Development and Assessment of Innovative High-Fidelity Simulation Vaccination Course Integrating Emergency Cases for Pharmacy Undergraduates—A Randomized Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Procedure
2.3. Objective Structured Clinical Examination
2.4. Training Sessions
2.5. Instruments
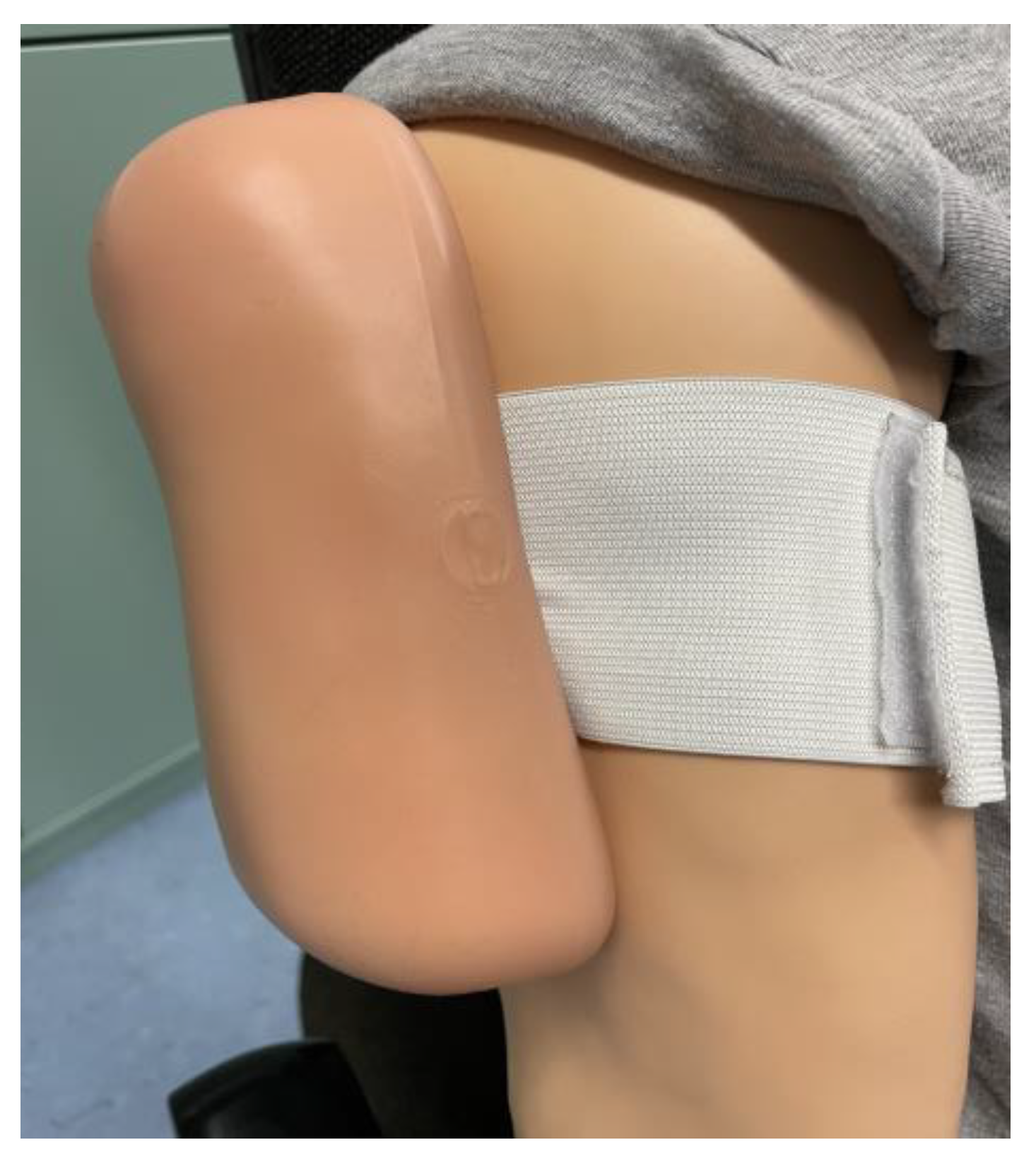
2.5.1. High- and Low-Fidelity Simulator
2.5.2. Cases for OSCEs
2.5.3. Analytical Checklist
2.5.4. Multiple-Choice Test
2.5.5. Self-Assessment Questionnaire
2.6. Statistical Methods
3. Results
3.1. Participant Characteristics
3.2. Analytical Checklist Score of OSCEs
3.3. Multiple-Choice Test
3.4. Self-Assessment Questionnaire
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Group | Pre-Training | Post-Training | p-Value (Intragroup) |
|---|---|---|---|
| Mean (CI) | Mean (CI) | ||
| Intervention | 32.45 3.10 | 41.60 2.77 | p < 0.01 |
| Control | 33.92 2.96 | 41.33 2.10 | p < 0.01 |
| p-Value (intergroup) | p = 0.505 | p = 0.568 |

References
- Vaccines work. Nat. Commun. 2018, 9, 1666. [CrossRef] [PubMed] [Green Version]
- Leidner, A.J.; Murthy, N.; Chesson, H.W.; Biggerstaff, M.; Stoecker, C.; Harris, A.M.; Acosta, A.; Dooling, K.; Bridges, C.B. Cost-effectiveness of adult vaccinations: A systematic review. Vaccine 2019, 37, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Ehreth, J. The global value of vaccination. Vaccine 2003, 21, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Bärnighausen, T.; Bloom, D.E.; Cafiero-Fonseca, E.T.; O’Brien, J.C. Valuing vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12313–12319. [Google Scholar] [CrossRef] [Green Version]
- Arsenault, C.; Harper, S.; Nandi, A. Effect of vaccination on children’s learning achievements: Findings from the India Human Development Survey. J. Epidemiol. Community Health 2020, 74, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Dye, C. The benefits of large scale covid-19 vaccination. BMJ 2022, 377, o867. [Google Scholar] [CrossRef]
