Yellow Fever Vaccine-Related Neurotropic Disease in Brazil Following Immunization with 17DD
Abstract
1. Introduction
2. Materials and Methods
3. Results
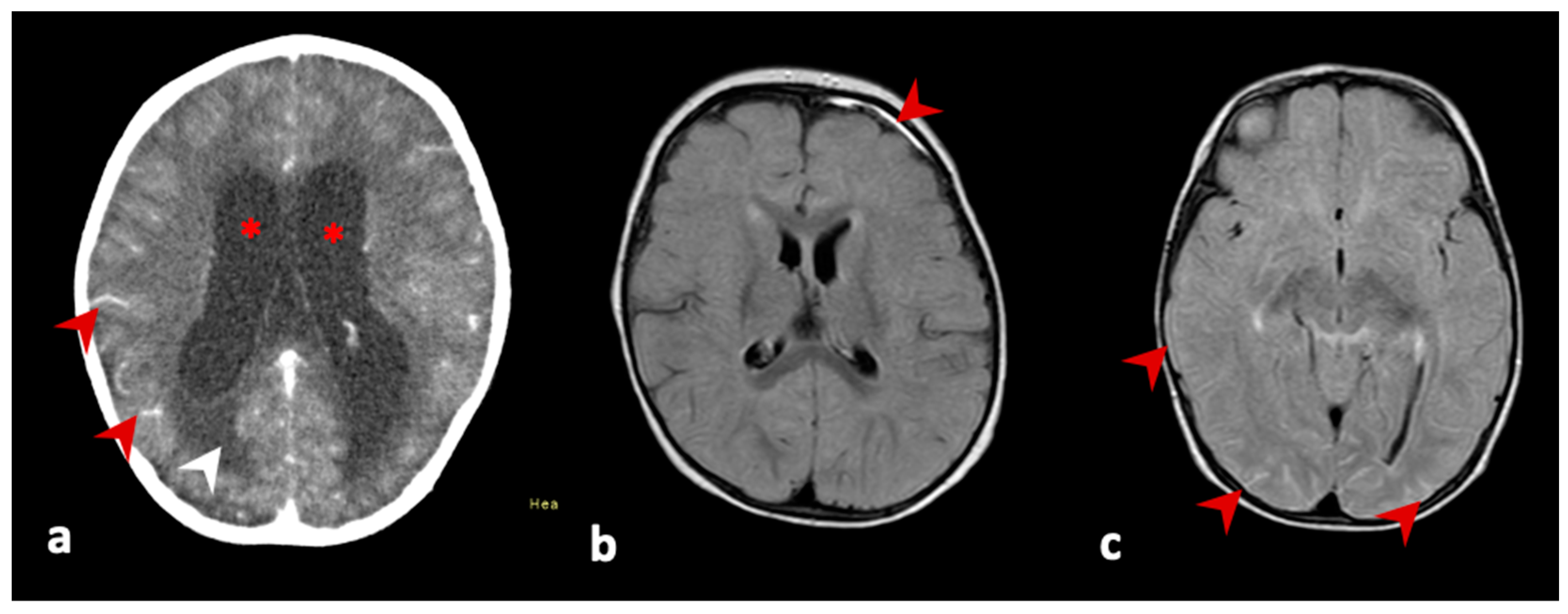
3.1. Case 1
3.2. Case 2
3.3. Case 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Reference ID | Gender, Age | Number of Cases, Clinical Presentation | Clinical Outcome | Yellow Fever Vaccine | Country, Year of Occurrence |
|---|---|---|---|---|---|
| [11] | both,16–71 years of age | 10, meningitis, encephalitis, Sd Guillain–Barrè Syndrome | recovered | 17D-204 | USA, 1990–2002 |
| [14] | male, 9 months of age | 1, meningitis | recovered | not reported | Brazil, not reported |
| [15] | both, 17–76 years of age | 17, encephalitis, Guillain–Barrè syndrome, aseptic meningitis, acute disseminated encephalomyelitis and transverse myelitis | recovered | 17D-204 | USA, 2007–2013 |
| [16] | male, 53–63 years of age | 2, transverse myelitis and cerebelar syndrome | recovered | 17D | Argentina, 2008 |
| [17] | male, 39 years of age. | 1, meningitis | recovered | 17D-204 | Poland, 2015 |
| [18] | male/female, not reported | 6, transverse myelitis, encephalitis, aseptic meningitis, facial paralysis, | 1 death and 5 recovered | 17D and 17DD | Benin, Burkina Faso, Cameroon, Guinea, Liberia, Mali, Senegal, Sierra Leone, and Togo, 2007-10 |
| [19] | male, 37 years of age | 1, intermediate and anterior uveitis | recovered | 17D-204 | France, 2018 |
| [20] | both, 0–85 years of age. | 67, Guillain–Barré syndrome, acute disseminated encephalomyelitis cases, transverse myelitis, bilateral optic neuritis, meningoencephalitis, polyradiculoneuritis, Kinsbourne syndrome | recovered | 17DD | Brazil, 2007–12 |
| [21] | female, 23 years of age. | 1, encephalitis (and viscerotropic disease) | recovered | 17D-204 | Brazil, 2006 |
| [22] | male, 70 years of age | 1, stroke-like syndrome | recovered | not reported | Portugal, not reported |
| [23] | male, 4 years of age | 1, meningoencephalitis | recovered | 17D-204 | France, not reported |
