] |
| Oral | Display of immunogenic proteins on microbial cell surfaces; Eno1p antigen from C. albicans displayed on E. coli and S. cerevisiae cells; protection against candidiasis. | Preclinical/Phase I/II | Preclinical studies demonstrate protection against candidiasis. Phase I/II trials show efficacy protection. | [73,74] |
| Multi-Epitope | In silico design of a multi-epitope vaccine against C. dubliniensis; potential immunogenicity; further in vivo investigations needed. | Preclinical | Immunoinformatic approach identifies eight epitopes for a C. dubliniensis vaccine candidate with potential immunogenicity. Further in vivo studies required for safety and efficacy assessment. | [79] |
| β-(1,3) Glucan | Synthesis of linear β-(1,3) glucan-CRM197 conjugate; elicits uniform IgG response in mice; exploration of protective potential. | Phase I/II | Linear β-(1,3) glucan-CRM197 conjugate induces a uniform IgG response in mice, demonstrating potential for C. albicans epitope coverage. Efforts are underway to explore protective potential. | [60] |
| Proteome-Wide Subunit | Identification of immunodominant epitopes in hyphal proteins; broad applicability; RS09 adjuvant inclusion. | Preclinical/Phase I/II | Proteome-wide immunoinformatic strategy to select 18 epitopes for C. albicans subunit vaccine. Epitopes are conserved and bound to multiple HLA class II alleles. RS09 used as an adjuvant enhances immune response. | [80] |
| Pan-Fungal Recombinant | Development of NXT-2, a pan-fungal recombinant protein vaccine; effectiveness against aspergillosis, candidiasis, and pneumocystosis; cross-reactivity. | Preclinical/Phase I/II | NXT-2 demonstrates effectiveness against multiple fungal infections in animal models. It elicits strong immune responses and shows cross-reactivity with various fungal pathogens. Further research needed for evaluation in humans. | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, G.; Chawla, S.; Kumar, P.; Singh, R. Advancing Vaccine Strategies against Candida Infections: Exploring New Frontiers. Vaccines 2023, 11, 1658. https://doi.org/10.3390/vaccines11111658
Kaur G, Chawla S, Kumar P, Singh R. Advancing Vaccine Strategies against Candida Infections: Exploring New Frontiers. Vaccines. 2023; 11(11):1658. https://doi.org/10.3390/vaccines11111658
Chicago/Turabian StyleKaur, Gurpreet, Sonam Chawla, Piyush Kumar, and Ritu Singh. 2023. "Advancing Vaccine Strategies against Candida Infections: Exploring New Frontiers" Vaccines 11, no. 11: 1658. https://doi.org/10.3390/vaccines11111658






