In Vitro Pre-Clinical Evaluation of a Gonococcal Trivalent Candidate Vaccine Identified by Transcriptomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antigens
2.2. Immunization of Mice
2.3. Bacterial Strains and Growth Conditions
2.4. Human Sera
2.5. Human Antibody ELISA
2.6. Mouse Antibody ELISA
2.7. Antibody Avidity
2.8. Cytokine ELISA
2.9. Serum Bactericidal Activity (SBA)
2.10. Statistical Analysis
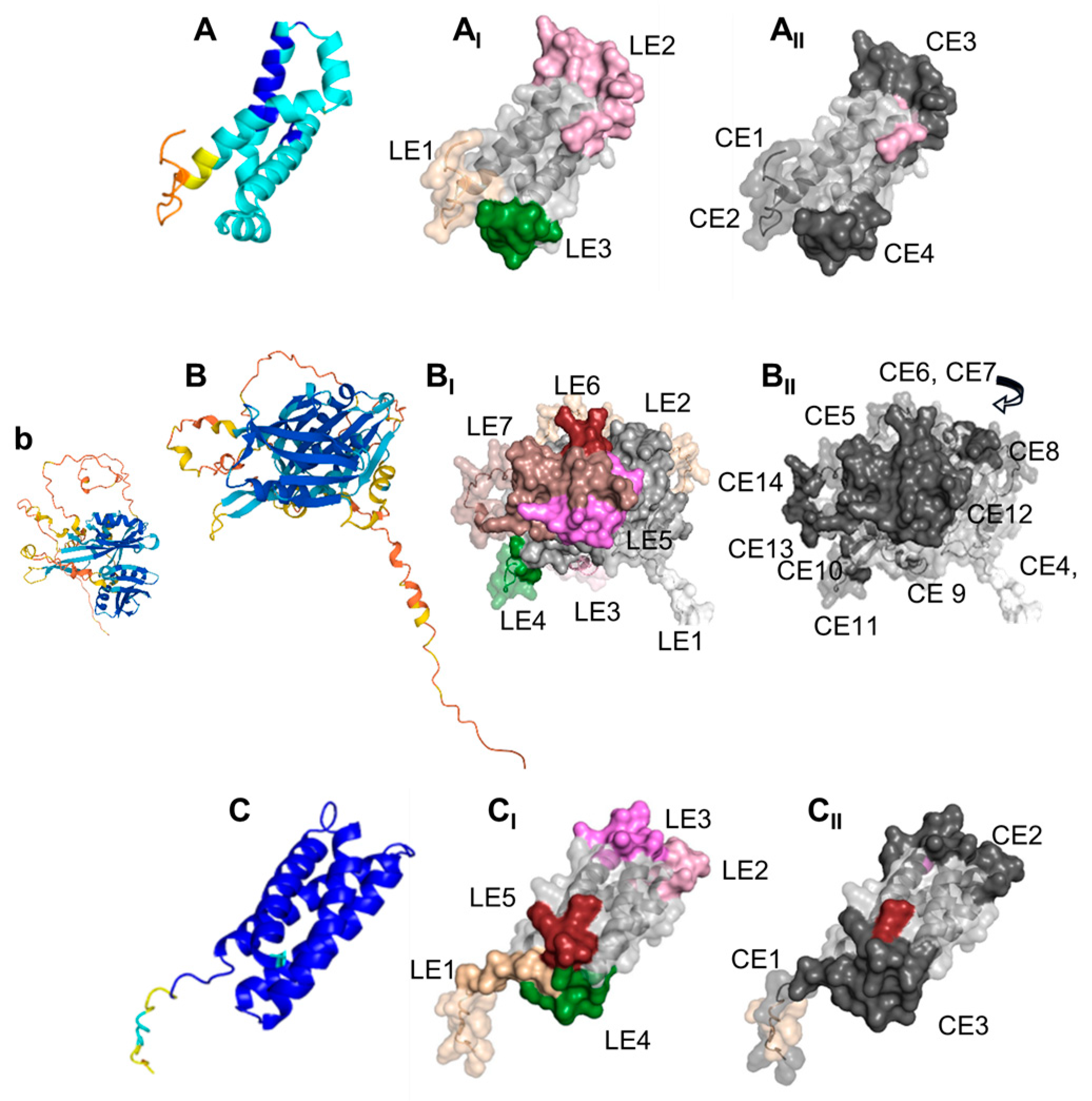
2.11. Modeling and B Cell Epitope Predictions
3. Results
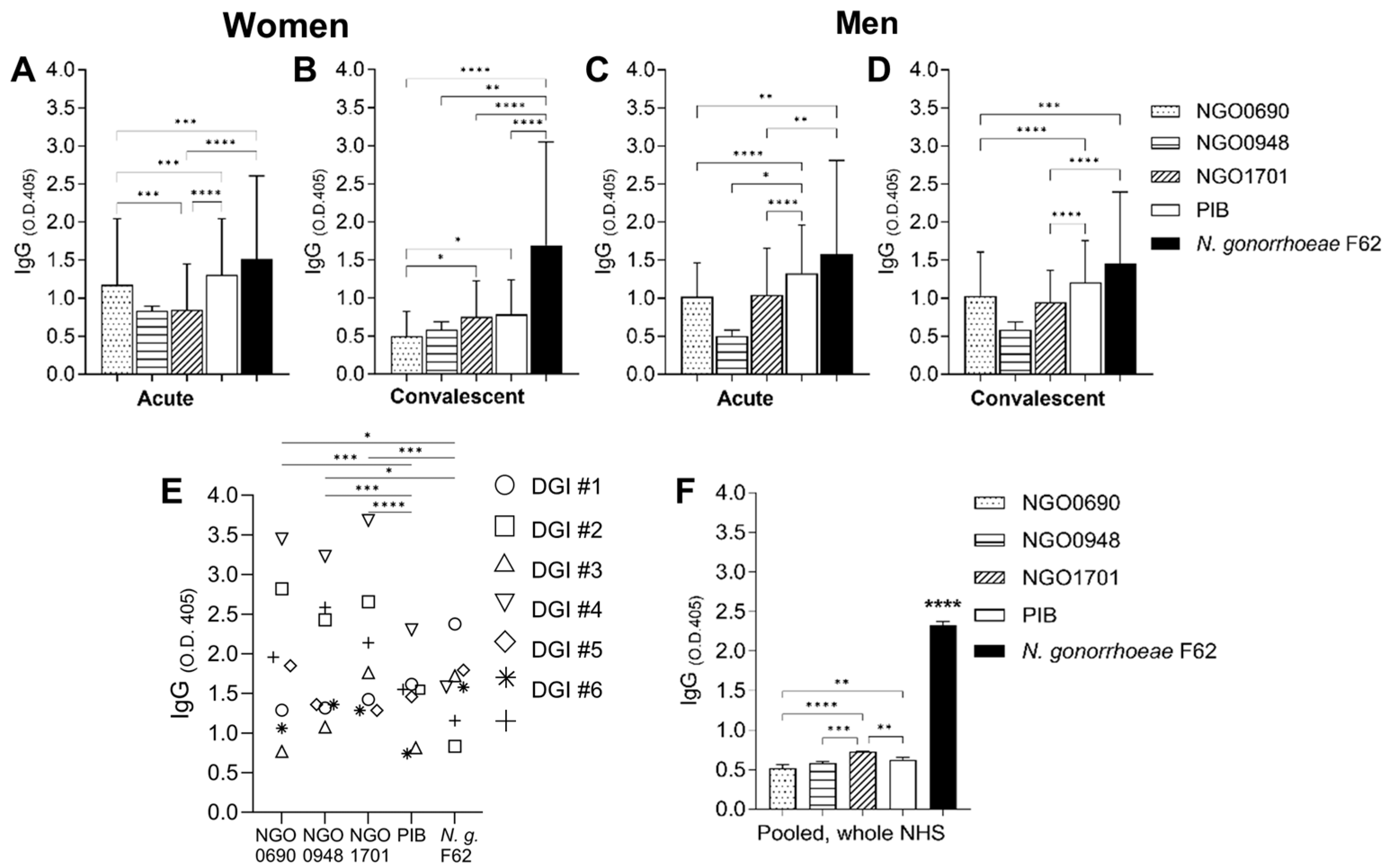
3.1. Antigen Recognition by Human Sera
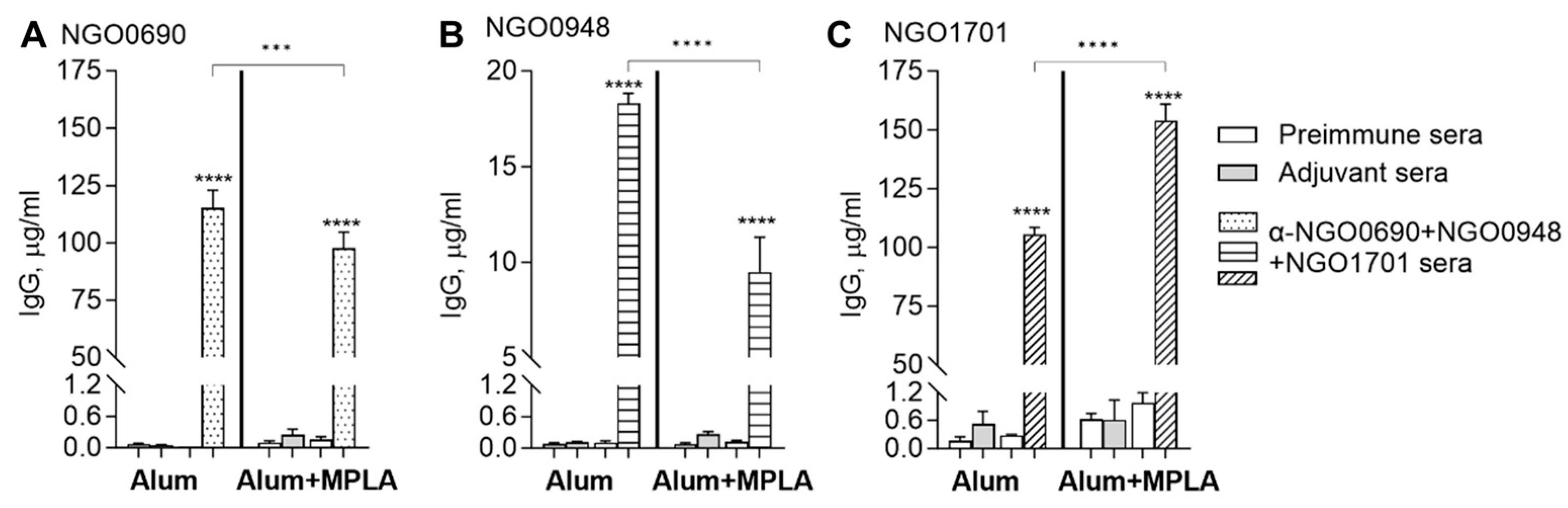
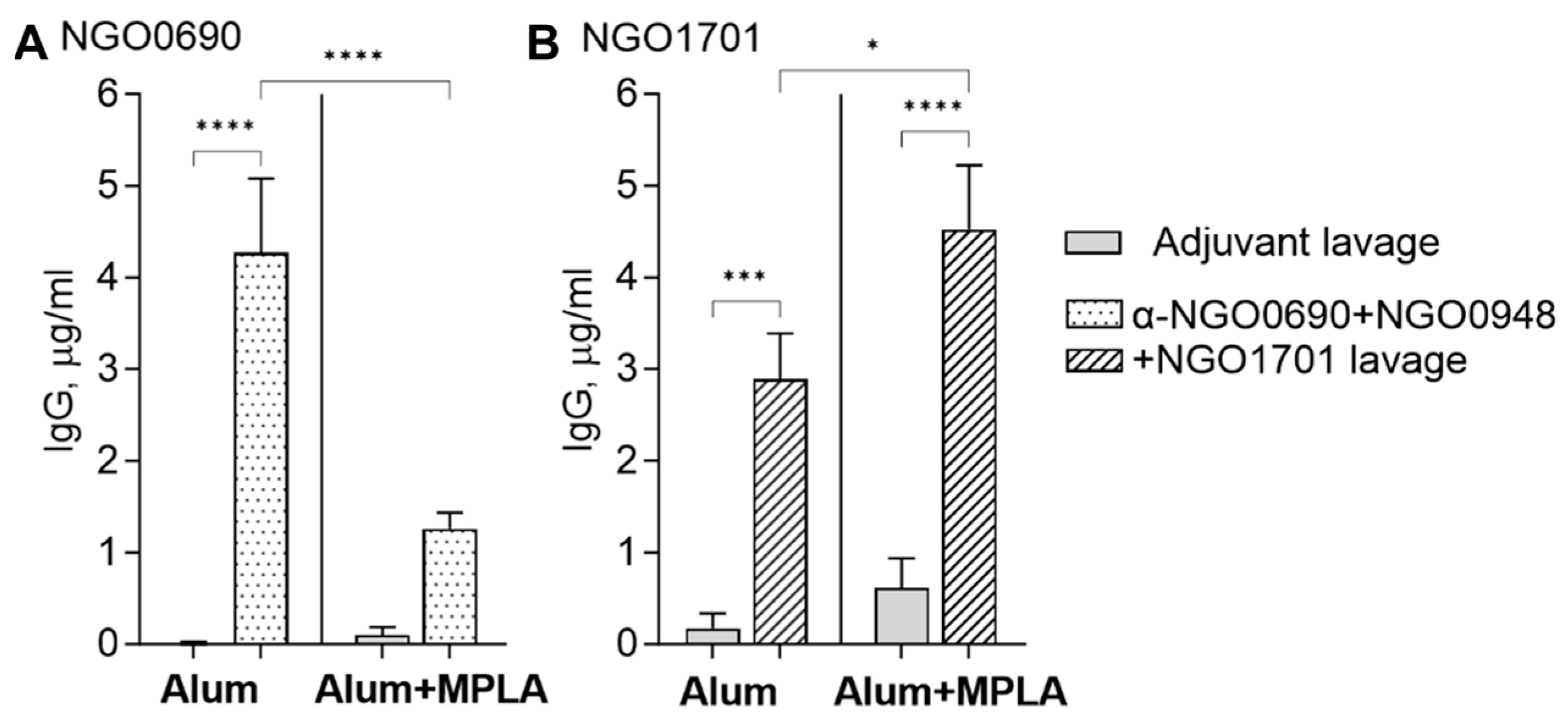
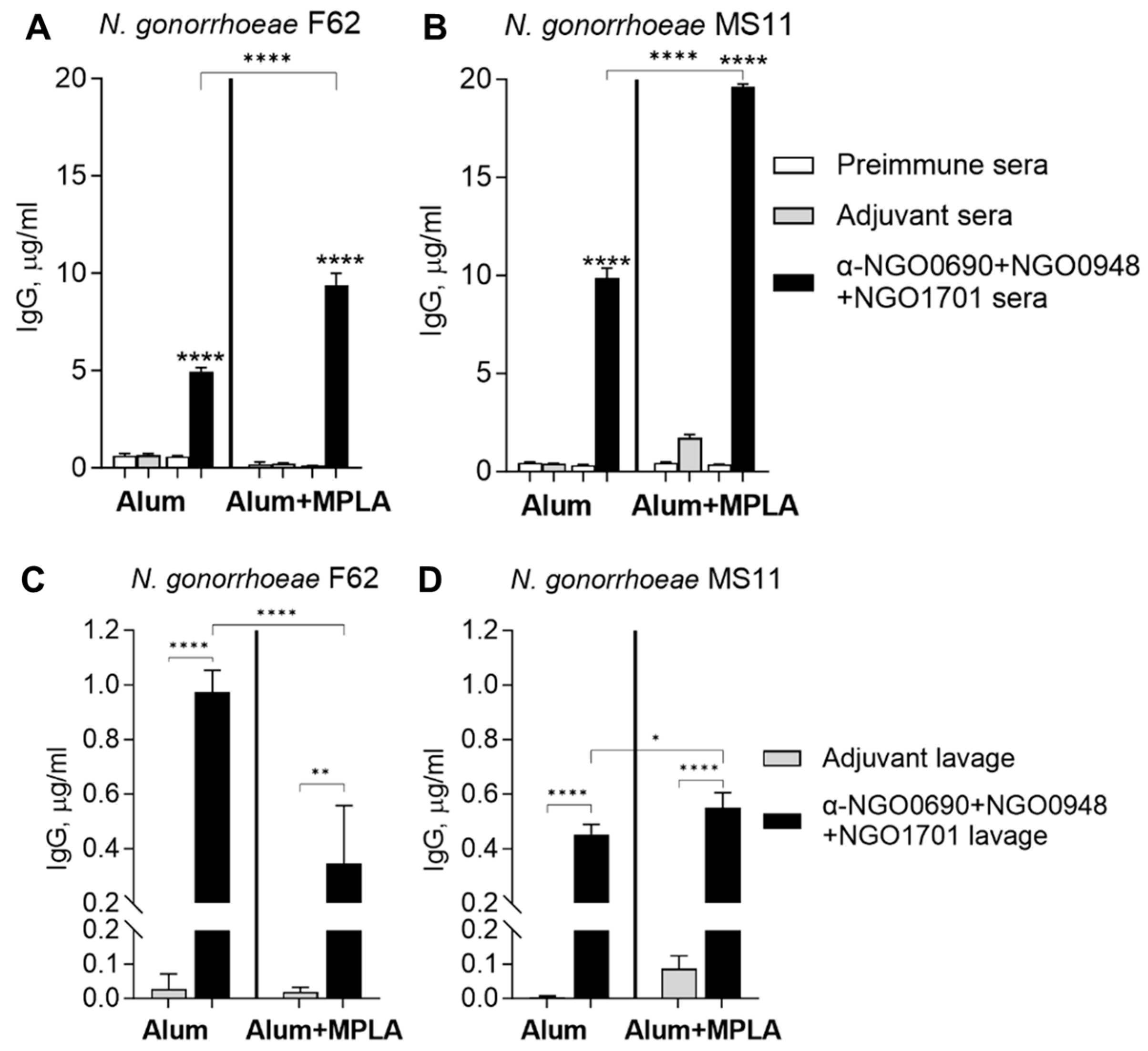
3.2. Antibody Responses in Mice to a Multi-Antigen Vaccine and Effects of Adjuvants
