The BNT162b2 mRNA COVID-19 Vaccine Increases the Contractile Sensitivity to Histamine and Parasympathetic Activation in a Human Ex Vivo Model of Severe Eosinophilic Asthma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Collection and Preparation
2.2. Passive Sensitization and Eosinophils Activation
2.3. Transmural Stimulation
2.4. Preparation of Drugs
2.5. Contraction Measurement
2.6. Study Characteristics
2.7. Endpoint
2.8. Study Design
2.9. Pharmacological and Statistical Analysis
2.10. Sample Size
3. Results
3.1. Baseline Characteristics
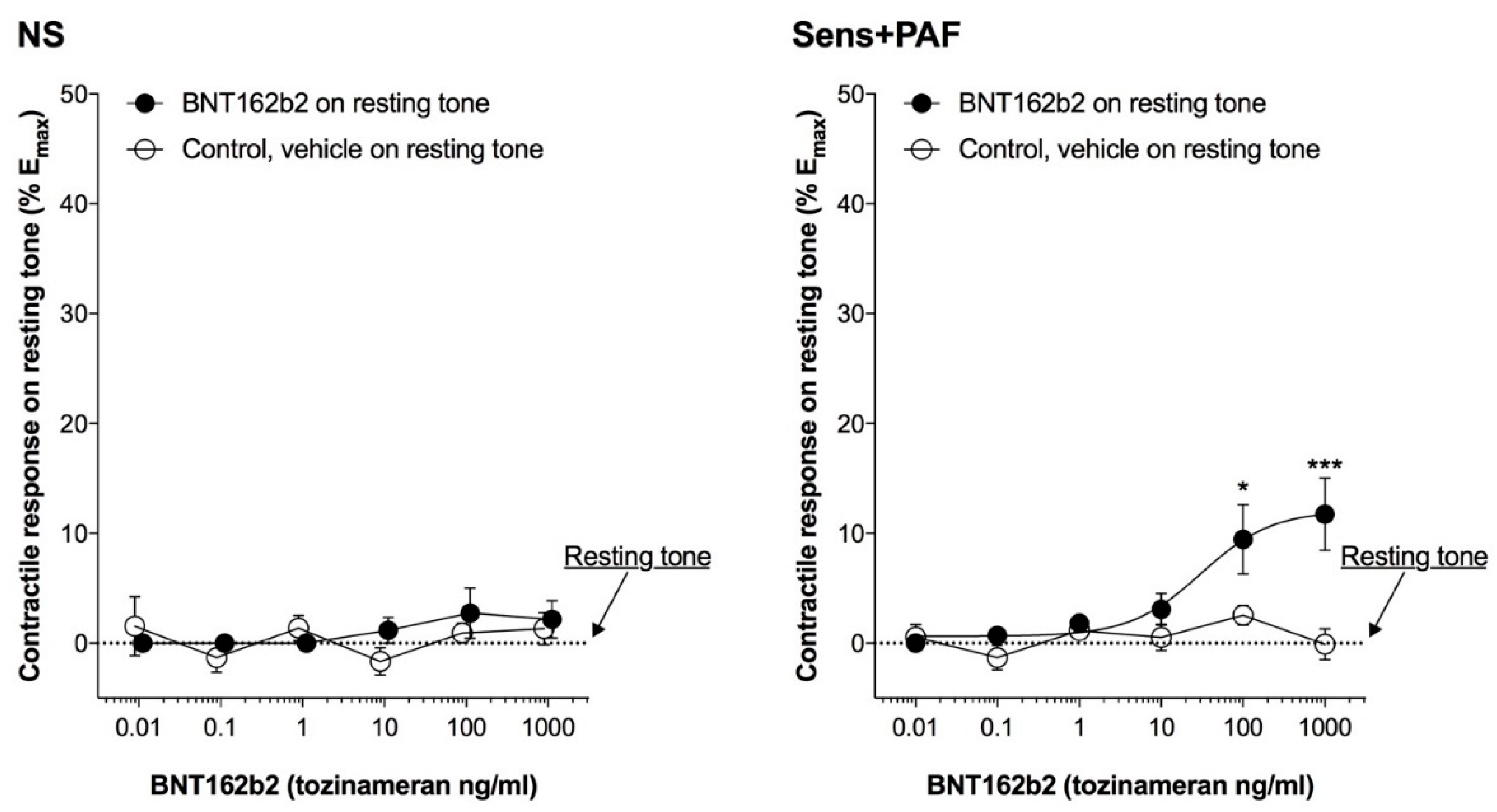
3.2. Effect of BNT162b2 on Isolated Airways Resting Tone
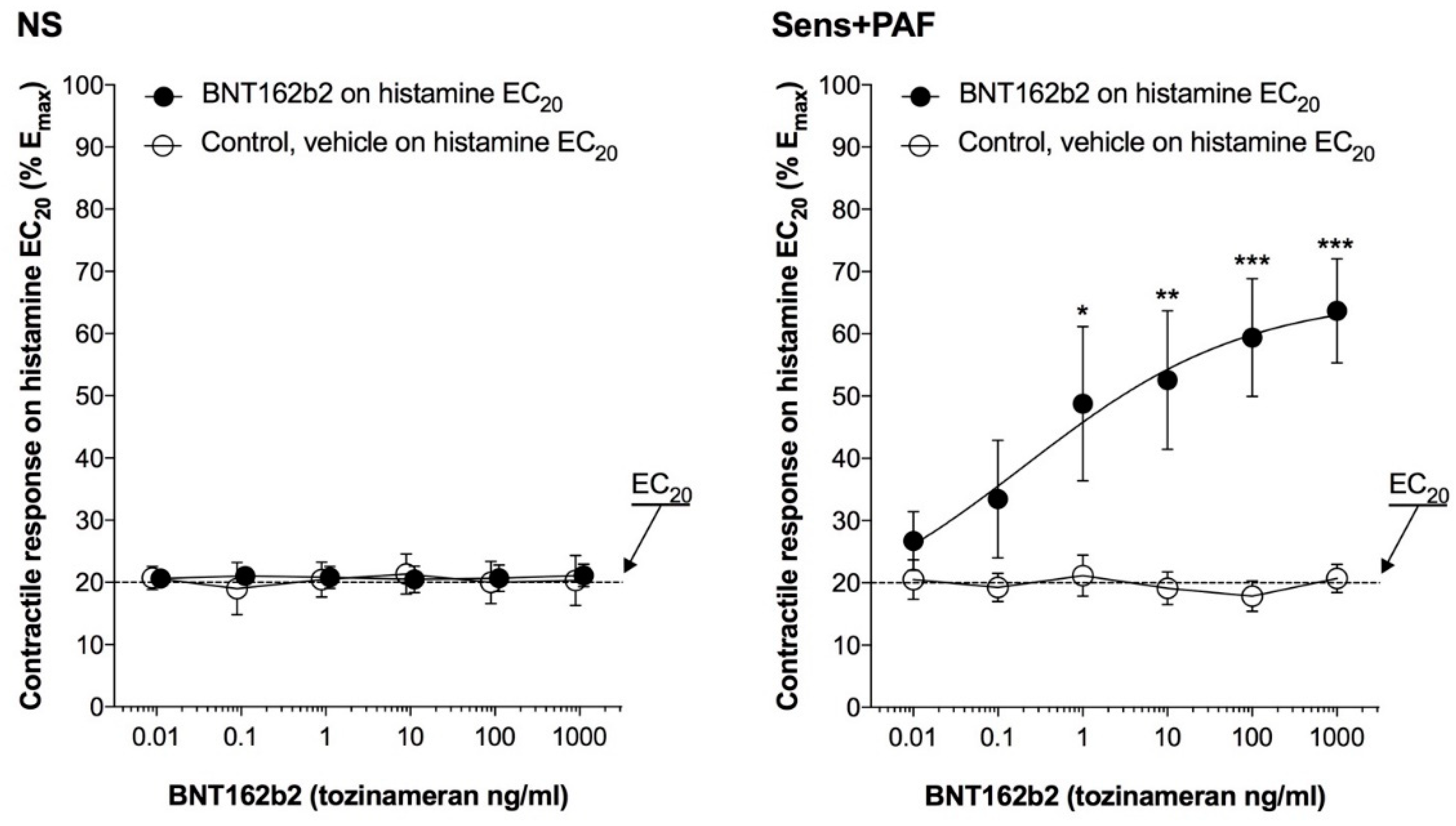
3.3. Effect of BNT162b2 on Isolated Airways Partially Pre-Contracted by Histamine
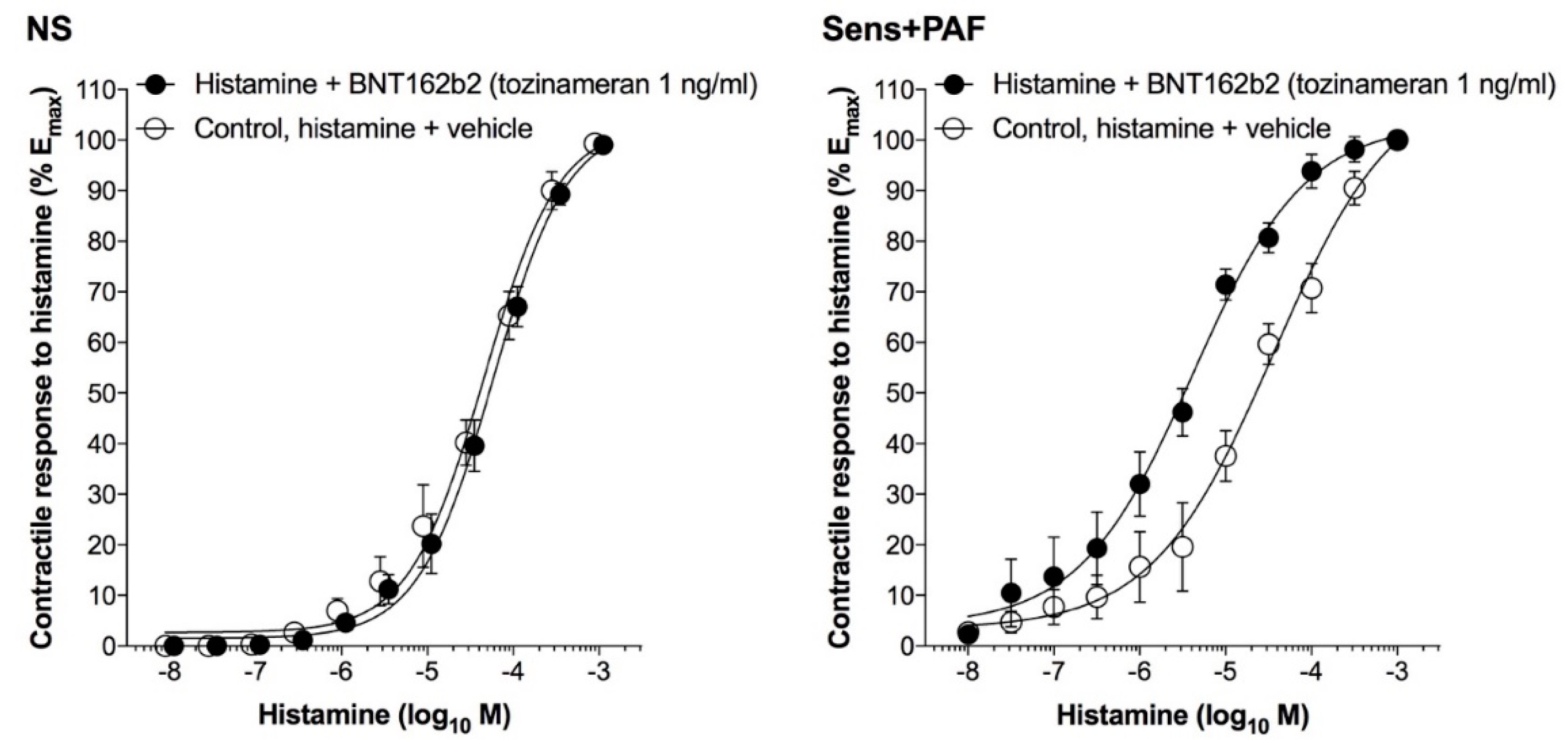
3.4. Effect of BNT162b2 on the CRC to Histamine
