Improved DNA Vaccine Delivery with Needle-Free Injection Systems
Abstract
:1. DNA as a Platform for Prophylactic Vaccine Development
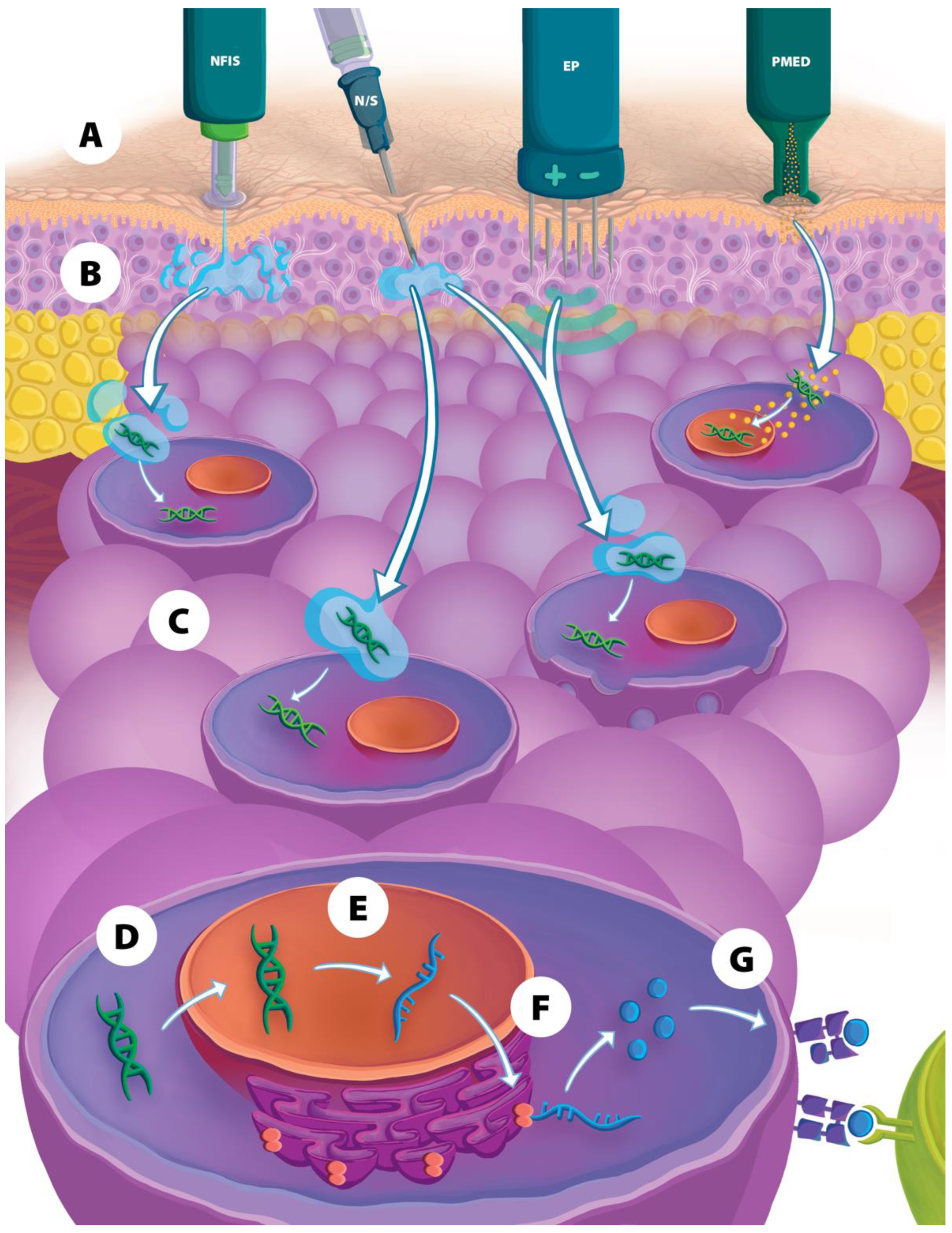
2. Physical Delivery Methods to Improve Intracellular DNA Vaccine Entry
2.1. Particle-Mediated Epidermal Delivery
2.2. Electroporation
2.3. Needle-Free Injection Systems
3. Comparison of NFIS to EP and PMED Vaccine Delivery
4. NFIS Delivery of DNA Vaccines for Pandemic Viruses
4.1. COVID-19
4.2. Influenza
4.3. HIV
5. NFIS Delivery of DNA Vaccines for Emerging Infectious Diseases
5.1. Venezuelan Equine Encephalitis Virus (VEEV)
5.2. Hantaviruses
5.3. Zika
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Shafaati, M.; Saidijam, M.; Soleimani, M.; Hazrati, F.; Mirzaei, R.; Amirheidari, B.; Tanzadehpanah, H.; Karampoor, S.; Kazemi, S.; Yavari, B.; et al. A brief review on DNA vaccines in the era of COVID-19. Future Virol. 2022, 17, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Eusebio, D.; Neves, A.R.; Costa, D.; Biswas, S.; Alves, G.; Cui, Z.; Sousa, A. Methods to improve the immunogenicity of plasmid DNA vaccines. Drug Discov. Today 2021, 26, 2575–2592. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Petrovsky, N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev. Vaccines 2016, 15, 313–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutzler, M.A.; Weiner, D.B. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Suschak, J.J.; Williams, J.A.; Schmaljohn, C.S. Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity. Hum. Vaccines Immunother. 2017, 13, 2837–2848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorritsma, S.H.T.; Gowans, E.J.; Grubor-Bauk, B.; Wijesundara, D.K. Delivery methods to increase cellular uptake and immunogenicity of DNA vaccines. Vaccine 2016, 34, 5488–5494. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef]
- Ledwith, B.J.; Manam, S.; Troilo, P.J.; Barnum, A.B.; Pauley, C.J.; Griffiths, T.G., 2nd; Harper, L.B.; Beare, C.M.; Bagdon, W.J.; Nichols, W.W. Plasmid DNA vaccines: Investigation of integration into host cellular DNA following intramuscular injection in mice. Intervirology 2000, 43, 258–272. [Google Scholar] [CrossRef]
- Saade, F.; Petrovsky, N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev. Vaccines 2012, 11, 189–209. [Google Scholar] [CrossRef]
- Liu, M.A. DNA vaccines: An historical perspective and view to the future. Immunol. Rev. 2011, 239, 62–84. [Google Scholar] [CrossRef]
- Redding, L.; Weiner, D.B. DNA vaccines in veterinary use. Expert Rev. Vaccines 2009, 8, 1251–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aida, V.; Pliasas, V.C.; Neasham, P.J.; North, J.F.; McWhorter, K.L.; Glover, S.R.; Kyriakis, C.S. Novel Vaccine Technologies in Veterinary Medicine: A Herald to Human Medicine Vaccines. Front. Vet. Sci. 2021, 8, 654289. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.; Paolino, K.M.; Mills, K.; Kwilas, S.; Josleyn, M.; Cohen, M.; Somerville, B.; Wisniewski, M.; Norris, S.; Hill, B.; et al. A phase 2a randomized, double-blind, dose-optimizing study to evaluate the immunogenicity and safety of a bivalent DNA vaccine for hemorrhagic fever with renal syndrome delivered by intramuscular electroporation. Vaccines 2020, 8, 377. [Google Scholar] [CrossRef] [PubMed]
- Hannaman, D.; Dupuy, L.C.; Ellefsen, B.; Schmaljohn, C.S. A Phase 1 clinical trial of a DNA vaccine for Venezuelan equine encephalitis delivered by intramuscular or intradermal electroporation. Vaccine 2016, 34, 3607–3612. [Google Scholar] [CrossRef]
- Elizaga, M.L.; Li, S.S.; Kochar, N.K.; Wilson, G.J.; Allen, M.A.; Tieu, H.V.N.; Frank, I.; Sobieszczyk, M.E.; Cohen, K.W.; Sanchez, B.; et al. Safety and tolerability of HIV-1 multiantigen pDNA vaccine given with IL-12 plasmid DNA via electroporation, boosted with a recombinant vesicular stomatitis virus HIV Gag vaccine in healthy volunteers in a randomized, controlled clinical trial. PLoS ONE 2018, 13, e0202753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houser, K.V.; Chen, G.L.; Carter, C.; Crank, M.C.; Nguyen, T.A.; Burgos Florez, M.C.; Berkowitz, N.M.; Mendoza, F.; Hendel, C.S.; Gordon, I.J.; et al. Safety and immunogenicity of a ferritin nanoparticle H2 influenza vaccine in healthy adults: A phase 1 trial. Nat. Med. 2022, 28, 383–391. [Google Scholar] [CrossRef]