3.2.1. Total IgG Antibody Responses to Purified Antigens
3.2.2. Serum and Vaginal Lavage Total IgG Induced by Combined Antigens against Whole N. gonorrhoeae
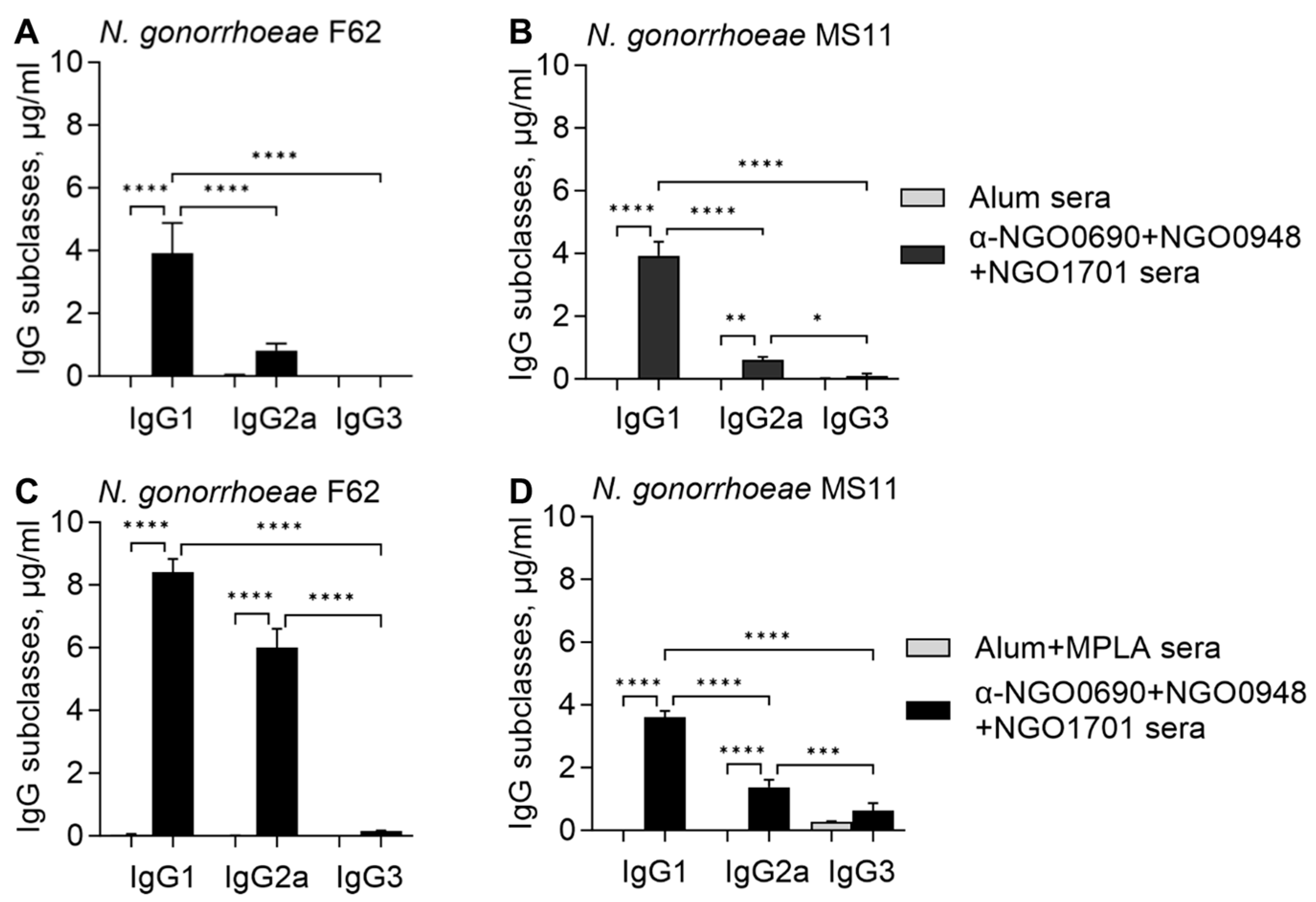
3.2.3. Serum IgG Antibody Subclasses against Whole N. gonorrhoeae
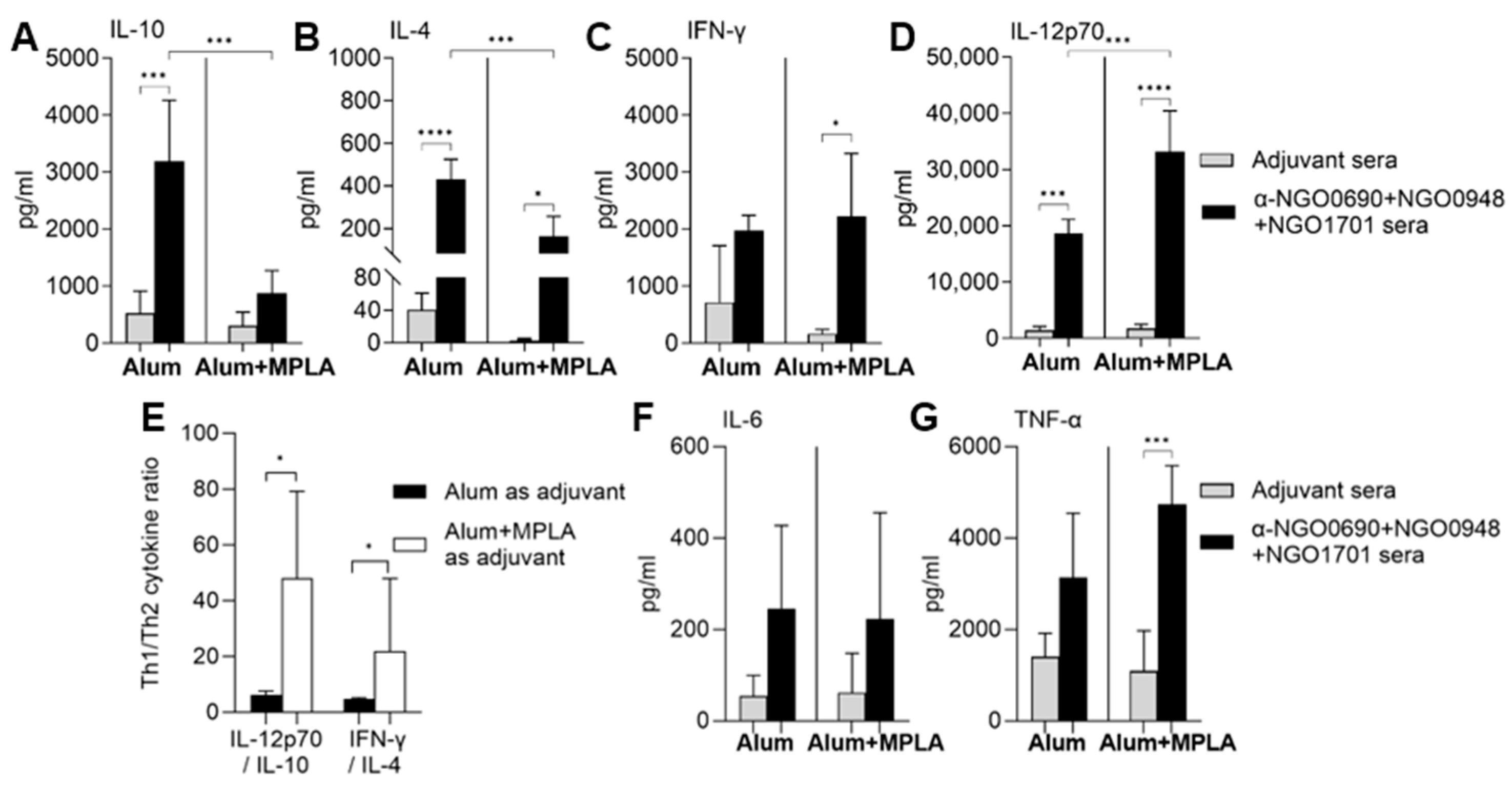
3.3. Serum Cytokine Production Induced by Combined Antigens
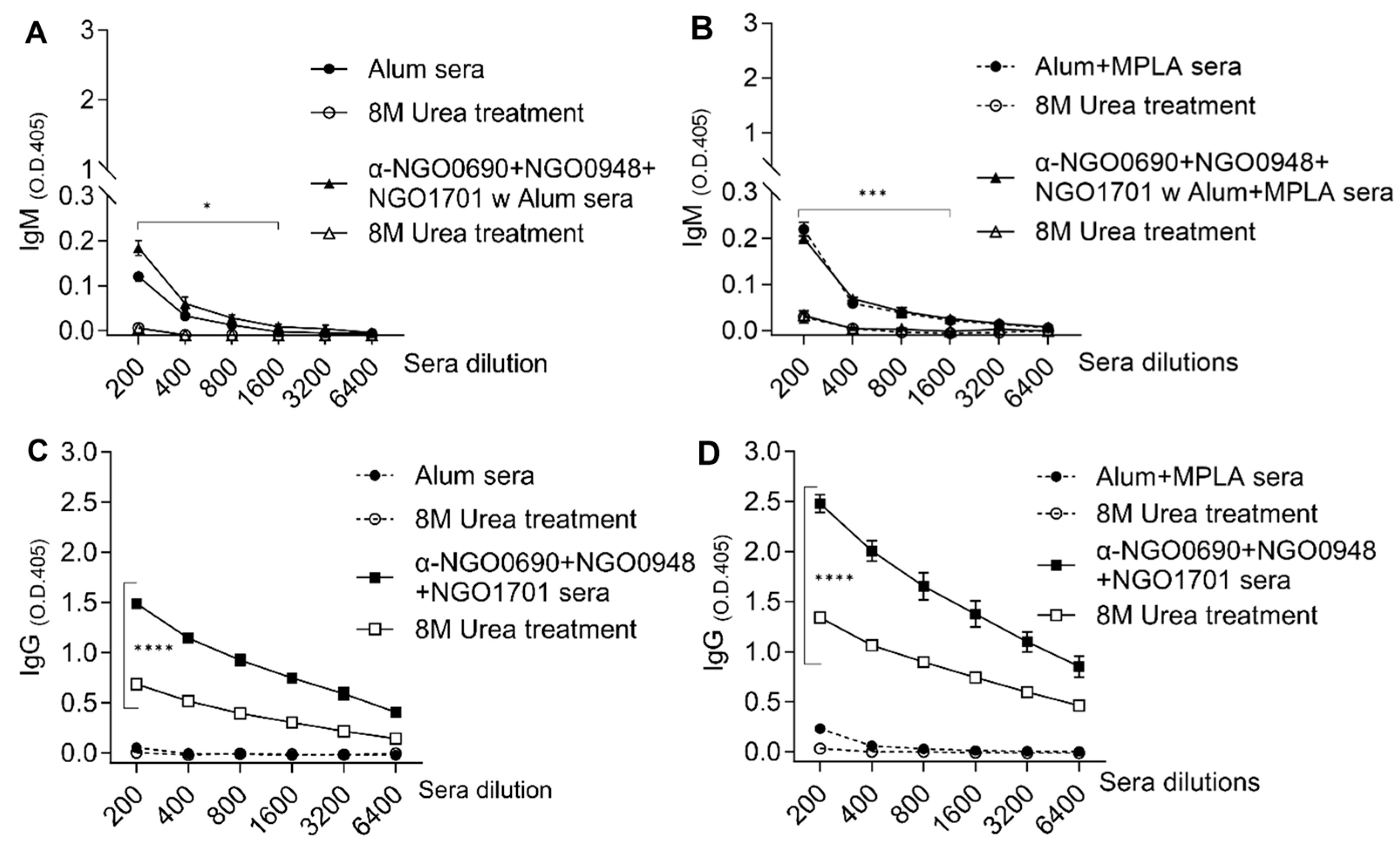