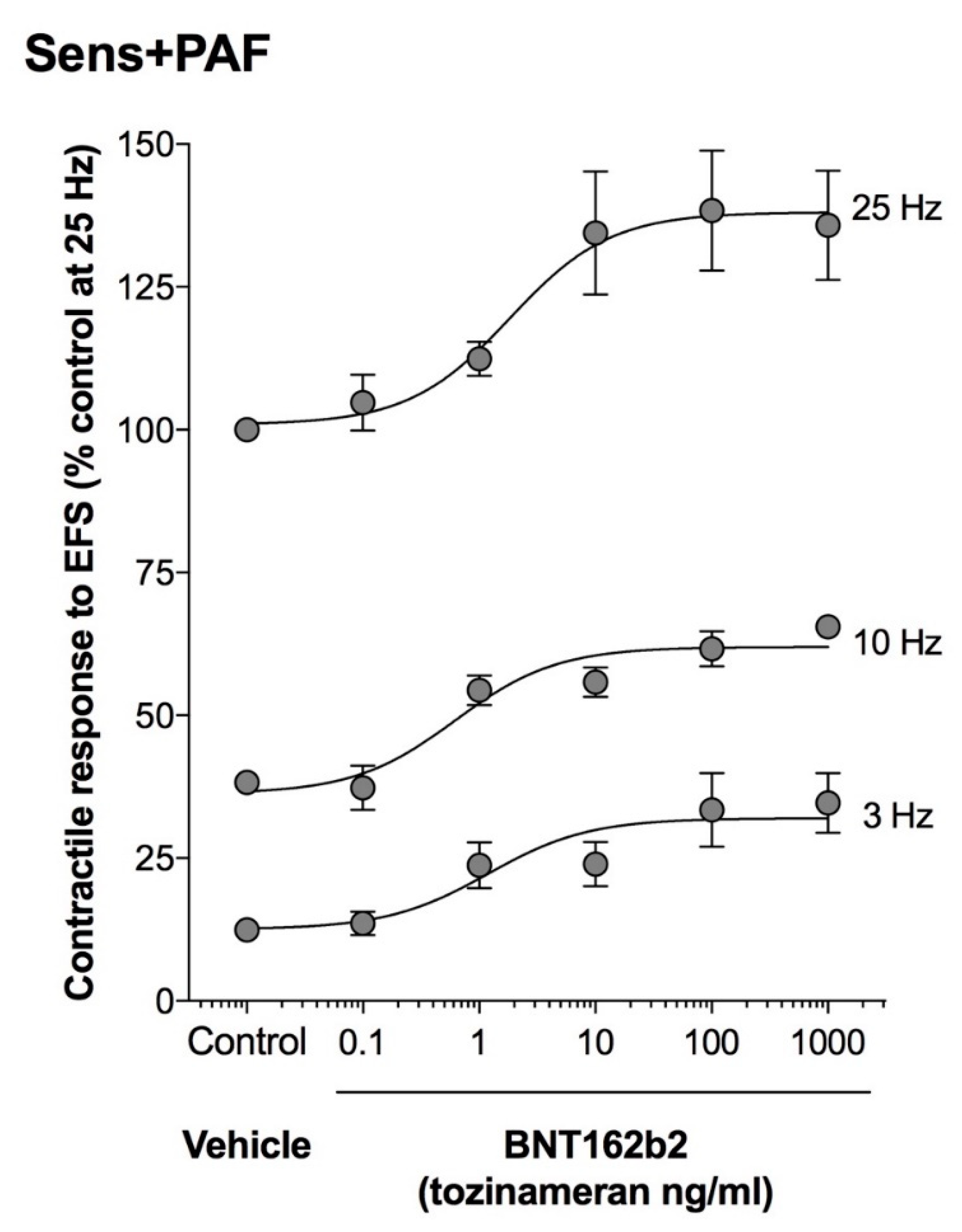
3.5. Effect of BNT162b2 on EFS3–25Hz
3.6. Effect of Denatured BNT162b2 on the CRC to Histamine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- GINA. 2022 GINA Main Report|Global Initiative for Asthma. 2022. Available online: https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf (accessed on 13 September 2022).
- Denlinger, L.C.; Phillips, B.R.; Ramratnam, S.; Ross, K.; Bhakta, N.R.; Cardet, J.C.; Castro, M.; Peters, S.P.; Phipatanakul, W.; Aujla, S.; et al. Inflammatory and Comorbid Features of Patients with Severe Asthma and Frequent Exacerbations. Am. J. Respir. Crit. Care Med. 2017, 195, 302–313. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, G.A.; Yacoub, M.-R.; Ripa, M.; Mannina, D.; Cariddi, A.; Saporiti, N.; Ciceri, F.; Castagna, A.; Colombo, G.; Dagna, L. Eosinophils from Physiology to Disease: A Comprehensive