- Gaudinski, M.R.; Houser, K.V.; Morabito, K.M.; Hu, Z.; Yamshchikov, G.; Rothwell, R.S.; Berkowitz, N.; Mendoza, F.; Saunders, J.G.; Novik, L.; et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: Randomised, open-label, phase 1 clinical trials. Lancet 2018, 391, 552–562. [Google Scholar] [CrossRef] [Green Version]
- Ledgerwood, J.E.; Pierson, T.C.; Hubka, S.A.; Desai, N.; Rucker, S.; Gordon, I.J.; Enama, M.E.; Nelson, S.; Nason, M.; Gu, W.; et al. A West Nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J. Infect. Dis. 2011, 203, 1396–1404. [Google Scholar] [CrossRef] [Green Version]
- Sarwar, U.N.; Costner, P.; Enama, M.E.; Berkowitz, N.; Hu, Z.; Hendel, C.S.; Sitar, S.; Plummer, S.; Mulangu, S.; Bailer, R.T.; et al. Safety and immunogenicity of DNA vaccines encoding Ebolavirus and Marburgvirus wild-type glycoproteins in a phase I clinical trial. J. Infect. Dis. 2015, 211, 549–557. [Google Scholar] [CrossRef]
- Tebas, P.; Kraynyak, K.A.; Patel, A.; Maslow, J.N.; Morrow, M.P.; Sylvester, A.J.; Knoblock, D.; Gillespie, E.; Amante, D.; Racine, T.; et al. Intradermal SynCon(R) Ebola GP DNA Vaccine Is Temperature Stable and Safely Demonstrates Cellular and Humoral Immunogenicity Advantages in Healthy Volunteers. J. Infect. Dis. 2019, 220, 400–410. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Lee, J.; Suh, Y.S.; Song, Y.G.; Choi, Y.J.; Lee, K.H.; Seo, S.H.; Song, M.; Oh, J.W.; Kim, M.; et al. Safety and immunogenicity of two recombinant DNA COVID-19 vaccines containing the coding regions of the spike or spike and nucleocapsid proteins: An interim analysis of two open-label, non-randomised, phase 1 trials in healthy adults. Lancet Microbe 2022, 3, e173–e183. [Google Scholar] [CrossRef] [PubMed]
- Kraynyak, K.A.; Blackwood, E.; Agnes, J.; Tebas, P.; Giffear, M.; Amante, D.; Reuschel, E.L.; Purwar, M.; Christensen-Quick, A.; Liu, N.; et al. SARS-CoV-2 DNA Vaccine INO-4800 Induces Durable Immune Responses Capable of Being Boosted in a Phase 1 Open-Label Trial. J. Infect. Dis. 2022, 225, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.; Kerr, D.; Fayer, R.; Wall, R. Serum and colostrum antibody responses induced by jet-injection of sheep with DNA encoding a Cryptosporidium parvum antigen. Vaccine 1995, 13, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Gul, C.; Karakavuk, T.; Karakavuk, M.; Can, H.; Degirmenci Doskaya, A.; Gul, A.; Erkunt Alak, S.; Guruz, A.Y.; Un, C.; Doskaya, M. An Overview of DNA Vaccines Development Studies Against Toxoplasma gondii. Turk. Parazitol. Derg. 2022, 46, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Sefidi-Heris, Y.; Jahangiri, A.; Mokhtarzadeh, A.; Shahbazi, M.A.; Khalili, S.; Baradaran, B.; Mosafer, J.; Baghbanzadeh, A.; Hejazi, M.; Hashemzaei, M.; et al. Recent progress in the design of DNA vaccines against tuberculosis. Drug Discov. Today 2020, 25, 1971–1987. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Arun Kumar, S.; Jhan, Y.Y.; Bishop, C.J. Engineering DNA vaccines against infectious diseases. Acta Biomater. 2018, 80, 31–47. [Google Scholar] [CrossRef]
- Carter, E.W.; Kerr, D.E. Optimization of DNA-based vaccination in cows using green fluorescent protein and protein A as a prelude to immunization against staphylococcal mastitis. J. Dairy Sci. 2003, 86, 1177–1186. [Google Scholar] [CrossRef] [Green Version]
- Scheiblhofer, S.; Weiss, R.; Thalhamer, J. Genetic vaccination approaches against malaria based on the circumsporozoite protein. Wien. Klin. Wochenschr. 2006, 118, 9–17. [Google Scholar] [CrossRef]
- Momin, T.; Kansagra, K.; Patel, H.; Sharma, S.; Sharma, B.; Patel, J.; Mittal, R.; Sanmukhani, J.; Maithal, K.; Dey, A.; et al. Safety and Immunogenicity of a DNA SARS-CoV-2 vaccine (ZyCoV-D): Results of an open-label, non-randomized phase I part of phase I/II clinical study by intradermal route in healthy subjects in India. EClinicalMedicine 2021, 38, 101020. [Google Scholar] [CrossRef]