| [24] | both, 21–55 years of age | 4, meningitis and meningoencephalitis | recovered | 17D | France, 2000–2008 |
| [25] | female, 14–42 years of age | 2, encephalitis and cerebelar syndrome | recovered | 17DD | Brazil, 2007–2008 |
| [26] | male, 56 years of age | 1, longitudinal myelitis | recovered | 17D-204 | Argentina, 2009 |
| [27] | both, 19–59 years of age | 2, meningoencephalitis | recovered | 17D | France, not reported |
| [28] | male, 16–71 years of age | 4, meningitis and white matter disease | recovered | not reported | USA, 2001–2002 |
| [29] | male, 56 years of age | 1, meningitis | recovered | 17D-204 | Belgium, 2018 |
| [30] | male, 56 years of age | 1, meningoencephalitis | recovered | 17D-204 | Germany, not reported |
| [31] | male, 76 years of age | 1, encephalitis (and viscerotropic disease) | recovered | 17D-204 | USA, 1998 |
| [32] | female, 3 years of age | 1, encephalitis | death | 17D | USA, 1965 |
| [33] | female, 67 years of age | 1, meningoencephalitis and variant Creutzfeldt Jakob disease | recovered | 17D-204 | USA, not reported |
| [34] | both genders, 17–68 years of age | 15, encephalitis, Guillain–Barrè syndrome, acute disseminated encephalomyelitis | recovered | 17D-204 | USA, 1990–2005 |
| [35] | both genders, 0–70 years of age | 12, not reported | not reported | 17D-204 | USA, 2000–2006 |
| [36] | not reported | 4, encephalitis, Guillain–Barrè syndrome, bulbar palsy | not reported | 17D-204 | not reported, 1991–2001 |
References
- WHO. Disease Outbreak News (DONS). Available online: https://www.who.int/emergencies/disease-outbreak-news. (accessed on 22 April 2022).
- Pierson, T.; Diamond, M. Fields Virology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; Volume 1, Chapter 26; pp. 747–794. [Google Scholar]
- Hansen, C.A.; Barrett, A.D.T. The Present and Future of Yellow Fever Vaccines. Pharmaceuticals 2021, 14, 891. [Google Scholar] [CrossRef]
- Eliminate Yellow fever Epidemics (EYE): A global strategy, 2017–2026. Wkly. Epidemiol. Rec. 2017, 92, 193–204.