Review. BioMed Res. Int. 2018, 2018, 9095275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fredberg, J.J. Bronchospasm and its biophysical basis in airway smooth muscle. Respir. Res. 2004, 5, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shavit, R.; Maoz-Segal, R.; Iancovici-Kidon, M.; Offengenden, I.; Yahia, S.H.; Maayan, D.M.; Lifshitz-Tunitsky, Y.; Niznik, S.; Frizinsky, S.; Deutch, M.; et al. Prevalence of Allergic Reactions After Pfizer-BioNTech COVID-19 Vaccination Among Adults With High Allergy Risk. JAMA Netw. Open 2021, 4, e2122255. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccine Doses Administered by Manufacturer, European Union, (n.d.). Available online: https://ourworldindata.org/grapher/covid-vaccine-doses-by-manufacturer (accessed on 24 December 2022).
- Caminati, M.; Guarnieri, G.; Batani, V.; Scarpieri, E.; Finocchiaro, A.; Chieco-Bianchi, F.; Senna, G.; Vianello, A. COVID-19 Vaccination in Patients with Severe Asthma on Biologic Treatment: Safety, Tolerability, and Impact on Disease Control. Vaccines 2021, 9, 853. [Google Scholar] [CrossRef]
- Pfaar, O.; Klimek, L.; Hamelmann, E.; Kleine-Tebbe, J.; Taube, C.; Wagenmann, M.; Werfel, T.; Brehler, R.; Novak, N.; Mülleneisen, N.; et al. COVID-19 vaccination of patients with allergies and type-2 inflammation with concurrent antibody therapy (biologicals)—A Position Paper of the German Society of Allergology and Clinical Immunology (DGAKI) and the German Society for Applied Allergo. Allergol. Sel. 2021, 5, 140–147. [Google Scholar] [CrossRef]
- Global Initiative for Asthma GINA Global Strategy for Asthma Management and Prevention GINA Guidance about COVID-19 and Asthma. 2022. Available online: www.ginasthma.org (accessed on 9 January 2023).