3.4. Serum IgM Antibodies against Whole N. gonorrhoeae Induced by Combined Antigens
3.5. Antibody Avidity
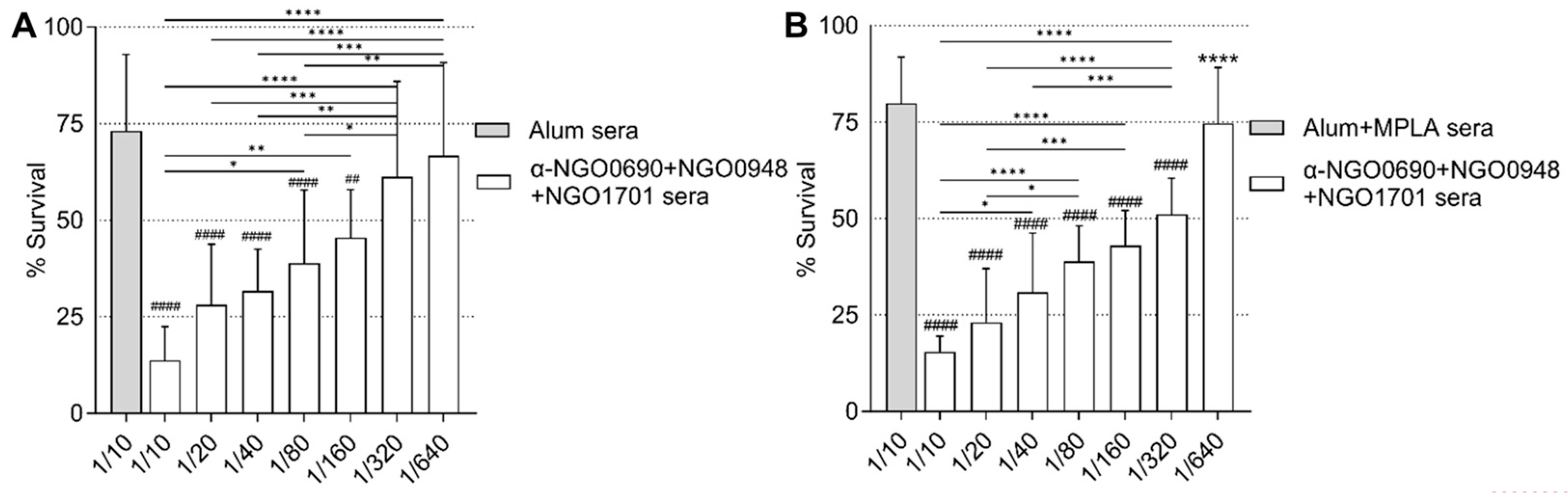
3.6. Serum Bactericidal Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- CDC. Sexually Transmitted Disease Surveillance 2021. Available online: https://www.cdc.gov/std/statistics/2021/overview.htm#Gonorrhea (accessed on 23 October 2023).