- de Menezes Martins, R.; Fernandes Leal Ma, L.; Homma, A. Serious adverse events associated with yellow fever vaccine. Hum. Vaccin. Immunother. 2015, 11, 2183–2187. [Google Scholar] [CrossRef]
- Staples, J.E.; Gershman, M.; Fischer, M. CDC Yellow fever vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2010, 59, 1–27. [Google Scholar]
- Brasil. Ministério da Saúde. Manual de Vigilância Epidemiológica de Eventos Adversos Pós-Vacinação, 3rd ed.; Ministério da Saúde, Secretaria de Vigilância em Saúde: Brasília, Brazil, 2021; 250p.
- Gianchecchi, E.; Cianchi, V.; Torelli, A.; Montomoli, E. Yellow Fever: Origin, Epidemiology, Preventive Strategies and Future Prospects. Vaccines 2022, 10, 372. [Google Scholar] [CrossRef]
- Frierson, J.G. The yellow fever vaccine: A history. Yale J. Biol. Med. 2010, 83, 77–85. [Google Scholar]
- Ferreira, C.D.C.; Campi-Azevedo, A.C.; Peruhype-Magalhāes, V.; Costa-Pereira, C.; De Albuquerque, C.P.; Muniz, L.F.; De Souza, T.Y.; Oliveira, A.C.V.; Martins-Filho, O.A.; Da Mota, L.M.H. The 17D-204 and 17DD yellow fever vaccines: An overview of major similarities and subtle differences. Expert. Rev. Vaccines 2017, 17, 79–90. [Google Scholar] [CrossRef]
- Khromava, A.Y.; Eidex, R.B.; Weld, L.H.; Kohl, K.S.; Bradshaw, R.D.; Chen, R.T.; Cetron, M.S. Yellow fever vaccine: An updated assessment of advanced age as a risk factor for serious adverse events. Vaccine 2005, 23, 3256–3263. [Google Scholar] [CrossRef]
- Porudominsky, R.; Gotuzzo, E.H. Yellow fever vaccine and risk of developing serious adverse events: A systematic review. Rev. Panam. Salud. Publica. 2018, 42, e75. [Google Scholar] [CrossRef]
- de Abreu, A.J.L.; Cavalcante, J.R.; de Araújo Lagos, L.W.; Caetano, R.; Braga, J.U. A Systematic Review and a Meta-Analysis of the Yellow Fever Vaccine in the Elderly Population. Vaccines 2022, 10, 711. [Google Scholar] [CrossRef]
- De Oliveira, H.S.B.; De Araujo, P.P.; De Sousa, J.R.P.; Donis, A.C.G.; Moreira, D.; Makssoudian, A. Serious adverse event: Late neurotropic disease associated with yellow fever vaccine. Einstein 2020, 18, eRC5041. [Google Scholar] [CrossRef]
- Lindsey, N.P.; Rabe, I.B.; Miller, E.R.; Fischer, M.; Staples, J.E. Adverse event reports following yellow fever vaccination, 2007–2013. J. Travel Med. 2016, 23, taw045. [Google Scholar] [CrossRef]
- Pires-Marczeski, F.C.; Martinez, V.P.; Nemirovsky, C.; Padula, P.J. Intrathecal antibody production in two cases of yellow fever vaccine associated neurotropic disease in Argentina. J. Med. Virol. 2011, 83, 2208–2212. [Google Scholar] [CrossRef]
- Florczak-Wyspiańska, J.; Nawotczyńska, E.; Kozubski, W. Yellow fever vaccine-associated neurotropic disease (YEL-AND)—A case report. Neurol. Neurochir. Pol. 2017, 51, 101–105. [Google Scholar] [CrossRef]
- Breugelmans, J.; Lewis, R.; Agbenu, E.; Veit, O.; Jackson, D.; Domingo, C.; Böthe, M.; Perea, W.; Niedrig, M.; Gessner, B.; et al. Adverse events following yellow fever preventive vaccination campaigns in eight African countries from 2007 to 2010. Vaccine 2013, 31, 1819–1829. [Google Scholar] [CrossRef]