- Paudyal, V.; Fialová, D.; Henman, M.C.; Hazen, A.; Okuyan, B.; Lutters, M.; Cadogan, C.; da Costa, F.A.; Galfrascoli, E.; Pudritz, Y.M.; et al. Pharmacists’ involvement in COVID-19 vaccination across Europe: A situational analysis of current practice and policy. Int. J. Clin. Pharm. 2021, 43, 1139–1148. [Google Scholar] [CrossRef]
- Betsch, C.; Böhm, R.; Korn, L.; Holtmann, C. On the benefits of explaining herd immunity in vaccine advocacy. Nat. Hum. Behav. 2017, 1, 0056. [Google Scholar] [CrossRef]
- Kadkhoda, K. Herd Immunity to COVID-19. Am. J. Clin. Pathol. 2021, 155, 471–472. [Google Scholar] [CrossRef]
- Krause, P.R.; Fleming, T.R.; Peto, R.; Longini, I.M.; Figueroa, J.P.; Sterne, J.A.C.; Cravioto, A.; Rees, H.; Higgins, J.P.T.; Boutron, I.; et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet 2021, 398, 1377–1380. [Google Scholar] [CrossRef]
- Federal Ministry of Health. Auffrischungsimpfung. 2022. Available online: https://www.zusammengegencorona.de/faqs/impfen/auffrischungsimpfung/ (accessed on 7 October 2022).
- Federal Ministry of Health. Aktueller Impfstatus: Wie ist der Fortschritt der COVID19 Impfung? Available online: https://impfdashboard.de/ (accessed on 7 October 2022).
- Infection Protection Act: IfSG. 2001. Available online: https://www.gesetze-im-internet.de/ifsg/__20c.html (accessed on 16 August 2022).
- International Pharmaceutical Federation. An Overview of Current Pharmacy Impact on Immunisation: A Global Report. Available online: https://www.fip.org/files/fip/publications/FIP_report_on_Immunisation.pdf (accessed on 16 August 2022).
- International Pharmaceutical Federation. An Overview of Pharmacy’s Impact on Immunisation Coverage: A Global Survey. The Hague. 2020. Available online: https://www.fip.org/file/4751 (accessed on 16 August 2022).