- Unemo, M.; Seifert, H.S.; Hook, E.W., 3rd; Hawkes, S.; Ndowa, F.; Dillon, J.R. Gonorrhoea. Nat. Rev. Dis. Primers 2019, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Lenz, J.D.; Dillard, J.P. Pathogenesis of Neisseria gonorrhoeae and the Host Defense in Ascending Infections of Human Fallopian Tube. Front. Immunol. 2018, 9, 2710. [Google Scholar] [CrossRef] [PubMed]
- Fairley, C.K.; Hocking, J.S.; Zhang, L.; Chow, E.P. Frequent Transmission of Gonorrhea in Men Who Have Sex with Men. Emerg. Infect. Dis. 2017, 23, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Lahra, M.M.; Escher, M.; Eremin, S.; Cole, M.J.; Galarza, P.; Ndowa, F.; Martin, I.; Dillon, J.R.; Galas, M.; et al. WHO global antimicrobial resistance surveillance for Neisseria gonorrhoeae 2017–2018: A retrospective observational study. Lancet Microbe 2021, 2, e627–e636. [Google Scholar] [CrossRef] [PubMed]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef] [PubMed]
- Plummer, F.A.; Simonsen, J.N.; Chubb, H.; Slaney, L.; Kimata, J.; Bosire, M.; Ndinya-Achola, J.O.; Ngugi, E.N. Epidemiologic evidence for the development of serovar-specific immunity after gonococcal infection. J. Clin. Investig. 1989, 83, 1472–1476. [Google Scholar] [CrossRef]
- Schmidt, K.A.; Schneider, H.; Lindstrom, J.A.; Boslego, J.W.; Warren, R.A.; Van de Verg, L.; Deal, C.D.; McClain, J.B.; Griffiss, J.M. Experimental gonococcal urethritis and reinfection with homologous gonococci in male volunteers. Sex Transm. Dis. 2001, 28, 555–564. [Google Scholar] [CrossRef]
- Darville, T. Pelvic Inflammatory Disease Due to Neisseria gonorrhoeae and Chlamydia trachomatis: Immune Evasion Mechanisms and Pathogenic Disease Pathways. J. Infect. Dis. 2021, 224, S39–S46. [Google Scholar] [CrossRef]
- Greenberg, L.; Diena, B.B.; Ashton, F.A.; Wallace, R.; Kenny, C.P.; Znamirowski, R.; Ferrari, H.; Atkinson, J. Gonococcal vaccine studies in Inuvik. Can. J. Public Health 1974, 65, 29–33. [Google Scholar]
- Boslego, J.W.; Tramont, E.C.; Chung, R.C.; McChesney, D.G.; Ciak, J.; Sadoff, J.C.; Piziak, M.V.; Brown, J.D.; Brinton, C.C., Jr.; Wood, S.W.; et al. Efficacy trial of a parenteral gonococcal pilus vaccine in men. Vaccine 1991, 9, 154–162. [Google Scholar] [CrossRef]
- McChesney, D.; Tramont, E.C.; Boslego, J.W.; Ciak, J.; Sadoff, J.; Brinton, C.C. Genital antibody response to a parenteral gonococcal pilus vaccine. Infect. Immun. 1982, 36, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Seifert, H.S.; Wright, C.J.; Jerse, A.E.; Cohen, M.S.; Cannon, J.G. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J. Clin. Investig. 1994, 93, 2744–2749. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.A.; Vayo, H.E.; Tam, M.R.; Blake, M.S. Immunoglobin G antibodies directed against protein III block killing of serum resistant Neisseria gonorrhoeae by immune sera. J. Exp. Med. 1986, 164, 1735–1748. [Google Scholar] [CrossRef] [PubMed]
- Rosenqvist, E.; Musacchio, A.; Aase, A.; Hoiby, E.A.; Namork, E.; Kolberg, J.; Wedege, E.; Delvig, A.; Dalseg, R.; Michaelsen, T.E.; et al. Functional activities and epitope specificity of human and murine antibodies against the class 4 outer membrane protein (Rmp) of Neisseria meningitidis. Infect. Immun. 1999, 67, 1267–1276. [Google Scholar] [CrossRef]
- Rice, P.A.; Hook, E.W.; Blake, M.S.; Kaslow, R.S.; Gulati, S.; Kohl, P.K.; VanRadden, M.; Buchanan, T.M. A possible influence of vaccine induced Por, LOS and Rmp antibodies on the outcome of intraurethral challenge with Neisseria gonorrhoeae. In Neisseria 94, Proceedings of the Ninth International Pathogenic Neisseria Conference, Winchester, UK, 26–30 September 1994; Maiden, M.C., Feavers, I.M., Evans, J.D., Yost, S.E., Eds.; Merieux: Winchester, UK, 1994; pp. 483–484. [Google Scholar]
- Gulati, S.; Shaughnessy, J.; Ram, S.; Rice, P.A. Targeting Lipooligosaccharide (LOS) for a Gonococcal Vaccine. Front. Immunol. 2019, 10, 321. [Google Scholar] [CrossRef]
- Gulati, S.; Pennington, M.W.; Czerwinski, A.; Carter, D.; Zheng, B.; Nowak, N.A.; DeOliveira, R.B.; Shaughnessy, J.; Reed, G.W.; Ram, S.; et al. Preclinical Efficacy of a Lipooligosaccharide Peptide Mimic Candidate Gonococcal Vaccine. mBio 2019, 10, e02552-19. [Google Scholar] [CrossRef]
- Pizza, M.; Scarlato, V.; Masignani, V.; Giuliani, M.M.; Arico, B.; Comanducci, M.; Jennings, G.T.; Baldi, L.; Bartolini, E.; Capecchi, B.; et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing [see comments]. Science 2000, 287, 1816–1820. [Google Scholar] [CrossRef]
- Jain, R.; Sonkar, S.C.; Chaudhry, U.; Bala, M.; Saluja, D. In-silico Hierarchical Approach for the Identification of Potential Universal Vaccine Candidates (PUVCs) from Neisseria gonorrhoeae. J. Theor. Biol. 2016, 410, 36–43. [Google Scholar] [CrossRef]
- Zielke, R.A.; Wierzbicki, I.H.; Baarda, B.I.; Gafken, P.R.; Soge, O.O.; Holmes, K.K.; Jerse, A.E.; Unemo, M.; Sikora, A.E. Proteomics-Driven Antigen Discovery for Development of Vaccines Against Gonorrhea. Mol. Cell. Proteom. 2016, 15, 2338–2355. [Google Scholar] [CrossRef]
- Connor, D.O.; Zantow, J.; Hust, M.; Bier, F.F.; von Nickisch-Rosenegk, M. Identification of Novel Immunogenic Proteins of Neisseria gonorrhoeae by Phage Display. PLoS ONE 2016, 11, e0148986. [Google Scholar] [CrossRef]
- El-Rami, F.E.; Zielke, R.A.; Wi, T.; Sikora, A.E.; Unemo, M. Quantitative Proteomics of the 2016 WHO Neisseria gonorrhoeae Reference Strains Surveys Vaccine Candidates and Antimicrobial Resistance Determinants. Mol. Cell. Proteom. 2019, 18, 127–150. [Google Scholar] [CrossRef]
- Zhu, T.; McClure, R.; Harrison, O.B.; Genco, C.; Massari, P. Integrated Bioinformatic Analyses and Immune Characterization of New Neisseria gonorrhoeae Vaccine Antigens Expressed during Natural Mucosal Infection. Vaccines 2019, 7, 153. [Google Scholar] [CrossRef]
- Jerse, A.E.; Wu, H.; Packiam, M.; Vonck, R.A.; Begum, A.A.; Garvin, L.E. Estradiol-Treated Female Mice as Surrogate Hosts for Neisseria gonorrhoeae Genital Tract Infections. Front. Microbiol. 2011, 2, 107. [Google Scholar] [CrossRef] [PubMed]
- Connolly, K.L.; Pilligua-Lucas, M.; Gomez, C.; Costenoble-Caherty, A.C.; Soc, A.; Underwood, K.; Macintyre, A.N.; Sempowski, G.D.; Jerse, A.E. Preclinical Testing of Vaccines and Therapeutics for Gonorrhea in Female Mouse Models of Lower and Upper Reproductive Tract Infection. J. Infect. Dis. 2021, 224, S152–S160. [Google Scholar] [CrossRef] [PubMed]