- Khobragade, A.; Bhate, S.; Ramaiah, V.; Deshpande, S.; Giri, K.; Phophle, H.; Supe, P.; Godara, I.; Revanna, R.; Nagarkar, R.; et al. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): The interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. Lancet 2022, 399, 1313–1321. [Google Scholar] [CrossRef]
- Government of India Press Information Bureau. DBT-BIRAC Supported ZyCoV-D Developed by Zydus Cadila Receives Emergency Use Authorization. Available online: https://pib.gov.in/PressReleasePage.aspx?PRID=1747669 (accessed on 18 July 2022).
- Feltquate, D.M.; Heaney, S.; Webster, R.G.; Robinson, H.L. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J. Immunol. 1997, 158, 2278–2284. [Google Scholar] [CrossRef] [PubMed]
- McCluskie, M.J.; Brazolot Millan, C.L.; Gramzinski, R.A.; Robinson, H.L.; Santoro, J.C.; Fuller, J.T.; Widera, G.; Haynes, J.R.; Purcell, R.H.; Davis, H.L. Route and method of delivery of DNA vaccine influence immune responses in mice and non-human primates. Mol. Med. 1999, 5, 287–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elnekave, M.; Furmanov, K.; Hovav, A.H. Intradermal naked plasmid DNA immunization: Mechanisms of action. Expert Rev. Vaccines 2011, 10, 1169–1182. [Google Scholar] [CrossRef] [PubMed]
- Weniger, B.; Papania, M. Alternative Vaccine Delivery Methods. In Vaccines, 6th ed.; Plotkin, S., Orenstein, W., Offit, P., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2013; pp. 1200–1231. [Google Scholar]
- Sachdev, S.; Potočnik, T.; Rems, L.; Miklavčič, D. Revisiting the role of pulsed electric fields in overcoming the barriers to in vivo gene electrotransfer. Bioelectrochemistry 2022, 144, 107994. [Google Scholar] [CrossRef]
- Van Drunen Littel-Van Den Hurk, S.; Hannaman, D. Electroporation for DNA immunization: Clinical application. Expert Rev. Vaccines 2010, 9, 503–517. [Google Scholar] [CrossRef]
- Chiarella, P.; Massi, E.; De Robertis, M.; Sibilio, A.; Parrella, P.; Fazio, V.M.; Signori, E. Electroporation of skeletal muscle induces danger signal release and antigen-presenting cell recruitment independently of DNA vaccine administration. Expert Opin. Biol. Ther. 2008, 8, 1645–1657. [Google Scholar] [CrossRef]
- Sardesai, N.Y.; Weiner, D.B. Electroporation delivery of DNA vaccines: Prospects for success. Curr. Opin. Immunol. 2011, 23, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Morrow, M.P.; Kraynyak, K.A.; Sylvester, A.J.; Shen, X.; Amante, D.; Sakata, L.; Parker, L.; Yan, J.; Boyer, J.; Roh, C.; et al. Augmentation of cellular and humoral immune responses to HPV16 and HPV18 E6 and E7 antigens by VGX-3100. Mol. Ther. Oncolytics 2016, 3, 16025. [Google Scholar] [CrossRef] [Green Version]
- Modjarrad, K.; Roberts, C.C.; Mills, K.T.; Castellano, A.R.; Paolino, K.; Muthumani, K.; Reuschel, E.L.; Robb, M.L.; Racine, T.; Oh, M.d.; et al. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: A phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect. Dis. 2019, 19, 1013–1022. [Google Scholar] [CrossRef] [Green Version]
- Algazi, A.P.; Twitty, C.G.; Tsai, K.K.; Le, M.; Pierce, R.; Browning, E.; Hermiz, R.; Canton, D.A.; Bannavong, D.; Oglesby, A.; et al. Phase II Trial of IL-12 Plasmid Transfection and PD-1 Blockade in Immunologically Quiescent Melanoma. Clin. Cancer Res. 2020, 26, 2827–2837. [Google Scholar] [CrossRef]
- Aggarwal, C.; Cohen, R.B.; Morrow, M.P.; Kraynyak, K.A.; Sylvester, A.J.; Knoblock, D.M.; Bauml, J.M.; Weinstein, G.S.; Lin, A.; Boyer, J.; et al. Immunotherapy Targeting HPV16/18 GeneratesPotent Immune Responses in HPV-AssociatedHead and Neck Cancer. Clin. Cancer Res. 2019, 25, 110–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitragotri, S. Current status and future prospects of needle-free liquid jet injectors. Nat. Rev. Drug Discov. 2006, 5, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Hogan, N.C.; Taberner, A.J.; Jones, L.A.; Hunter, I.W. Needle-free delivery of macromolecules through the skin using controllable jet injectors. Expert Opin. Drug Deliv. 2015, 12, 1637–1648. [Google Scholar] [CrossRef]
- Papania, M.J.; Zehrung, D.; Jarrahian, C. Technologies to Improve Immunization. In Plotkin’s Vaccines; Elsevier: Amsterdam, The Netherlands, 2018; p. 1320. [Google Scholar] [CrossRef]