- Social Code, Book Five (V): SGB. 1989. Available online: https://www.gesetze-im-internet.de/sgb_5/__132j.html (accessed on 16 August 2022).
- Eickhoff, C.; Griese-Mammen, N.; Müeller, U.; Said, A.; Schulz, M. Primary healthcare policy and vision for community pharmacy and pharmacists in Germany. Pharm. Pract. (Granada) 2021, 19, 2248. [Google Scholar] [CrossRef] [PubMed]
- Paules, C.; Subbarao, K. Influenza. Lancet 2017, 390, 697–708. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Seasonal Influenza Vaccines: An Overview for Decision-Makers. Available online: https://apps.who.int/iris/bitstream/handle/10665/336951/9789240010154-eng.pdf (accessed on 30 August 2022).
- OECD. Influenza Vaccination Rate (indicator). 2023. Available online: https://www.oecd-ilibrary.org/social-issues-migration-health/influenza-vaccination-rates/indicator/english_e452582e-en (accessed on 20 January 2023).
- Kelling, S.E. Exploring Accessibility of Community Pharmacy Services. Innov. Pharm. 2015, 6. [Google Scholar] [CrossRef]
- Isenor, J.E.; Edwards, N.T.; Alia, T.A.; Slayter, K.L.; MacDougall, D.M.; McNeil, S.A.; Bowles, S.K. Impact of pharmacists as immunizers on vaccination rates: A systematic review and meta-analysis. Vaccine 2016, 34, 5708–5723. [Google Scholar] [CrossRef] [PubMed]
- The Pharmaceutical Society of Ireland. Report on the Evaluation of the Seasonal Influenza Vaccination Service in Pharmacy 2014/2015. 2015. Available online: https://www.thepsi.ie/Libraries/Pharmacy_Practice/PSI_2014_15_Report_on_Seasonal_Influenza_Vaccination_Service.sflb.ashx (accessed on 18 August 2022).
- Czech, M.; Balcerzak, M.; Antczak, A.; Byliniak, M.; Piotrowska-Rutkowska, E.; Drozd, M.; Juszczyk, G.; Religioni, U.; Vaillancourt, R.; Merks, P. Flu Vaccinations in Pharmacies-A Review of Pharmacists Fighting Pandemics and Infectious Diseases. Int. J. Environ. Res. Public Health 2020, 17, 7945. [Google Scholar] [CrossRef] [PubMed]
- Steyer, T.E.; Ragucci, K.R.; Pearson, W.S.; Mainous, A.G. The role of pharmacists in the delivery of influenza vaccinations. Vaccine 2004, 22, 1001–1006. [Google Scholar] [CrossRef]
- Ecarnot, F.; Crepaldi, G.; Juvin, P.; Grabenstein, J.; Del Giudice, G.; Tan, L.; O’Dwyer, S.; Esposito, S.; Bosch, X.; Gavazzi, G.; et al. Pharmacy-based interventions to increase vaccine uptake: Report of a multidisciplinary stakeholders meeting. BMC Public Health 2019, 19, 1698. [Google Scholar] [CrossRef] [Green Version]
- Bushell, M.-J.A.; Yee, K.C.; Ball, P.A. Case for Pharmacist Administered Vaccinations in Australia. J. Pharm. Pract. Res. 2013, 43, 292–296. [Google Scholar] [CrossRef]
- Prescott, W.A.; Bernhardi, C. Immunization Education in US Pharmacy Colleges and Schools. Am. J. Pharm. Educ. 2019, 83, 6765. [Google Scholar] [CrossRef]
- Turner, C.J.; Ellis, S.; Giles, J.; Altiere, R.; Sintek, C.; Ulrich, H.; Valdez, C.; Zadvorny, E. An introductory pharmacy practice experience emphasizing student-administered vaccinations. Am. J. Pharm. Educ. 2007, 71, 3. [Google Scholar] [CrossRef] [Green Version]
- Terriff, C.M.; McKeirnan, K. Training student pharmacists to administer emergency pediatric influenza vaccine: A comparison of traditional vs. just-in-time training. Curr. Pharm. Teach. Learn. 2017, 9, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.; Emmerton, L.; Sim, T.F. Immunization training for pharmacy students: A student-centered evaluation. Pharm. Pract. (Granada) 2021, 19, 2427. [Google Scholar] [CrossRef]