- Volkov, L.; Grard, G.; Bollaert, P.-E.; Durand, G.A.; Cravoisy, A.; Conrad, M.; Nace, L.; Courte, G.; Marnai, R.; Leparc-Goffart, I.; et al. Viscerotropic disease and acute uveitis following yellow fever vaccination: A case report. BMC Infect. 2020, 20, 1–5. [Google Scholar] [CrossRef]
- Martins, R.D.M.; Pavão, A.L.B.; de Oliveira, P.M.N.; dos Santos, P.R.G.; Carvalho, S.M.D.; Mohrdieck, R.; Fernandes, A.; Sato, H.K.; de Figueiredo, P.M.; Doellinger, V.D.R.V.; et al. Adverse events following yellow fever immunization: Report and analysis of 67 neurological cases in Brazil. Vaccine 2014, 32, 6676–6682. [Google Scholar] [CrossRef]
- Silva, M.L.; Espírito-Santo, L.R.; Martins, M.A.; Lemos, D.S.; Peruhype-Magalhāes, V.; Caminha, R.C.; Maranhāo-Filho, P.D.A.; Auxiliadora-Martins, M.; Martins, R.D.M.; Galler, R.; et al. Clinical and immunological insights on severe, adverse neurotropic and viscerotropic disease following 17D yellow fever vaccination. Clin. Vaccine. Immunol. 2010, 17, 118–126. [Google Scholar] [CrossRef]
- Beirão, P.; Pereira, P.; Nunes, A.; Antunes, P. Yellow fever vaccine-associated neurological disease, a suspicious case. BMJ Case Rep. 2017, 2017, bcr2016218706. [Google Scholar] [CrossRef]
- Gerin, M.; Wroblewski, I.; Bost-Bru, C.; N’guyen, M.A.; Debillon, T. YEL-AND meningoencephalitis in a 4-year-old boy consecutive to a yellow-fever vaccine. Arch. Pediatr. 2014, 21, 384–387. [Google Scholar] [CrossRef]
- Guimard, T.; Minjolle, S.; Polard, E.; Fily, F.; Zeller, H.; Michelet, C.; Tattevin, P. Short report: Incidence of yellow fever vaccine-associated neurotropic disease. Am. J. Trop. Med. Hyg. 2009, 81, 1141–1143. [Google Scholar] [CrossRef] [PubMed]
- Guedes, B.F.; Ribeiro, A.F.; Pinto, L.F.; Vidal, J.E.; de Oliveira, F.G.; Sztajnbok, J.; de Oliveira, A.C.P.; Simabukuro, M.M. Potential autoimmune encephalitis following yellow fever vaccination: A report of three cases. J. Neuroimmunol. 2021, 355, 577548. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.; Riccio, P.; Patrucco, L.; Rojas, J.I.; Cristiano, E. Longitudinal myelitis associated with yellow fever vaccination. J. Neurovirol. 2009, 15, 348–350. [Google Scholar] [CrossRef]
- Drouet, A.; Chagnon, A.; Valance, J.; Carli, P.; Muzellec, Y.; Paris, J. Meningoencephalitis after vaccination against yellow fever with the 17 D strain: 2 cases. Rev. Med. Interne. 1993, 14, 257–259. [Google Scholar] [CrossRef] [PubMed]
- CDC. Adverse events associated with 17D-derived yellow fever vaccination—United States, 2001-2002. MMWR Morb. Mortal. Wkly. Rep. 2002, 51, 989–993. [Google Scholar]
- LeComte, E.; Laureys, G.; Verbeke, F.; Carrasco, C.D.; Van Esbroeck, M.; Huits, R. A clinician’s perspective on yellow fever vaccine-associated neurotropic disease. J. Travel. Med. 2020, 27. [Google Scholar] [CrossRef]