- Wang, R.; Bian, Q.; Xu, Y.; Xu, D.; Gao, J. Recent advances in mechanical force-assisted transdermal delivery of macromolecular drugs. Int. J. Pharm. 2021, 602, 120598. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Hong, J.Y.; Kwon, T.R.; Lee, S.E.; Yoo, K.H.; Choi, S.Y.; Kim, B.J. Mechanism and clinical applications of needle-free injectors in dermatology: Literature review. J. Cosmet. Dermatol. 2021, 20, 3793–3801. [Google Scholar] [CrossRef]
- Canter, J.; Mackey, K.; Good, L.S.; Roberto, R.R.; Chin, J.; Bond, W.W.; Alter, M.J.; Horan, J.M. An outbreak of hepatitis B associated with jet injections in a weight reduction clinic. Arch. Intern. Med. 1990, 150, 1923–1927. [Google Scholar] [CrossRef] [PubMed]
- Manam, S.; Ledwith, B.J.; Barnum, A.B.; Troilo, P.J.; Pauley, C.J.; Harper, L.B.; Griffiths, T.G., 2nd; Niu, Z.; Denisova, L.; Follmer, T.T.; et al. Plasmid DNA vaccines: Tissue distribution and effects of DNA sequence, adjuvants and delivery method on integration into host DNA. Intervirology 2000, 43, 273–281. [Google Scholar] [CrossRef]
- Marston, J.O.; Lacerda, C.M.R. Characterization of jet injection efficiency with mouse cadavers. J. Control. Release 2019, 305, 101–109. [Google Scholar] [CrossRef]
- Bernelin-Cottet, C.; Urien, C.; Fretaud, M.; Langevin, C.; Trus, I.; Jouneau, L.; Blanc, F.; Leplat, J.J.; Barc, C.; Boulesteix, O.; et al. A DNA Prime Immuno-Potentiates a Modified Live Vaccine against the Porcine Reproductive and Respiratory Syndrome Virus but Does Not Improve Heterologous Protection. Viruses 2019, 11, 576. [Google Scholar] [CrossRef]
- Graham, B.S.; Enama, M.E.; Nason, M.C.; Gordon, I.J.; Peel, S.A.; Ledgerwood, J.E.; Plummer, S.A.; Mascola, J.R.; Bailer, R.T.; Roederer, M.; et al. DNA vaccine delivered by a needle-free injection device improves potency of priming for antibody and CD8+ T-cell responses after rAd5 boost in a randomized clinical trial. PLoS ONE 2013, 8, e59340. [Google Scholar] [CrossRef]
- Alberer, M.; Gnad-Vogt, U.; Hong, H.S.; Mehr, K.T.; Backert, L.; Finak, G.; Gottardo, R.; Bica, M.A.; Garofano, A.; Koch, S.D.; et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: An open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet 2017, 390, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Alamri, S.S.; Alluhaybi, K.A.; Alhabbab, R.Y.; Basabrain, M.; Algaissi, A.; Almahboub, S.; Alfaleh, M.A.; Abujamel, T.S.; Abdulaal, W.H.; ElAssouli, M.Z.; et al. Synthetic SARS-CoV-2 Spike-Based DNA Vaccine Elicits Robust and Long-Lasting Th1 Humoral and Cellular Immunity in Mice. Front. Microbiol. 2021, 12, 727455. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.; Medioni, J.; Garibal, J.; Adotevi, O.; Doucet, L.; Durey, M.D.; Ghrieb, Z.; Kiladjian, J.J.; Brizard, M.; Laheurte, C.; et al. A First-in-Human Phase I Study of INVAC-1, an Optimized Human Telomerase DNA Vaccine in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2020, 26, 588–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashorun, A.O.; Badjie Hydara, M.; Adigweme, I.; Umesi, A.; Danso, B.; Johnson, N.; Sambou, N.A.; Fofana, S.; Kanu, F.J.; Jeyaseelan, V.; et al. Intradermal administration of fractional doses of the inactivated poliovirus vaccine in a campaign: A pragmatic, open-label, non-inferiority trial in The Gambia. Lancet Glob. Health 2022, 10, e257–e268. [Google Scholar] [CrossRef] [PubMed]
- Jarrahian, C.; Rein-Weston, A.; Saxon, G.; Creelman, B.; Kachmarik, G.; Anand, A.; Zehrung, D. Vial usage, device dead space, vaccine wastage, and dose accuracy of intradermal delivery devices for inactivated poliovirus vaccine (IPV). Vaccine 2017, 35, 1789–1796. [Google Scholar] [CrossRef]
- Daly, C.; Molodecky, N.A.; Sreevatsava, M.; Belayneh, A.D.; Chandio, S.A.; Partridge, J.; Shaikh, A.; Laghari, M.; Agbor, J.; Safdar, R.M.; et al. Needle-free injectors for mass administration of fractional dose inactivated poliovirus vaccine in Karachi, Pakistan: A survey of caregiver and vaccinator acceptability. Vaccine 2020, 38, 1893–1898. [Google Scholar] [CrossRef]