- Donohoe, K.L.; Mawyer, T.M.; Stevens, J.T.; Morgan, L.A.; Harpe, S.E. An active-learning laboratory on immunizations. Am. J. Pharm. Educ. 2012, 76, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, P. The history of simulation in medical education and possible future directions. Med. Educ. 2006, 40, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Korayem, G.B.; Alshaya, O.A.; Kurdi, S.M.; Alnajjar, L.I.; Badr, A.F.; Alfahed, A.; Cluntun, A. Simulation-Based Education Implementation in Pharmacy Curriculum: A Review of the Current Status. Adv. Med. Educ. Pract. 2022, 13, 649–660. [Google Scholar] [CrossRef]
- Lin, K.; Travlos, D.V.; Wadelin, J.W.; Vlasses, P.H. Simulation and introductory pharmacy practice experiences. Am. J. Pharm. Educ. 2011, 75, 209. [Google Scholar] [CrossRef] [Green Version]
- Basak, T.; Unver, V.; Moss, J.; Watts, P.; Gaioso, V. Beginning and advanced students’ perceptions of the use of low- and high-fidelity mannequins in nursing simulation. Nurse Educ. Today 2016, 36, 37–43. [Google Scholar] [CrossRef]
- Vyas, D.; Bray, B.S.; Wilson, M.N. Use of simulation-based teaching methodologies in US colleges and schools of pharmacy. Am. J. Pharm. Educ. 2013, 77, 53. [Google Scholar] [CrossRef] [Green Version]
- Morris, A.; Young, G.; Roller, L.; Li, F.; Takamoto, P.; Baumgartner, L. High-fidelity simulation increases pharmacy resident perceived competence during medical emergencies. Curr. Pharm. Teach. Learn. 2019, 11, 1016–1021. [Google Scholar] [CrossRef]
- Vyas, D.; Wombwell, E.; Russell, E.; Caligiuri, F. High-fidelity patient simulation series to supplement introductory pharmacy practice experiences. Am. J. Pharm. Educ. 2010, 74, 169. [Google Scholar] [CrossRef] [Green Version]
- Bingham, A.L.; Sen, S.; Finn, L.A.; Cawley, M.J. Retention of advanced cardiac life support knowledge and skills following high-fidelity mannequin simulation training. Am. J. Pharm. Educ. 2015, 79, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awaisu, A.; Abd Rahman, N.S.; Nik Mohamed, M.H.; Bux Rahman Bux, S.H.; Mohamed Nazar, N.I. Malaysian pharmacy students’ assessment of an objective structured clinical examination (OSCE). Am. J. Pharm. Educ. 2010, 74, 34. [Google Scholar] [CrossRef] [PubMed]
- Harden, R.M. Revisiting ‘Assessment of clinical competence using an objective structured clinical examination (OSCE)’. Med. Educ. 2016, 50, 376–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harden, R.M.; Gleeson, F.A. Assessment of clinical competence using an objective structured clinical examination (OSCE). Med. Educ. 1979, 13, 39–54. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA. 2021. Available online: http://www.rstudio.com/ (accessed on 30 August 2022).
- Sharkas, A. Vaccination Training Project Using High-Fidelity Simulation for Pharmacy Undergraduates. Master’s Thesis, Heinrich-Heine-University, Düsseldorf, Germany, Martin-Luther-University, Halle, Germany, September 2021. Available online: https://www.pharmazie.hhu.de/institut-fuer-klinische-pharmazie-und-pharmakotherapie/veroeffentlichungen/diplomarbeiten (accessed on 30 August 2022).
- Bundesapothekerkammer. Durchführung von Grippeschutzimpfungen in Öffentlichen Apotheken. Available online: https://www.abda.de/fuer-apotheker/qualitaetssicherung/leitlinien/leitlinien-und-arbeitshilfen/ (accessed on 20 September 2022).
- Microsoft Excel 2019; Microsoft Corporation: Redmond, WA, USA, 2019.