- Piekarowicz, A.; Klyz, A.; Stein, D.C. A New Vaccination Method Based on Phage NgoPhi6 and Its Phagemid Derivatives. Front. Microbiol. 2022, 13, 793205. [Google Scholar] [CrossRef]
- Christodoulides, M.; Humbert, M.V.; Heckels, J.E. The potential utility of liposomes for Neisseria vaccines. Expert Rev. Vaccines 2021, 20, 1235–1256. [Google Scholar] [CrossRef] [PubMed]
- Bagwe, P.; Bajaj, L.; Menon, I.; Braz Gomes, K.; Kale, A.; Patil, S.; Vijayanand, S.; Gala, R.; D’Souza, M.J.; Zughaier, S.M. Gonococcal microparticle vaccine in dissolving microneedles induced immunity and enhanced bacterial clearance in infected mice. Int. J. Pharm. 2023, 642, 123182. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Jerse, A.E. Alpha-2,3-sialyltransferase enhances Neisseria gonorrhoeae survival during experimental murine genital tract infection. Infect. Immun. 2006, 74, 4094–4103. [Google Scholar] [CrossRef]
- Hobbs, M.M.; Anderson, J.E.; Balthazar, J.T.; Kandler, J.L.; Carlson, R.W.; Ganguly, J.; Begum, A.A.; Duncan, J.A.; Lin, J.T.; Sparling, P.F.; et al. Lipid A’s structure mediates Neisseria gonorrhoeae fitness during experimental infection of mice and men. mBio 2013, 4, e00892-13. [Google Scholar] [CrossRef]
- Gulati, S.; Beurskens, F.J.; de Kreuk, B.J.; Roza, M.; Zheng, B.; DeOliveira, R.B.; Shaughnessy, J.; Nowak, N.A.; Taylor, R.P.; Botto, M.; et al. Complement alone drives efficacy of a chimeric antigonococcal monoclonal antibody. PLoS Biol. 2019, 17, e3000323. [Google Scholar] [CrossRef]
- Lewis, L.A.; Gulati, S.; Zelek, W.M.; Morgan, B.P.; Song, W.C.; Zheng, B.; Nowak, N.; DeOliveira, R.B.; Sanchez, B.; DeSouza Silva, L.; et al. Efficacy of an Experimental Gonococcal Lipooligosaccharide Mimitope Vaccine Requires Terminal Complement. J. Infect. Dis. 2022, 225, 1861–1864. [Google Scholar] [CrossRef] [PubMed]
- Borrow, R.; Carlone, G.M.; Rosenstein, N.; Blake, M.; Feavers, I.; Martin, D.; Zollinger, W.; Robbins, J.; Aaberge, I.; Granoff, D.M.; et al. Neisseria meningitidis group B correlates of protection and assay standardization—International meeting report Emory University, Atlanta, Georgia, United States, 16–17 March 2005. Vaccine 2006, 24, 5093–5107. [Google Scholar] [CrossRef]
- Gulati, S.; Rice, P.A.; Ram, S. Complement-Dependent Serum Bactericidal Assays for Neisseria gonorrhoeae. Methods Mol. Biol. 2019, 1997, 267–280. [Google Scholar] [CrossRef]
- Semchenko, E.A.; Jen, F.E.; Jennings, M.P.; Seib, K.L. Assessment of Serum Bactericidal and Opsonophagocytic Activity of Antibodies to Gonococcal Vaccine Targets. Methods Mol. Biol. 2022, 2414, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Girgis, M.M.; Christodoulides, M. Vertebrate and Invertebrate Animal and New In Vitro Models for Studying Neisseria Biology. Pathogens 2023, 12, 782. [Google Scholar] [CrossRef]
- Petousis-Harris, H.; Paynter, J.; Morgan, J.; Saxton, P.; McArdle, B.; Goodyear-Smith, F.; Black, S. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: A retrospective case-control study. Lancet 2017, 390, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Paynter, J.; Goodyear-Smith, F.; Morgan, J.; Saxton, P.; Black, S.; Petousis-Harris, H. Effectiveness of a Group B Outer Membrane Vesicle Meningococcal Vaccine in Preventing Hospitalization from Gonorrhea in New Zealand: A Retrospective Cohort Study. Vaccines 2019, 7, 5. [Google Scholar] [CrossRef]
- Reyes Diaz, L.M.; Lastre Gonzalez, M.; Cuello, M.; Sierra-Gonzalez, V.G.; Ramos Pupo, R.; Lantero, M.I.; Harandi, A.M.; Black, S.; Perez, O. VA-MENGOC-BC Vaccination Induces Serum and Mucosal Anti Neisseria gonorrhoeae Immune Responses and Reduces the Incidence of Gonorrhea. Pediatr. Infect. Dis. J. 2021, 40, 375–381. [Google Scholar] [CrossRef]
- Bruxvoort, K.J.; Lewnard, J.A.; Chen, L.H.; Tseng, H.F.; Chang, J.; Veltman, J.; Marrazzo, J.; Qian, L. Prevention of Neisseria gonorrhoeae with Meningococcal B Vaccine: A Matched Cohort Study in Southern California. Clin. Infect. Dis. 2023, 76, e1341–e1349. [Google Scholar] [CrossRef]
- Wang, B.; Giles, L.; Andraweera, P.; McMillan, M.; Almond, S.; Beazley, R.; Mitchell, J.; Ahoure, M.; Denehy, E.; Flood, L.; et al. 4CMenB sustained vaccine effectiveness against invasive meningococcal B disease and gonorrhoea at three years post programme implementation. J. Infect. 2023, 87, 95–102. [Google Scholar] [CrossRef]
- Abara, W.E.; Bernstein, K.T.; Lewis, F.M.T.; Schillinger, J.A.; Feemster, K.; Pathela, P.; Hariri, S.; Islam, A.; Eberhart, M.; Cheng, I.; et al. Effectiveness of a serogroup B outer membrane vesicle meningococcal vaccine against gonorrhoea: A retrospective observational study. Lancet Infect. Dis. 2022, 22, 1021–1029. [Google Scholar] [CrossRef]
- Raccagni, A.R.; Galli, L.; Spagnuolo, V.; Bruzzesi, E.; Muccini, C.; Bossolasco, S.; Ranzenigo, M.; Gianotti, N.; Lolatto, R.; Castagna, A.; et al. Meningococcus B Vaccination Effectiveness Against Neisseria gonorrhoeae Infection in People Living with HIV: A Case-Control Study. Sex Transm. Dis. 2023, 50, 247–251. [Google Scholar] [CrossRef]
- Ruiz Garcia, Y.; Sohn, W.Y.; Seib, K.L.; Taha, M.K.; Vazquez, J.A.; de Lemos, A.P.S.; Vadivelu, K.; Pizza, M.; Rappuoli, R.; Bekkat-Berkani, R. Looking beyond meningococcal B with the 4CMenB vaccine: The Neisseria effect. NPJ Vaccines 2021, 6, 130. [Google Scholar] [CrossRef] [PubMed]
- Leduc, I.; Connolly, K.L.; Begum, A.; Underwood, K.; Darnell, S.; Shafer, W.M.; Balthazar, J.T.; Macintyre, A.N.; Sempowski, G.D.; Duncan, J.A.; et al. The serogroup B meningococcal outer membrane vesicle-based vaccine 4CMenB induces cross-species protection against Neisseria gonorrhoeae. PLoS Pathog. 2020, 16, e1008602. [Google Scholar] [CrossRef]
- Semchenko, E.A.; Tan, A.; Borrow, R.; Seib, K.L. The Serogroup B Meningococcal Vaccine Bexsero Elicits Antibodies to Neisseria gonorrhoeae. Clin. Infect. Dis. 2019, 69, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.C.; Thomas, K.S.; Lamb, E.R.; Werner, L.M.; Connolly, K.L.; Jerse, A.E.; Criss, A.K. Evaluating vaccine-elicited antibody activities against Neisseria gonorrhoeae: Cross-protective responses elicited by the 4CMenB meningococcal vaccine. Infect. Immun. 2023, e0030923. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.N.; Weynants, V.; Poolman, J.T.; Heckels, J.E.; Christodoulides, M. Immuno-proteomic analysis of human immune responses to experimental Neisseria meningitidis outer membrane vesicle vaccines identifies potential cross-reactive antigens. Vaccine 2014, 32, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Serruto, D.; Bottomley, M.J.; Ram, S.; Giuliani, M.M.; Rappuoli, R. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: Immunological, functional and structural characterization of the antigens. Vaccine 2012, 30 (Suppl. S2), B87–B97. [Google Scholar] [CrossRef]