- Yousafzai, M.T.; Saleem, A.F.; Mach, O.; Baig, A.; Sutter, R.W.; Zaidi, A.K.M. Feasibility of conducting intradermal vaccination campaign with inactivated poliovirus vaccine using Tropis intradermal needle free injection system, Karachi, Pakistan. Heliyon 2017, 3, e00395. [Google Scholar] [CrossRef] [Green Version]
- Sokolowski, C.J.; Giovannitti, J.A., Jr.; Boynes, S.G. Needle phobia: Etiology, adverse consequences, and patient management. Dent. Clin. N. Am. 2010, 54, 731–744. [Google Scholar] [CrossRef]
- Love, A.S.; Love, R.J. Considering Needle Phobia among Adult Patients during Mass COVID-19 Vaccinations. J. Prim. Care Community Health 2021, 12, 21501327211007393. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Sharps Safety for Healthcare Settings. Available online: https://www.cdc.gov/sharpssafety/index.html (accessed on 22 February 2022).
- Mengistu, D.A.; Tolera, S.T.; Demmu, Y.M. Worldwide Prevalence of Occupational Exposure to Needle Stick Injury among Healthcare Workers: A Systematic Review and Meta-Analysis. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 9019534. [Google Scholar] [CrossRef]
- Bullo, U.F.; Mehraj, J.; Raza, S.M.; Rasool, S.; Ansari, N.N.; Shaikh, A.A.; Phul, Z.A.; Memon, S.A.; Baloch, R.I.; Baloch, Z.A.; et al. An experience of mass administration of fractional dose inactivated polio vaccine through intradermal needle-free injectors in Karachi, Sindh, Pakistan. BMC Public Health 2021, 21, 44. [Google Scholar] [CrossRef] [PubMed]
- Mvundura, M.; Hsu, J.S.; Frivold, C.; Kristensen, D.; Boyle, S.; Zehrung, D.; Jarrahian, C. Evaluating the cost per child vaccinated with full versus fractional-dose inactivated poliovirus vaccine. Vaccine X 2019, 2, 100032. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.; van Diepen, M.; Galant, S.; Kruse, E.; Margolin, E.; Ximba, P.; Hermanus, T.; Moore, P.; Douglass, N.; Williamson, A.L.; et al. Immunogenicity of HIV-1 Vaccines Expressing Chimeric Envelope Glycoproteins on the Surface of Pr55 Gag Virus-Like Particles. Vaccines 2020, 8, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, L.A.; Rupp, R.; Papadimitriou, A.; Wallace, D.; Raanan, M.; Moss, K.J. A phase 1 study of safety and immunogenicity following intradermal administration of a tetravalent dengue vaccine candidate. Vaccine 2018, 36, 3976–3983. [Google Scholar] [CrossRef] [PubMed]
- Bakari, M.; Aboud, S.; Nilsson, C.; Francis, J.; Buma, D.; Moshiro, C.; Aris, E.A.; Lyamuya, E.F.; Janabi, M.; Godoy-Ramirez, K.; et al. Broad and potent immune responses to a low dose intradermal HIV-1 DNA boosted with HIV-1 recombinant MVA among healthy adults in Tanzania. Vaccine 2011, 29, 8417–8428. [Google Scholar] [CrossRef] [Green Version]
- Beckett, C.G.; Tjaden, J.; Burgess, T.; Danko, J.R.; Tamminga, C.; Simmons, M.; Wu, S.J.; Sun, P.; Kochel, T.; Raviprakash, K.; et al. Evaluation of a prototype dengue-1 DNA vaccine in a Phase 1 clinical trial. Vaccine 2011, 29, 960–968. [Google Scholar] [CrossRef] [PubMed]
- McAllister, L.; Anderson, J.; Werth, K.; Cho, I.; Copeland, K.; Le Cam Bouveret, N.; Plant, D.; Mendelman, P.M.; Cobb, D.K. Needle-free jet injection for administration of influenza vaccine: A randomised non-inferiority trial. Lancet 2014, 384, 674–681. [Google Scholar] [CrossRef]
- Petrovsky, N.; Honda-Okubo, Y.; Royals, M.; Bragg, K.; Sajkovd, D. A randomized controlled study to assess the immunogenicity and tolerability of a 2012 trivalent seasonal inactivated influenza vaccine administered via a disposable syringe jet injector device versus a traditional pre-filled syringe and needle. Trials Vaccinol. 2013, 2, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Bavdekar, A.; Oswal, J.; Ramanan, P.V.; Aundhkar, C.; Venugopal, P.; Kapse, D.; Miller, T.; McGray, S.; Zehrung, D.; Kulkarni, P.S.; et al. Immunogenicity and safety of measles-mumps-rubella vaccine delivered by disposable-syringe jet injector in India: A randomized, parallel group, non-inferiority trial. Vaccine 2018, 36, 1220–1226. [Google Scholar] [CrossRef]
- Seqirus Package Insert—Afluria Quadrivalent. Available online: https://www.fda.gov/media/117022/download (accessed on 15 October 2022).