- Origin(Pro); Version 2021; OriginLab Corporation: Northampton, MA, USA, 2021.
- Yuan, H.B.; Williams, B.A.; Fang, J.B.; Ye, Q.H. A systematic review of selected evidence on improving knowledge and skills through high-fidelity simulation. Nurse Educ. Today 2012, 32, 294–298. [Google Scholar] [CrossRef]
- Bushell, M.; Frost, J.; Deeks, L.; Kosari, S.; Hussain, Z.; Naunton, M. Evaluation of Vaccination Training in Pharmacy Curriculum: Preparing Students for Workforce Needs. Pharmacy 2020, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Skoy, E.T.; Eukel, H.N.; Frenzel, J.E. Comparison of Low- and Higher-Fidelity Simulation to Train and Assess Pharmacy Students’ Injection Technique. Am. J. Pharm. Educ. 2013, 77, 33. [Google Scholar] [CrossRef] [PubMed]
- Carroll, P.R.; Hanrahan, J. Development and evaluation of an interprofessional student-led influenza vaccination clinic for medical, nursing and pharmacy students. Pharm. Pract. (Granada) 2021, 19, 2449. [Google Scholar] [CrossRef]
- Lau, E.T.L.; Rochin, M.E.; DelDot, M.; Glass, B.D.; Nissen, L.M. “There’s No Touching in Pharmacy”: Training Pharmacists for Australia’s First Pharmacist Immunization Pilot. Can. J. Hosp. Pharm. 2017, 70, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Abajas-Bustillo, R.; Amo-Setién, F.; Aparicio, M.; Ruiz-Pellón, N.; Fernández-Peña, R.; Silio-García, T.; Leal-Costa, C.; Ortego-Mate, C. Using High-Fidelity Simulation to Introduce Communication Skills about End-of-Life to Novice Nursing Students. Healthcare 2020, 8, 238. [Google Scholar] [CrossRef]
- Tokunaga, J.; Takamura, N.; Ogata, K.; Yoshida, H.; Setoguchi, N.; Matsuoka, T.; Hirokane, T.; Yamaoka, A.; Sato, K. Vital sign monitoring using human patient simulators at pharmacy schools in Japan. Am. J. Pharm. Educ. 2010, 74, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massoth, C.; Röder, H.; Ohlenburg, H.; Hessler, M.; Zarbock, A.; Pöpping, D.M.; Wenk, M. High-fidelity is not superior to low-fidelity simulation but leads to overconfidence in medical students. BMC Med. Educ. 2019, 19, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Cerra, C.; Dante, A.; Caponnetto, V.; Franconi, I.; Gaxhja, E.; Petrucci, C.; Alfes, C.M.; Lancia, L. Effects of high-fidelity simulation based on life-threatening clinical condition scenarios on learning outcomes of undergraduate and postgraduate nursing students: A systematic review and meta-analysis. BMJ Open 2019, 9, e025306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alinier, G.; Hunt, B.; Gordon, R.; Harwood, C. Effectiveness of intermediate-fidelity simulation training technology in undergraduate nursing education. J. Adv. Nurs. 2006, 54, 359–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCoy, C.E.; Rahman, A.; Rendon, J.C.; Anderson, C.L.; Langdorf, M.I.; Lotfipour, S.; Chakravarthy, B. Randomized Controlled Trial of Simulation vs. Standard Training for Teaching Medical Students High-quality Cardiopulmonary Resuscitation. West. J. Emerg. Med. 2019, 20, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branch, C. Pharmacy Students’ Learning and Satisfaction with High-Fidelity Simulation to Teach Drug-Induced Dyspepsia. Am. J. Pharm. Educ. 2013, 77, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilliland, I.; Frei, B.L.; McNeill, J.; Stovall, J. Use of high-fidelity simulation to teach end-of-life care to pharmacy students in an interdisciplinary course. Am. J. Pharm. Educ. 2012, 76, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee Chin, K.; Ling Yap, Y.; Leng Lee, W.; Chang Soh, Y. Comparing effectiveness of high-fidelity human patient simulation vs case-based learning in pharmacy education. Am. J. Pharm. Educ. 2014, 78, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.