- Runnstrom, M.C.; Morrison-Porter, A.; Ravindran, M.; Quehl, H.; Ramonell, R.P.; Woodruff, M.; Patel, R.; Kim, C.; Haddad, N.S.; Lee, F.E.-H. Reduced COVID-19 Vaccine Response in Patients Treated with Biologic Therapies for Asthma. Am. J. Respir. Crit. Care Med. 2022, 205, 1243–1245. [Google Scholar] [CrossRef]
- Liao, S.; Gerber, A.; Zelarney, P.; Make, B.; Wechsler, M. SARS-CoV-2 mRNA Vaccine Antibody Response in Patients with Asthma Receiving Biologic Therapy: A Real-World Analysis. Am. J. Respir. Crit. Care Med. 2022, 206, 644–647. [Google Scholar] [CrossRef]
- Podrazil, M.; Taborska, P.; Stakheev, D.; Rataj, M.; Lastovicka, J.; Vlachova, A.; Pohunek, P.; Bartunkova, J.; Smrz, D. Effectiveness and Durability of mRNA Vaccine-Induced SARS-CoV-2-Specific Humoral and Cellular Immunity in Severe Asthma Patients on Biological Therapy. Front. Immunol. 2022, 13, 2375. [Google Scholar] [CrossRef]
- Da Cruz, J.P.G.L.; Carvalho, C.d.C.R.d.; Silva, P.A.d.C.; Guerreiro, L.F.C.; Bento, T.V.; Costa, L.V.C.C.M.; Simões, R.F.M.M.D.; Marques, R.P.P.G.; Fernandes, A.C.C.; Galaio, L.M.C.d.M.; et al. Spontaneous Adverse Event Reporting by COVID-19 Vaccinated Healthcare Professionals Through an Electronic Form Implemented by the Hospital Pharmacy. Hosp. Pharm. 2022, 57, 744–751. [Google Scholar] [CrossRef]
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A., Jr.; Robinson, L.B.; Long, A.A.; Wolfson, A.R.; Williams, P.; Khan, D.A.; Phillips, E.; et al. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.J. Allergic Reactions After COVID-19 Vaccination—Putting Risk Into Perspective. JAMA Netw. Open 2021, 4, e2122326. [Google Scholar] [CrossRef] [PubMed]
- Colaneri, M.; De Filippo, M.; Licari, A.; Marseglia, A.; Maiocchi, L.; Ricciardi, A.; Corsico, A.; Marseglia, G.; Mondelli, M.U.; Bruno, R. COVID vaccination and asthma exacerbation: Might there be a link? Int. J. Infect. Dis. 2021, 112, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Sueyoshi, K.; Nakamura, Y.; Okamoto, K.; Tanaka, H. Can the coronavirus disease-2019 vaccine induce an asthma attack? Acute Med. Surg. 2021, 8, e714. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Shasha, D.; Bareket, R.; Sikron, F.H.; Gertel, O.; Tsamir, J.; Dvir, D.; Mossinson, D.; Heymann, A.D.; Zacay, G. Real-world safety data for the Pfizer BNT162b2 SARS-CoV-2 vaccine: Historical cohort study. Clin. Microbiol. Infect. 2022, 28, 130–134. [Google Scholar] [CrossRef]
- Chapman, D.G.; Irvin, C.G. Mechanisms of airway hyper-responsiveness in asthma: The past, present and yet to come. Clin. Exp. Allergy 2015, 45, 706–719. [Google Scholar] [CrossRef] [Green Version]
- Calzetta, L.; Matera, M.G.; Facciolo, F.; Cazzola, M.; Rogliani, P. Beclomethasone dipropionate and formoterol fumarate synergistically interact in hyperresponsive medium bronchi and small airways. Respir. Res. 2018, 19, 65. [Google Scholar] [CrossRef] [Green Version]
- 2023 GOLD Reports-Global Initiative for Chronic Obstructive Lung Disease-GOLD, (n.d.). Available online: https://goldcopd.org/2023-gold-report-2/# (accessed on 14 March 2022).