- Government of India Central Drugs Standard Control Organization. Zydus Lifesciences ZyCoV-D Summary of Product Characteristics. Available online: https://cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadSmPC/ZyCoV-D%20SmPc%20&%20Factsheet.pdf (accessed on 15 October 2022).
- Grodeland, G.; Fredriksen, A.B.; Loset, G.A.; Vikse, E.; Fugger, L.; Bogen, B. Antigen Targeting to Human HLA Class II Molecules Increases Efficacy of DNA Vaccination. J. Immunol. 2016, 197, 3575–3585. [Google Scholar] [CrossRef] [Green Version]
- Mucker, E.M.; Golden, J.W.; Hammerbeck, C.D.; Kishimori, J.M.; Royals, M.; Joselyn, M.D.; Ballantyne, J.; Nalca, A.; Hooper, J.W. A Nucleic Acid-Based Orthopoxvirus Vaccine Targeting the Vaccinia Virus L1, A27, B5, and A33 Proteins Protects Rabbits against Lethal Rabbitpox Virus Aerosol Challenge. J. Virol. 2022, 96, e0150421. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.W.; Moon, J.E.; Paolino, K.M.; Newcomer, R.; McLain, D.E.; Josleyn, M.; Hannaman, D.; Schmaljohn, C. A Phase 1 clinical trial of Hantaan virus and Puumala virus M-segment DNA vaccines for haemorrhagic fever with renal syndrome delivered by intramuscular electroporation. Clin. Microbiol. Infect. 2014, 20 (Suppl. 5), 110–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brocato, R.L.; Kwilas, S.A.; Josleyn, M.D.; Long, S.; Zeng, X.; Perley, C.C.; Principe, L.M.; Somerville, B.; Cohen, M.V.; Hooper, J.W. Small animal jet injection technique results in enhanced immunogenicity of hantavirus DNA vaccines. Vaccine 2021, 39, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Ewing, D.; Blevins, M.; Sun, P.; Sundaram, A.K.; Raviprakash, K.S.; Porter, K.R.; Sanders, J.W. Enhanced immunogenicity and protective efficacy of a tetravalent dengue DNA vaccine using electroporation and intradermal delivery. Vaccine 2019, 37, 4444–4453. [Google Scholar] [CrossRef] [PubMed]
- Mooij, P.; Grodeland, G.; Koopman, G.; Andersen, T.K.; Mortier, D.; Nieuwenhuis, I.G.; Verschoor, E.J.; Fagrouch, Z.; Bogers, W.M.; Bogen, B. Needle-free delivery of DNA: Targeting of hemagglutinin to MHC class II molecules protects rhesus macaques against H1N1 influenza. Vaccine 2019, 37, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Chozhavel Rajanathan, T.M.; Chandra, H.; Pericherla, H.P.R.; Kumar, S.; Choonia, H.S.; Bajpai, M.; Singh, A.K.; Sinha, A.; Saini, G.; et al. Immunogenic potential of DNA vaccine candidate, ZyCoV-D against SARS-CoV-2 in animal models. Vaccine 2021, 39, 4108–4116. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Kumar, S.; Agarwal, K.; Jain, M.; Patil, D.R.; Maithal, K.; Mathapati1, B.; Giri, S.; Mohandas, S.; Shete, A.; et al. Assessment of immunogenicity and protective efficacy of ZyCoV-D DNA vaccine candidates in Rhesus macaques against SARS-CoV-2 infection. bioRxiv 2021. [Google Scholar] [CrossRef]
- Mucker, E.M.; Brocato, R.L.; Principe, L.M.; Kim, R.K.; Zeng, X.; Smith, J.M.; Kwilas, S.A.; Kim, S.; Horton, H.; Caproni, L.; et al. SARS-CoV-2 Doggybone DNA Vaccine Produces Cross-Variant Neutralizing Antibodies and Is Protective in a COVID-19 Animal Model. Vaccines 2022, 10, 1104. [Google Scholar] [CrossRef]
- Brocato, R.L.; Kwilas, S.A.; Kim, R.K.; Zeng, X.; Principe, L.M.; Smith, J.M.; Hooper, J.W. Protective efficacy of a SARS-CoV-2 DNA vaccine in wild-type and immunosuppressed Syrian hamsters. NPJ Vaccines 2021, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Lassauniere, R.; Polacek, C.; Gram, G.J.; Frische, A.; Tingstedt, J.L.; Kruger, M.; Dorner, B.G.; Cook, A.; Brown, R.; Orekov, T.; et al. Preclinical evaluation of a candidate naked plasmid DNA vaccine against SARS-CoV-2. NPJ Vaccines 2021, 6, 156. [Google Scholar] [CrossRef]
- Alluhaybi, K.A.; Alharbi, R.H.; Alhabbab, R.Y.; Aljehani, N.D.; Alamri, S.S.; Basabrain, M.; Alharbi, R.; Abdulaal, W.H.; Alfaleh, M.A.; Tamming, L.; et al. Cellular and Humoral Immunogenicity of a Candidate DNA Vaccine Expressing SARS-CoV-2 Spike Subunit 1. Vaccines 2021, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Margolin, E.; Allen, J.D.; Verbeek, M.; Chapman, R.; Meyers, A.; van Diepen, M.; Ximba, P.; Motlou, T.; Moore, P.L.; Woodward, J.; et al. Augmenting glycosylation-directed folding pathways enhances the fidelity of HIV Env immunogen production in plants. Biotechnol. Bioeng. 2022, 119, 2919–2937. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.; van Diepen, M.; Douglass, N.; Galant, S.; Jaffer, M.; Margolin, E.; Ximba, P.; Hermanus, T.; Moore, P.L.; Williamson, A.L. Assessment of an LSDV-Vectored Vaccine for Heterologous Prime-Boost Immunizations against HIV. Vaccines 2021, 9, 1281. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.; Starke, C.E.; Ortiz, A.M.; Ransier, A.; Darko, S.; Douek, D.C.; Brenchley, J.M. Multiple modes of antigen exposure induce clonotypically diverse epitope-specific CD8+ T cells across multiple tissues in nonhuman primates. PLoS Pathog. 2022, 18, e1010611. [Google Scholar] [CrossRef]
- Food and Drug Administration. COVID-19 Vaccines. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines (accessed on 20 September 2022).