- Slesak, G.; Gabriel, M.; Domingo, C.; Schäfer, J. Severe Yellow fever vaccine-associated disease: A case report and current overview. Dtsch. Med. Wochenschr. 2017, 142, 1219–1222. [Google Scholar]
- Martin, M. Fever and multisystem organ failure associated with 17D-204 yellow fever vaccination: A report of four cases. Lancet 2001, 358, 98–104. [Google Scholar] [CrossRef]
- Receveur, M.C.; Bruyand, M.; Pistone, T.; Malvy, D. Yellow fever vaccination: Update on rare and severe adverse effects. Med. Mal. Infect. 2009, 39, 234–241. [Google Scholar] [CrossRef]
- Cohen, M.; Nguyen, M.; Nix, C.D.; Case, B.; Nickerson, J.P.; Bernard, J.; Durrant, J.; Safarpour, D.; Tucker, T.; Vagnerova, K.; et al. Case Report: Yellow Fever Vaccine-Associated Neurotropic Disease and Associated MRI, EEG, and CSF Findings. Front. Neurol. 2021, 12, 779014. [Google Scholar] [CrossRef]
- McMahon, A.W.; Eidex, R.B.; Marfin, A.A.; Russell, M.; Sejvar, J.J.; Markoff, L.; Hayes, E.B.; Chen, R.T.; Ball, R.; Braun, M.M.; et al. Neurologic disease associated with 17D-204 yellow fever vaccination: A report of 15 cases. Vaccine 2007, 25, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, N.P.; Schroeder, B.A.; Miller, E.R.; Braun, M.M.; Hinckley, A.F.; Marano, N.; Slade, B.A.; Barnett, E.D.; Brunette, G.W.; Horan, K.; et al. Adverse event reports following yellow fever vaccination. Vaccine 2008, 26, 6077–6082. [Google Scholar] [CrossRef] [PubMed]
- Kitchener, S. Viscerotropic and neurotropic disease following vaccination with the 17D yellow fever vaccine, ARILVAX. Vaccine 2004, 22, 2103–2105. [Google Scholar] [CrossRef] [PubMed]
| ID | Gender, Age | YFV Vaccine Date, Dose | Date of Hospitalization | Time between Vaccination and Symptoms Onset | Signs and Symptoms | CSF Observations | Radiologic Observations | Other Observations | Diagnostic Tests |
|---|---|---|---|---|---|---|---|---|---|
| 1; | female, 4 years of age | 20 July 2021, second dose | 25 August 2021 | 36 days | vomiting, hyporexia, non-responsiveness, weight loss of 3 kg, bradypnea, bradycardia. outcome: follow-up on 12 November 2021, no recurrence of signs and symptoms, without sequelae | leukocytes 17 cells/mm3, lymphocytes 86%, neutrophils 0%, protein 153 mg/dL, glucose 37 mg/dL, culture negative | brain CT: signs of hypertensive hydrocephaly (supra and infratentorial). brain NMR: increased dimensions of the ventricular system, with ependymal transudation and interstitial edema, in addition to hyperdynamic flow within the third and fourth ventricle and mesencephalic aqueduct. Note leptomeningeal enhancement in the base cysteines and cranial pairs. brain biopsy: granulomatous chronic inflammatory process with focal necrosis and histiocytes | plasma/serum: Ht 33.2%; Hb 11.8 g/dL; plat 304,000/mm3; leuko: 8810/mm3; 24.9% neutro; TB: 0.22 mg/dL; DB: 0.1 mg/dL, Cr: 0.4 mg/dL; AST: 21 UI/L; ALT: 11 UI/L; GGT: 10 UI/L; AP: 112 UI/L; INR 1.17; anti-CMV