- Schaafsma, D.; Zuidhof, A.B.; Nelemans, S.; Zaagsma, J.; Meurs, H. Inhibition of Rho-kinase normalizes nonspecific hyperresponsiveness in passively sensitized airway smooth muscle preparations. Eur. J. Pharmacol. 2006, 531, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Ruehlmann, E.; Branscheid, D.; Magnussen, H.; Rabe, K. Passive sensitization of human airways increases responsiveness to leukotriene C4. Eur. Respir. J. 1999, 14, 315–319. [Google Scholar] [CrossRef]
- Rabe, K.F.; Muñoz, N.M.; Vita, A.J.; Morton, B.E.; Magnussen, H.; Leff, A.R. Contraction of human bronchial smooth muscle caused by activated human eosinophils. Am. J. Physiol. Cell. Mol. Physiol. 1994, 267, L326–L334. [Google Scholar] [CrossRef]
- Kroegel, C.; A Giembycz, M.; Matthys, H.; Westwick, J.; Barnes, P.J. Modulatory role of protein kinase C on the signal transduction pathway utilized by platelet-activating factor in eosinophil activation. Am. J. Respir. Cell Mol. Biol. 1994, 11, 593–599. [Google Scholar] [CrossRef]
- Mesnil, C.; Raulier, S.; Paulissen, G.; Xiao, X.; Birrell, M.A.; Pirottin, D.; Janss, T.; Starkl, P.; Ramery, E.; Henket, M.; et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J. Clin. Investig. 2016, 126, 3279–3295. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, D.; Dent, G.; Ruhlmann, E.; Munoz, N.; Leff, A.; Rabe, K. Studying human airway pharmacology in microsections: Application of videomicrometry. Eur. Respir. J. 2002, 19, 991–996. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, R.W.; Rabe, K.F.; Magnussen, H.; Leff, A.R. Passive sensitization of human airways induces myogenic contractile responses in vitro. J. Appl. Physiol. 1997, 83, 1276–1281. [Google Scholar] [CrossRef]
- Johnson, P.; Armour, C.; Black, J. The action of platelet activating factor and its antagonism by WEB 2086 on human isolated airways. Eur. Respir. J. 1990, 3, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Black, J.; Armour, C. Platelet-activating factor-induced contraction of human isolated bronchus. Eur. Respir. J. 1992, 5, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Ritondo, B.L.; Matera, M.G.; Facciolo, F.; Rogliani, P. Targeting IL-5 pathway against airway hyperresponsiveness: A challenge between benralizumab and mepolizumab. Br. J. Pharmacol. 2020, 177, 4750–4765. [Google Scholar] [CrossRef]
- Rogliani, P.; Matera, M.G.; Facciolo, F.; Page, C.; Cazzola, M.; Calzetta, L. Beclomethasone dipropionate, formoterol fumarate and glycopyrronium bromide: Synergy of triple combination therapy on human airway smooth muscle ex vivo. Br. J. Pharmacol. 2020, 177, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Kim, C.; Kwon, D. Thermal degradation of poly(ethyleneglycol). Polym. Degrad. Stab. 1995, 47, 203–208. [Google Scholar] [CrossRef]
- Fabre, A.-L.; Colotte, M.; Luis, A.; Tuffet, S.; Bonnet, J. An efficient method for long-term room temperature storage of RNA. Eur. J. Hum. Genet. 2014, 22, 379–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matera, M.G.; Calzetta, L.; Passeri, D.; Facciolo, F.; Rendina, E.A.; Page, C.; Cazzola, M.; Orlandi, A. Epithelium integrity is crucial for the relaxant activity of brain natriuretic peptide in human isolated bronchi. Br. J. Pharmacol. 2011, 163, 1740–1754. [Google Scholar] [CrossRef] [Green Version]
- Calzetta, L.; Pietroiusti, A.; Page, C.; Bussolati, O.; Chetta, A.; Facciolo, F.; Rogliani, P. Multi-walled carbon nanotubes induce airway hyperresponsiveness in human bronchi by stimulating sensory C-fibers and increasing the release of neuronal acetylcholine. Expert Rev. Respir. Med. 2021, 15, 1473–1481. [Google Scholar] [CrossRef]
- Motulsky, H.; Christopoulos, A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Motulsky, H.J.; Michel, M.C. Commentary on the BJP’s new statistical reporting guidelines. Br. J. Pharmacol. 2018, 175, 3636–3637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Li, Y.; Li, S.; Jia, L.; Wang, H.; Li, M.; Deng, J.; Zhu, A.; Ma, L.; Li, W.; et al. The nano delivery systems and applications of mRNA. Eur. J. Med. Chem. 2022, 227, 113910. [Google Scholar] [CrossRef]
- Rolla, G.; Brussino, L.; Badiu, I. Maintaining Safety with SARS-CoV-2 Vaccines. N. Engl. J. Med. 2021, 384, e37. [Google Scholar] [CrossRef]
- Cabanillas, B.; Akdis, C.A.; Novak, N. Allergic reactions to the first COVID-19 vaccine: A potential role of polyethylene glycol? Allergy 2020, 76, 1617–1618. [Google Scholar] [CrossRef] [PubMed]
- Giavina-Bianchi, P.; Kalil, J. May polyethylene glycol be the cause of anaphylaxis to mRNA COVID-19 vaccines? World Allergy Organ. J. 2021, 14, 100532. [Google Scholar] [CrossRef]
- Wenande, E.; Garvey, L.H. Immediate-type hypersensitivity to polyethylene glycols: A review. Clin. Exp. Allergy 2016, 46, 907–922. [Google Scholar] [CrossRef]
- Borderé, A.; Stockman, A.; Boone, B.; Franki, A.-S.; Coppens, M.J.; Lapeere, H.; Lambert, J. A case of anaphylaxis caused by macrogol 3350 after injection of a corticosteroid. Contact Dermat. 2012, 67, 376–378. [Google Scholar] [CrossRef]
- Sellaturay, P.; Nasser, S.; Ewan, P. Polyethylene Glycol–Induced Systemic Allergic Reactions (Anaphylaxis). J. Allergy Clin. Immunol. Pr. 2021, 9, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Calzetta, L.; Ora, J.; Cazzola, M.; Matera, M.G. Efficacy and safety profile of doxofylline compared to theophylline in asthma: A meta-analysis. Multidiscip. Respir. Med. 2019, 14, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calzetta, L.; Pistocchini, E.; Cito, G.; Ritondo, B.L.; Verri, S.; Rogliani, P. Inflammatory and contractile profile in LPS-challenged equine isolated bronchi: Evidence for IL-6 as a potential target against AHR in equine asthma. Pulm. Pharmacol. Ther. 2022, 73–74, 102125. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, M.; Yang, W.H.; Hébert, J.; de Takacsy, F.; Stril, J.-L. The real world effect of omalizumab add on therapy for patients with moderate to severe allergic asthma: The ASTERIX Observational study. PLoS ONE 2017, 12, e0183869. [Google Scholar] [CrossRef] [Green Version]
- Kistemaker, L.E.M.; Prakash, Y.S. Airway Innervation and Plasticity in Asthma. Physiology 2019, 34, 283–298. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- CHMP. Committee for Medicinal Products for Human Use (CHMP) Assessment Report Comirnaty Common Name: COVID-19 mRNA Vaccine (Nucleoside-Modified), (n.d.). Available online: www.ema.europa.eu/contact (accessed on 21 January 2022).