- Marjuki, H.; Topaz, N.; Joseph, S.J.; Gernert, K.M.; Kersh, E.N.; Antimicrobial-Resistant Neisseria gonorrhoeae Working, G.; Wang, X. Genetic Similarity of Gonococcal Homologs to Meningococcal Outer Membrane Proteins of Serogroup B Vaccine. mBio 2019, 10, e01668-19. [Google Scholar] [CrossRef]
- Liu, Y.; Hammer, L.A.; Liu, W.; Hobbs, M.M.; Zielke, R.A.; Sikora, A.E.; Jerse, A.E.; Egilmez, N.K.; Russell, M.W. Experimental vaccine induces Th1-driven immune responses and resistance to Neisseria gonorrhoeae infection in a murine model. Mucosal Immunol. 2017, 10, 1594–1608. [Google Scholar] [CrossRef]
- Liu, Y.; Perez, J.; Hammer, L.A.; Gallagher, H.C.; De Jesus, M.; Egilmez, N.K.; Russell, M.W. Intravaginal Administration of Interleukin 12 during Genital Gonococcal Infection in Mice Induces Immunity to Heterologous Strains of Neisseria gonorrhoeae. mSphere 2018, 3, e00421-17. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.S.; Novy, P.L.; Friedland, L.R. Potential benefits of using a multicomponent vaccine for prevention of serogroup B meningococcal disease. Int. J. Infect. Dis. 2019, 85, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Hollingshead, S.; Jongerius, I.; Exley, R.M.; Johnson, S.; Lea, S.M.; Tang, C.M. Structure-based design of chimeric antigens for multivalent protein vaccines. Nat. Commun. 2018, 9, 1051. [Google Scholar] [CrossRef] [PubMed]
- Dorosti, H.; Eskandari, S.; Zarei, M.; Nezafat, N.; Ghasemi, Y. Design of a multi-epitope protein vaccine against herpes simplex virus, human papillomavirus and Chlamydia trachomatis as the main causes of sexually transmitted diseases. Infect. Genet. Evol. 2021, 96, 105136. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.P.; Goldenberg, D.L.; Rice, P.A. Disseminated Gonococcal Infection: A Prospective Analysis of 49 Patients and a Review of the Pathophysiology and Immune Mechanisms. Medicine 1983, 62, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Massari, P.; King, C.A.; Macleod, H.; Wetzler, L.M. Improved purification of native meningococcal porin PorB and studies on its structure/function. Protein Expr. Purif. 2005, 44, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Medhane, M.; Tunheim, G.; Naess, L.M.; Mihret, W.; Bedru, A.; Norheim, G.; Petros, B.; Aseffa, A.; Rosenqvist, E. Avidity of IgG antibodies against meningococcal serogroup a polysaccharide and correlations with bactericidal activity in sera from meningitis patients and controls from Ethiopia. Scand. J. Immunol. 2014, 79, 267–275. [Google Scholar] [CrossRef]
- Vermont, C.L.; van Dijken, H.H.; van Limpt, C.J.; de Groot, R.; van Alphen, L.; van Den Dobbelsteen, G.P. Antibody avidity and immunoglobulin G isotype distribution following immunization with a monovalent meningococcal B outer membrane vesicle vaccine. Infect. Immun. 2002, 70, 584–590. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Ponomarenko, J.; Bui, H.H.; Li, W.; Fusseder, N.; Bourne, P.E.; Sette, A.; Peters, B. ElliPro: A new structure-based tool for the prediction of antibody epitopes. BMC Bioinform. 2008, 9, 514. [Google Scholar] [CrossRef] [PubMed]
- Clifford, J.N.; Hoie, M.H.; Deleuran, S.; Peters, B.; Nielsen, M.; Marcatili, P. BepiPred-3.0: Improved B-cell epitope prediction using protein language models. Protein Sci. 2022, 31, e4497. [Google Scholar] [CrossRef]
- The PyMOL Molecular Graphics System, V.r.p., Schrödinger, LLC. Available online: https://pymol.org/2/ (accessed on 29 November 2023).
- McClure, R.; Sunkavalli, A.; Balzano, P.M.; Massari, P.; Cho, C.; Nauseef, W.M.; Apicella, M.A.; Genco, C.A. Global Network Analysis of Neisseria gonorrhoeae Identifies Coordination between Pathways, Processes, and Regulators Expressed during Human Infection. mSystems 2020, 5, e00729-19. [Google Scholar] [CrossRef] [PubMed]
- Nudel, K.; McClure, R.; Moreau, M.; Briars, E.; Abrams, A.J.; Tjaden, B.; Su, X.H.; Trees, D.; Rice, P.A.; Massari, P.; et al. Transcriptome Analysis of Neisseria gonorrhoeae during Natural Infection Reveals Differential Expression of Antibiotic Resistance Determinants between Men and Women. mSphere 2018, 3, e00312-18. [Google Scholar] [CrossRef] [PubMed]
- McClure, R.; Nudel, K.; Massari, P.; Tjaden, B.; Su, X.; Rice, P.A.; Genco, C.A. The Gonococcal Transcriptome during Infection of the Lower Genital Tract in Women. PLoS ONE 2015, 10, e0133982. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.F.; Lammel, C.J. Humoral immune response to gonococcal infections. Clin. Microbiol. Rev. 1989, 2, S5–S10. [Google Scholar] [CrossRef] [PubMed]
- Hedges, S.R.; Mayo, M.S.; Mestecky, J.; Hook, E.W., 3rd; Russell, M.W. Limited local and systemic antibody responses to Neisseria gonorrhoeae during uncomplicated genital infections. Infect. Immun. 1999, 67, 3937–3946. [Google Scholar] [CrossRef]
- Hook, E.W.; Olsen, D.A.; Buchanan, T.M. Analysis of the Antigen Specificity of the Human Serum Immunoglobulin GG Immune Response to Complicated Gonococcal Infection. Infect. Immun. 1984, 43, 706–709. [Google Scholar] [CrossRef]
- Cannon, J.G.; Buchanan, T.M.; Sparling, P.F. Confirmation of association of protein I serotype of Neisseria gonorrhoeae with ability to cause disseminated infection. Infect. Immun. 1983, 40, 816–819. [Google Scholar] [CrossRef]
- Oleszycka, E.; Lavelle, E.C. Immunomodulatory properties of the vaccine adjuvant alum. Curr. Opin. Immunol. 2014, 28, 1–5. [Google Scholar] [CrossRef]
- Casella, C.R.; Mitchell, T.C. Putting endotoxin to work for us: Monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell. Mol. Life Sci. 2008, 65, 3231–3240. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, T.E.; Kolberg, J.; Aase, A.; Herstad, T.K.; Hoiby, E.A. The four mouse IgG isotypes differ extensively in bactericidal and opsonophagocytic activity when reacting with the P1.16 epitope on the outer membrane PorA protein of Neisseria meningitidis. Scand. J. Immunol. 2004, 59, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Malburet, C.; Leclercq, L.; Cotte, J.F.; Thiebaud, J.; Marco, S.; Nicolai, M.C.; Cottet, H. Antigen-Adjuvant Interactions in Vaccines by Taylor Dispersion Analysis: Size Characterization and Binding Parameters. Anal. Chem. 2021, 93, 6508–6515. [Google Scholar] [CrossRef] [PubMed]