-W.; Ku, S.-C.; Ma, M.H.-M.; Chu, T.-S.; Chang, S.-C. Application of high-fidelity simulation in critical care residency training as an effective learning, assessment, and prediction tool for clinical performance. J. Formos. Med. Assoc. 2019, 118, 1347–1355. [Google Scholar] [CrossRef]
- Zamami, Y.; Imai, T.; Imanishi, M.; Takechi, K.; Shiraishi, N.; Koyama, T.; Sagara, H.; Shiino, Y.; Sendo, T.; Ishizawa, K. Evaluation of pharmaceutical lifesaving skills training oriented pharmaceutical intervention. J. Pharm. Health Care Sci. 2016, 2, 21. [Google Scholar] [CrossRef] [Green Version]






| Control Group (n = 21) | Intervention Group (n = 21) | p-Values | |
|---|---|---|---|
| Age | |||
| Mean (±SD) Median Range | 25 (±2.67) 24 22–32 | 24.38 (±2.35) 23 22–31 | 0.337 |
| Gender | |||
| Female, n (%) Male, n (%) | 17 (81) 4 (19) | 18 (86) 3 (14) | 1 |
| Previous or current experience (e.g., pharmaceutical technician, vaccination centre) | |||
| Yes (%) No (%) | 6 (29) 15 (71) | 0 (0) 21 (100) | 0.021 |
| Group | Pre-Training OSCE-Score | Post-Training OSCE-Score | Score Difference |
|---|---|---|---|
| Mean (SD) % | Mean (SD) % | Mean (SD) % | |
| Station 1 | |||
| Intervention | 26.06 (14.81) | 69.38 (15.72) | 43.34 (23.18) |
| Control | 27.65 (18.53) | 63.89 (18.92) | 36.24 (21.54) |
| Station 2 | |||
| Intervention | 7.54 (9.83) | 45.64 (17.00) | 38.10 (16.79) |
| Control | 22.22 (13.26) | 43.25 (28.34) | 21.03 (32.13) |
| Station 3 | |||
| Intervention | 49.60 (15.47) | 84.52 (11.87) | 34.92 (17.60) |
| Control | 53.18 (17.38) | 67.86 (22.10) | 14.68 (26.10) |
| Station 4 | |||
| Intervention | 51.70 (19.42) | 65.99 (17.77) | 14.29 (27.85) |
| Control | 61.22 (26.40) | 63.95 (27.71) | 2.72 (19.50) |
| Total | |||
| Intervention | 32.10 (7.70) | 66.12 (8.89) | 34.03 (11.66) |
| Control | 39.73 (8.21) | 58.79 (19.84) | 19.06 (18.20) |
| Group | Pre-Training | Post-Training |
|---|---|---|
| Mean (%) | Mean (%) | |
| Intervention | 3.00 (60) | 3.43 (68.57) |
| Control | 2.7 (55.24) | 2.86 (57.14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayyed, S.A.; Sharkas, A.R.; Ali Sherazi, B.; Dabidian, A.; Schwender, H.; Laeer, S. Development and Assessment of Innovative High-Fidelity Simulation Vaccination Course Integrating Emergency Cases for Pharmacy Undergraduates—A Randomized Controlled Study. Vaccines 2023, 11, 324. https://doi.org/10.3390/vaccines11020324
Sayyed SA, Sharkas AR, Ali Sherazi B, Dabidian A, Schwender H, Laeer S. Development and Assessment of Innovative High-Fidelity Simulation Vaccination Course Integrating Emergency Cases for Pharmacy Undergraduates—A Randomized Controlled Study. Vaccines. 2023; 11(2):324. https://doi.org/10.3390/vaccines11020324
Chicago/Turabian StyleSayyed, Shahzad Ahmad, Ahmed Reda Sharkas, Bushra Ali Sherazi, Armin Dabidian, Holger Schwender, and Stephanie Laeer. 2023. "Development and Assessment of Innovative High-Fidelity Simulation Vaccination Course Integrating Emergency Cases for Pharmacy Undergraduates—A Randomized Controlled Study" Vaccines 11, no. 2: 324. https://doi.org/10.3390/vaccines11020324