- Latypova, S. Did Pfizer Perform Adequate Safety Testing for Its Covid-19 mRNA Vaccine in Preclinical Studies? Evidence of Scientific and Regulatory Fraud. 2022. Available online: https://img1.wsimg.com/blobby/go/d0283b38-61d3-48f6-bd2a-d67ae57c091a/Review%20of%20Pfizer%27s%20Non-Clinical%20Program_Regula.pdf (accessed on 21 December 2022).
- Palmer, M.; Bhakdi, S. The Pfizer mRNA Vaccine: Pharmacokinetics and Toxicity, Letsbfree.Com. 2021. Available online: https://letsbfree.com/content/uploads/files/2021/10/letsbfree_79fe2c12d77a388dc66e225bf18b3f2d.pdf (accessed on 21 December 2022).
- Maldiney, T.; Richard, C.; Seguin, J.; Wattier, N.; Bessodes, M.; Scherman, D. Effect of Core Diameter, Surface Coating, and PEG Chain Length on the Biodistribution of Persistent Luminescence Nanoparticles in Mice. ACS Nano 2011, 5, 854–862. [Google Scholar] [CrossRef]
- Molina, D.K.; DiMaio, V.J. Normal organ weights in men: Part II—the brain, lungs, liver, spleen, and kidneys. Am. J. Forensic Med. Pathol. 2012, 33, 368–372. [Google Scholar] [CrossRef]
- Molina, D.K.; DiMaio, V.J.M. Normal organ weights in women: Part II—The Brain, Lungs, Liver, Spleen, and Kidneys. Am. J. Forensic Med. Pathol. 2015, 36, 182–187. [Google Scholar] [CrossRef]
- Calzetta, L.; Pistocchini, E.; Ritondo, B.L.; Roncada, P.; Cito, G.; Britti, D.; Matera, M.G. Isolated airways in equine respiratory pharmacology: They never lie. Pulm. Pharmacol. Ther. 2019, 59, 101849. [Google Scholar] [CrossRef]
- Ritondo, B.L.; Rogliani, P.; Facciolo, F.; Falco, S.; Vocale, A.; Calzetta, L. Beclomethasone dipropionate and sodium cromoglycate protect against airway hyperresponsiveness in a human ex vivo model of cow’s milk aspiration. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100010. [Google Scholar] [CrossRef]
- Cazzola, M.; Calzetta, L.; Ora, J.; Puxeddu, E.; Rogliani, P.; Matera, M.G. Searching for the synergistic effect between aclidinium and formoterol: From bench to bedside. Respir. Med. 2015, 109, 1305–1311. [Google Scholar] [CrossRef] [Green Version]
- Cazzola, M.; Calzetta, L.; Segreti, A.; Facciolo, F.; Rogliani, P.; Matera, M.G. Translational Study Searching for Synergy between Glycopyrronium and Indacaterol. COPD: J. Chronic Obstr. Pulm. Dis. 2015, 12, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Ora, J.; Girolami, A.; Rossi, I.; de Guido, I.; Facciolo, F.; Cazzola, M.; Calzetta, L. Ceiling effect of beclomethasone/formoterol/glycopyrronium triple fixed-dose combination in COPD: A translational bench-to-bedside study. Pulm. Pharmacol. Ther. 2021, 69, 102050. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.-P.; Yang, M.; Lai, C.-L. COVID-19 Vaccines: A Review of the Safety and Efficacy of Current Clinical Trials. Pharmaceuticals 2021, 14, 406. [Google Scholar] [CrossRef]
- Rogliani, P.; Cavalli, F.; Chetta, A.; Cazzola, M.; Calzetta, L. Potential Drawbacks of ICS/LABA/LAMA Triple Fixed-Dose Combination Therapy in the Treatment of Asthma: A Quantitative Synthesis of Safety Profile. J. Asthma Allergy 2022, 15, 565–577. [Google Scholar] [CrossRef]