- Van Diepen, M.T.; Chapman, R.; Douglass, N.; Galant, S.; Moore, P.L.; Margolin, E.; Ximba, P.; Morris, L.; Rybicki, E.P.; Williamson, A.-L. Prime-Boost Immunizations with DNA, Modified Vaccinia Virus Ankara, and Protein-Based Vaccines Elicit Robust HIV-1 Tier 2 Neutralizing Antibodies against the CAP256 Superinfecting Virus. J. Virol. 2019, 93, 2155–2173. [Google Scholar] [CrossRef] [Green Version]
- Ximba, P.; Chapman, R.; Meyers, A.; Margolin, E.; van Diepen, M.T.; Sander, A.F.; Woodward, J.; Moore, P.L.; Williamson, A.L.; Rybicki, E.P. Development of a synthetic nanoparticle vaccine presenting the HIV-1 envelope glycoprotein. Nanotechnology 2022, 33, 485102. [Google Scholar] [CrossRef]
- Suschak, J.J.; Bixler, S.L.; Badger, C.V.; Spik, K.W.; Kwilas, S.A.; Rossi, F.D.; Twenhafel, N.; Adams, M.L.; Shoemaker, C.J.; Spiegel, E.; et al. A DNA vaccine targeting VEE virus delivered by needle-free jet-injection protects macaques against aerosol challenge. NPJ Vaccines 2022, 7, 46. [Google Scholar] [CrossRef]
- Kwilas, S.; Kishimori, J.M.; Josleyn, M.; Jerke, K.; Ballantyne, J.; Royals, M.; Hooper, J.W. A hantavirus pulmonary syndrome (HPS) DNA vaccine delivered using a spring-powered jet injector elicits a potent neutralizing antibody response in rabbits and nonhuman primates. Curr. Gene Ther. 2014, 14, 200–210. [Google Scholar] [CrossRef] [Green Version]
- Mucker, E.M.; Karmali, P.P.; Vega, J.; Kwilas, S.A.; Wu, H.; Joselyn, M.; Ballantyne, J.; Sampey, D.; Mukthavaram, R.; Sullivan, E.; et al. Lipid Nanoparticle Formulation Increases Efficiency of DNA-Vectored Vaccines/Immunoprophylaxis in Animals Including Transchromosomic Bovines. Sci. Rep. 2020, 10, 8764. [Google Scholar] [CrossRef]
- Dowd, K.A.; Ko, S.Y.; Morabito, K.M.; Yang, E.S.; Pelc, R.S.; DeMaso, C.R.; Castilho, L.R.; Abbink, P.; Boyd, M.; Nityanandam, R.; et al. Rapid development of a DNA vaccine for Zika virus. Science 2016, 354, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Van Rompay, K.K.A.; Keesler, R.I.; Ardeshir, A.; Watanabe, J.; Usachenko, J.; Singapuri, A.; Cruzen, C.; Bliss-Moreau, E.; Murphy, A.M.; Yee, J.L.; et al. DNA vaccination before conception protects Zika virus-exposed pregnant macaques against prolonged viremia and improves fetal outcomes. Sci. Transl. Med. 2019, 11, eaay2736. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, S.; Ruckwardt, T.J.; Morabito, K.M.; Foreman, B.M.; Burgomaster, K.E.; Gordon, D.N.; Pelc, R.S.; DeMaso, C.R.; Ko, S.Y.; Fisher, B.E.; et al. Distinct neutralizing antibody correlates of protection among related Zika virus vaccines identify a role for antibody quality. Sci. Transl. Med. 2020, 12, eaaw9066. [Google Scholar] [CrossRef] [PubMed]
| Indication | Name (Immunogen) | Species | Device (Route) | EP Device or PMED | Dose | Vaccine Schedule | Vs. NS | NFIS Notes |
|---|---|---|---|---|---|---|---|---|
| PRRSV [52] | N, Nsp1β, RdRp | Pig | Tropis (ID) | CUY21EDIT | 0.4 mg * | 0, 34 d C: 63 d | No |
|
| Hantavirus (various) [79] | M | Hamster | Tropis (SC, IM) | PMED | 0.2 mg, 0.42 mg | 0, 4, 8 wk C: 21 wk | Yes |
|
| Poxvirus [77] | 4pox (L1, A27, B5, A33) | Rabbit | Tropis (ID) Stratis (IM) | TriGrid | 0.4 mg | 0, 4, 8 wk | Yes |
|
| Influenza [76] | HA | Pig | Tropis (ID) | AgilePulse | 0.025 −0.4 mg | 0, 3–4 wk | No |
|
| Dengue [80] | TVDV (Pre-M, E) | NHP | Tropis (ID) Stratis (IM) | TriGrid | 1 mg, 5 mg | 0, 28, 91 d C: 392 d | No |
|
| Indication | Name (Immunogen) | Species | Device (Route) | Dose | Vaccination Schedule | Vs. NS | NFIS Notes |