- Correa, V.A.; Rodrigues, T.S.; Portilho, A.I.; Trzewikoswki de Lima, G.; De Gaspari, E. Modified ELISA for antibody avidity evaluation: The need for standardization. Biomed. J. 2021, 44, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.A.; Shafer, W.M.; Ram, S.; Jerse, A.E. Neisseria gonorrhoeae: Drug Resistance, Mouse Models, and Vaccine Development. Annu. Rev. Microbiol. 2017, 71, 665–686. [Google Scholar] [CrossRef]
- Semchenko, E.A.; Seib, K.L. Outer membrane vesicle vaccines for Neisseria gonorrhoeae. Nat. Rev. Urol. 2022, 19, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Mattsson, A.H.; Schussek, S.; Zheng, B.; DeOliveira, R.B.; Shaughnessy, J.; Lewis, L.A.; Rice, P.A.; Comstedt, P.; Ram, S. Preclinical efficacy of a cell division protein candidate gonococcal vaccine identified by artificial intelligence. mBio 2023, e0250023. [Google Scholar] [CrossRef]
- Vu, D.M.; Wong, T.T.; Granoff, D.M. Cooperative serum bactericidal activity between human antibodies to meningococcal factor H binding protein and neisserial heparin binding antigen. Vaccine 2011, 29, 1968–1973. [Google Scholar] [CrossRef]
- Giuliani, M.; Bartolini, E.; Galli, B.; Santini, L.; Lo Surdo, P.; Buricchi, F.; Bruttini, M.; Benucci, B.; Pacchiani, N.; Alleri, L.; et al. Human protective response induced by meningococcus B vaccine is mediated by the synergy of multiple bactericidal epitopes. Sci. Rep. 2018, 8, 3700. [Google Scholar] [CrossRef]
- Gulati, S.; Zheng, B.; Reed, G.W.; Su, X.; Cox, A.D.; St, M.F.; Stupak, J.; Lewis, L.A.; Ram, S.; Rice, P.A. Immunization against a saccharide epitope accelerates clearance of experimental gonococcal infection. PLoS Pathog. 2013, 9, e1003559. [Google Scholar] [CrossRef]
- Liu, Y.; Hammer, L.A.; Daamen, J.; Stork, M.; Egilmez, N.K.; Russell, M.W. Microencapsulated IL-12 Drives Genital Tract Immune Responses to Intranasal Gonococcal Outer Membrane Vesicle Vaccine and Induces Resistance to Vaginal Infection with Diverse Strains of Neisseria gonorrhoeae. mSphere 2023, 8, e0038822. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Morel, S.; Lockman, L.; Giannini, S.L.; Bisteau, M.; Carlsen, H.; Kielland, A.; Vosters, O.; Vanderheyde, N.; Schiavetti, F.; et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J. Immunol. 2009, 183, 6186–6197. [Google Scholar] [CrossRef]
- Bouvet, J.P.; Belec, L.; Pires, R.; Pillot, J. Immunoglobulin G antibodies in human vaginal secretions after parenteral vaccination. Infect. Immun. 1994, 62, 3957–3961. [Google Scholar] [CrossRef]
- Li, Z.; Palaniyandi, S.; Zeng, R.; Tuo, W.; Roopenian, D.C.; Zhu, X. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc. Natl. Acad. Sci. USA 2011, 108, 4388–4393. [Google Scholar] [CrossRef] [PubMed]
- Price, R.J.; Boettcher, B. The presence of complement in human cervical mucus and its possible relevance to infertility in women with complement-dependent sperm-immobilizing antibodies. Fertil. Steril. 1979, 32, 61–66. [Google Scholar] [CrossRef]
- Ma, X.; Yan, W.; Zheng, H.; Du, Q.; Zhang, L.; Ban, Y.; Li, N.; Wei, F. Regulation of IL-10 and IL-12 production and function in macrophages and dendritic cells. F1000Res 2015, 4, 1465. [Google Scholar] [CrossRef] [PubMed]
- Haas, K.M.; Poe, J.C.; Steeber, D.A.; Tedder, T.F. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 2005, 23, 7–18. [Google Scholar] [CrossRef]
- Dyevoich, A.M.; Disher, N.S.; Haro, M.A.; Haas, K.M. A TLR4-TRIF-dependent signaling pathway is required for protective natural tumor-reactive IgM production by B1 cells. Cancer Immunol. Immunother. 2020, 69, 2113–2124. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.R. Natural and immune human antibodies reactive with antigens of virulent Neisseria gonorrhoeae: Immunoglobulins G, M, and A. J. Bacteriol. 1967, 94, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Joseph, H.; Miller, E.; Dawson, M.; Andrews, N.; Feavers, I.; Borrow, R. Meningococcal serogroup a avidity indices as a surrogate marker of priming for the induction of immunologic memory after vaccination with a meningococcal A/C conjugate vaccine in infants in the United Kingdom. J. Infect. Dis. 2001, 184, 661–662. [Google Scholar] [CrossRef]
- Pollard, A.J.; Galassini, R.; van der Voort, E.M.; Booy, R.; Langford, P.; Nadel, S.; Ison, C.; Kroll, J.S.; Poolman, J.; Levin, M. Humoral immune responses to Neisseria meningitidis in children. Infect. Immun. 1999, 67, 2441–2451. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Kelly, D.F.; Yu, L.M.; Slack, M.P.; Booy, R.; Heath, P.T.; Siegrist, C.A.; Moxon, R.E.; Pollard, A.J. Haemophilus influenzae type b vaccine failure in children is associated with inadequate production of high-quality antibody. Clin. Infect. Dis. 2008, 46, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Mu, X.; Zheng, B.; Reed, G.W.; Ram, S.; Rice, P.A. Antibody to reduction modifiable protein increases the bacterial burden and the duration of gonococcal infection in a mouse model. J. Infect. Dis. 2015, 212, 311–315. [Google Scholar] [CrossRef] [PubMed]


| Adjuvant Group | N. gonorrhoeae F62 | N. gonorrhoeae MS11 |
|---|---|---|
| Alum | 0.35 ± 0.02 a | 0.31 ± 0.006 a |
| Alum+MPLA | 0.93 ± 0.02 b | 0.51 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roe, S.K.; Felter, B.; Zheng, B.; Ram, S.; Wetzler, L.M.; Garges, E.; Zhu, T.; Genco, C.A.; Massari, P. In Vitro Pre-Clinical Evaluation of a Gonococcal Trivalent Candidate Vaccine Identified by Transcriptomics. Vaccines 2023, 11, 1846. https://doi.org/10.3390/vaccines11121846
Roe SK, Felter B, Zheng B, Ram S, Wetzler LM, Garges E, Zhu T, Genco CA, Massari P. In Vitro Pre-Clinical Evaluation of a Gonococcal Trivalent Candidate Vaccine Identified by Transcriptomics. Vaccines. 2023; 11(12):1846. https://doi.org/10.3390/vaccines11121846
Chicago/Turabian StyleRoe, Shea K., Brian Felter, Bo Zheng, Sanjay Ram, Lee M. Wetzler, Eric Garges, Tianmou Zhu, Caroline A. Genco, and Paola Massari. 2023. "In Vitro Pre-Clinical Evaluation of a Gonococcal Trivalent Candidate Vaccine Identified by Transcriptomics" Vaccines 11, no. 12: 1846. https://doi.org/10.3390/vaccines11121846