- Rogliani, P.; Lauro, D.; Di Daniele, N.; Chetta, A.; Calzetta, L. Reduced risk of COVID-19 hospitalization in asthmatic and COPD patients: A benefit of inhaled corticosteroids? Expert Rev. Respir. Med. 2021, 15, 561–568. [Google Scholar] [CrossRef]
- Aldakheel, F.M. Allergic Diseases: A Comprehensive Review on Risk Factors, Immunological Mechanisms, Link with COVID-19, Potential Treatments, and Role of Allergen Bioinformatics. Int. J. Environ. Res. Public Heal. 2021, 18, 12105. [Google Scholar] [CrossRef]
- Garner, O.; Ramey, J.S.; Hanania, N.A. Management of Life-Threatening Asthma. Chest 2022, 162, 747–756. [Google Scholar] [CrossRef]

| Characteristics | Tissue Used as Ex Vivo Model of Severe Eosinophilic Asthma | Normal Range |
|---|---|---|
| Gender (male/female) | 5/5 | / |
| Age (years) | 53.5 ± 3.1 | / |
| Height (cm) | 168.2 ± 2.3 | / |
| Weight (Kg) | 67.4 ± 2.4 | / |
| BMI | 23.8 ± 0.8 | 18.5–24.9 |
| Smoking status | ||
| current | 0 | / |
| former | 10 | / |
| never | 0 | / |
| IgE | 45.3 ± 6.4 | <100 |
| Pack years | 16.5 ± 2.1 | / |
| FEV1 (L) | 2.75 ± 0.12 | / |
| FEV1 (% predicted) | 88.0 ± 3.8 | >80 |
| FEV1 reversibility (%) | 4.8 ± 1.1 | <12% |
| FVC (L) | 3.58 ± 0.15 | / |
| FEV1/FVC | 0.77 ± 0.01 | >0.7 |
| NS | Sens+PAF | |||
|---|---|---|---|---|
| Emax | pEC50 | Emax | pEC50 | |
| Control, histamine + vehicle | 103.10 ± 3.56 | 4.29±0.07 | 96.57 ± 3.63 | 4.63 ± 0.09 ** |
| Histamine + BNT162b2 1 ng/ml | 101.30 ± 2.71 | 4.27±0.05 | 97.20 ± 2.73 | 5.39 ± 0.09 *** § |
| Histamine + denatured BNT162b2 1 ng/ml | / | / | 101.0 ± 6.10 | 5.25 ± 0.14 § |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calzetta, L.; Chetta, A.; Aiello, M.; Frizzelli, A.; Ora, J.; Melis, E.; Facciolo, F.; Ippoliti, L.; Magrini, A.; Rogliani, P. The BNT162b2 mRNA COVID-19 Vaccine Increases the Contractile Sensitivity to Histamine and Parasympathetic Activation in a Human Ex Vivo Model of Severe Eosinophilic Asthma. Vaccines 2023, 11, 282. https://doi.org/10.3390/vaccines11020282
Calzetta L, Chetta A, Aiello M, Frizzelli A, Ora J, Melis E, Facciolo F, Ippoliti L, Magrini A, Rogliani P. The BNT162b2 mRNA COVID-19 Vaccine Increases the Contractile Sensitivity to Histamine and Parasympathetic Activation in a Human Ex Vivo Model of Severe Eosinophilic Asthma. Vaccines. 2023; 11(2):282. https://doi.org/10.3390/vaccines11020282
Chicago/Turabian StyleCalzetta, Luigino, Alfredo Chetta, Marina Aiello, Annalisa Frizzelli, Josuel Ora, Enrico Melis, Francesco Facciolo, Lorenzo Ippoliti, Andrea Magrini, and Paola Rogliani. 2023. "The BNT162b2 mRNA COVID-19 Vaccine Increases the Contractile Sensitivity to Histamine and Parasympathetic Activation in a Human Ex Vivo Model of Severe Eosinophilic Asthma" Vaccines 11, no. 2: 282. https://doi.org/10.3390/vaccines11020282
APA StyleCalzetta, L., Chetta, A., Aiello, M., Frizzelli, A., Ora, J., Melis, E., Facciolo, F., Ippoliti, L., Magrini, A., & Rogliani, P. (2023). The BNT162b2 mRNA COVID-19 Vaccine Increases the Contractile Sensitivity to Histamine and Parasympathetic Activation in a Human Ex Vivo Model of Severe Eosinophilic Asthma. Vaccines, 11(2), 282. https://doi.org/10.3390/vaccines11020282