|---|---|---|---|---|---|---|---|
| COVID-19 [82] | ZyCoV-D (Spike) | Rabbit | Tropis (ID) | 0.5 mg | 0, 2, 4 wk | No |
|
| COVID-19 [83] | ZyCoV-D (Spike) | NHP | Tropis (ID) | 1 mg, 2 mg * | 0, 4, 8 wk C: 15 wk | Yes |
|
| COVID-19 [29] | ZyCoV-D (Spike) | Human Ph 1/2 | Tropis (ID) | 1 mg, 2 mg * | 0, 4, 8 wk | Yes |
|
| COVID-19 [30] | ZyCoV-D (Spike) | Human Ph 3 | Tropis (ID) | 2 mg * | 0, 4, 8 wk | No |
|
| COVID-19 [84] | nCoV-S(JET), dbDNAS(JET), dbDNAS(ST-JET) (Spike) | Hamster | Tropis (IM) | 0.05 mg, 0.2 mg | 0, 3 wk C: 6 wk | No |
|
| COVID-19 [85] | nCoV-S(JET) (Spike) | Hamster | Tropis (IM) | 0.2 mg | 0, 3 wk C: 6 wk | No |
|
| COVID-19 [86] | pNTC-Spike (Spike) | Rabbit NHP | Tropis (ID) Stratis (IM) | Rabbit: 0.125 mg NHP: 2 mg * | 0, 2, 4 wk C (NHP): 8 wk | Yes |
|
| COVID-19 [55] | VIU-1005 (Spike) | Mouse | Tropis † (ID, IM) | 0.025 mg, 0.05 mg, 0.1 mg | 0, 2, 4 wk | Yes |
|
| COVID-19 [87] | pVAX-S1 (Spike S1) | Mouse | Tropis † (IM) | 0.025 mg, 0.05 mg | 0, 3, 6 wk | No |
|
| Influenza [16] | VRC-FLUDNA082-00-VP (HA) | Human Ph 1 | Stratis (IM) | 4 mg | 0, 16 wk | No |
|
| Influenza [81] | HA | NHP | Tropis (ID) | 0.075 mg | 0, 6, 12 wk C: 16 wk | No |
|
| HIV [88] | DNAGC5 (Env, Gag) | Rabbit | Stratis (IM) | 0.2 mg | 0, 4, 8, 12, 20, 28 wk | No |
|
| HIV [89] | DNAGC5 (Env, Gag) | Rabbit | Stratis (IM) | 0.2 mg | 0, 4, 8, 12, 16, 20 wk | No |
|
| HIV ‡ | DNAGC5 (Env, Gag) | Rabbit | Stratis (IM) | 0.2 mg | 0, 4, 8, 12 wk | Yes |
|
| HIV [90] | SIV-gag (Gag) | NHP | Stratis (IM) | 1 mg | 0, 28, 56, 84, 211 d | No |
|
| Indication | Name (Immunogen) | Species | Device (Route) | Dose | Schedule | Vs NS | NFIS Notes |
|---|---|---|---|---|---|---|---|
| VEEV [94] | pWRG/VEE (E3-E2-6K-E1) | NHP | Tropis (ID) Stratis (IM) | 0.4 mg, 2 mg | 0, 4 wk | No |
|
| Hantavirus (SNV, ANDV) [95] | M | Rabbit, NHP | Stratis (IM) IMv1 IDv1 | Rabbit 0.4 mg, 2 mg, 4 mg * NHP 1 mg *, 2 mg * | 0, 4, 8 wk | Yes |
|
| Hantavirus (ANDV) Zika [96] | ANDV: M ZIKV: PrM, E | Rabbit, NHP | Stratis (IM) | Rabbit LNP: 0.001–1 mg No LNP: 0.1 mg NHP LNP: 0.1 mg, 0.3 mg No LNP: 0.1 mg, 0.3 mg, 2 mg | Rabbit 0, 27 d 0, 42 d NHP 0, 28, 56 d | No |
|
| Zika [97] | VRC5283, VRC5288 (PrM, E) | NHP | Stratis (IM) | 1 mg, 4 mg | 0, 4 wk C: 8 wk | No |
|
| Zika [98] | VRC5283 (PrM, E) | NHP | Stratis (IM) | 1 mg | 0, 4 wk C: 30, 60, 90 d | No |
|
| Zika [17] | VRC5283 (PrM, E) | Human Ph 1 | Stratis (IM) | 4 mg * | 0, 4, 8 wk | Yes |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ledesma-Feliciano, C.; Chapman, R.; Hooper, J.W.; Elma, K.; Zehrung, D.; Brennan, M.B.; Spiegel, E.K. Improved DNA Vaccine Delivery with Needle-Free Injection Systems. Vaccines 2023, 11, 280. https://doi.org/10.3390/vaccines11020280
Ledesma-Feliciano C, Chapman R, Hooper JW, Elma K, Zehrung D, Brennan MB, Spiegel EK. Improved DNA Vaccine Delivery with Needle-Free Injection Systems. Vaccines. 2023; 11(2):280. https://doi.org/10.3390/vaccines11020280
Chicago/Turabian StyleLedesma-Feliciano, Carmen, Ros Chapman, Jay W. Hooper, Kira Elma, Darin Zehrung, Miles B. Brennan, and Erin K. Spiegel. 2023. "Improved DNA Vaccine Delivery with Needle-Free Injection Systems" Vaccines 11, no. 2: 280. https://doi.org/10.3390/vaccines11020280
APA StyleLedesma-Feliciano, C., Chapman, R., Hooper, J. W., Elma, K., Zehrung, D., Brennan, M. B., & Spiegel, E. K. (2023). Improved DNA Vaccine Delivery with Needle-Free Injection Systems. Vaccines, 11(2), 280. https://doi.org/10.3390/vaccines11020280