IgM negative and IgG positive; PCR for CMV: negative; anti-HIV I/II: negative; anti-Rubella IgM negative and IgG positive; FTA-abs negative; anti-toxoplasmosis IgM negative; anti-measles IgM negative; anti-EBV IgM negative; anti-HSV I/II IgM negative CSF: real-time PCR TB negative; anti-dengue IgM negative; anti-Zika IgM negative; anti-SLEV IgM negative; anti-WNV negative; anti-ROCV IgM negative; anti-CHIKV IgM negative | anti-YFV IgM positive in CSF |
| 2; | female, 10 months of age | 17 June 2021, first dose | 7 July 2021 | 20 days | vomiting, somnolence, responsiveness, tonic-clonic seizures, fever, and left hemiparesis. outcome: follow-up on 12 August 2021, patient with no recurrence of signs and symptoms, without sequelae | leukocytes 150 cells/mm3, lymphocytes 72%, neutrophils 4%, protein 110 mg/dL, glucose 45 mg/dL, culture negative | EEG: bilateral degree I slowing, without non-epileptiform disorder brain NMR: focal pachymeningeal enhancement in the frontal brain area | plasma/serum: Ht 28.2%; Hb 10.1 g/dl; plat 244,000/mm3; leuko: 6950/mm3; 35.6% neutro; TB: 0.15 mg/dL; DB: 0.08 mg/dL, Cr: 0.3 mg/dL; AST: 30 UI/L; ALT: 15 UI/L; GGT: 16 UI/L; AP: 164 UI/L; INR 1.27; anti-dengue IgM negative CSF: anti-dengue IgM negative; anti-Zika IgM negative; anti-SLEV IgM negative; anti-WNV IgM negative; anti-ROCV IgM negative; RT-PCR CHIKV negative; RT-PCR DENV negative; RT-PCR SLEV negative; RT-PCR YFV negative; RT-PCR WNV negative; RT-PCR ZIKV negative | anti-YFV IgM positive in CSF |
| 3. | female, 9 months of age | 23 July 2021, first dose | 6 August 2021 | 14 days | fever, hyporexia, adynamia, emesis, lethargy, diarrhea, irritability. outcome: follow-up on 4 October 2021, patient without sequelae | leukocytes 70 cells/mm3, lymphocytes 73%, monocytes 25%, protein 35 mg/dL, glucose 51 mg/dL, culture negative | brain NMR: diffuse leptomeningeal enhancement | plasma/serum: Ht 39.1%; Hb 13g/dL; plat 499,000/mm3; leuko: 20,160/mm3; 71.8% neutro; TB: 0.17 mg/dL; DB: 0.01 mg/dL, Cr: 0.5 mg/dl; ALT: 11 UI/L; INR 1.20 CSF: anti-dengue IgM negative | anti-YFV IgM positive in CSF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Andrade Gandolfi, F.; Estofolete, C.F.; Wakai, M.C.; Negri, A.F.; Barcelos, M.D.; Vasilakis, N.; Nogueira, M.L. Yellow Fever Vaccine-Related Neurotropic Disease in Brazil Following Immunization with 17DD. Vaccines 2023, 11, 445. https://doi.org/10.3390/vaccines11020445
de Andrade Gandolfi F, Estofolete CF, Wakai MC, Negri AF, Barcelos MD, Vasilakis N, Nogueira ML. Yellow Fever Vaccine-Related Neurotropic Disease in Brazil Following Immunization with 17DD. Vaccines. 2023; 11(2):445. https://doi.org/10.3390/vaccines11020445
Chicago/Turabian Stylede Andrade Gandolfi, Flora, Cassia Fernanda Estofolete, Marcia Catelan Wakai, Andreia Francesli Negri, Michela Dias Barcelos, Nikos Vasilakis, and Mauricio Lacerda Nogueira. 2023. "Yellow Fever Vaccine-Related Neurotropic Disease in Brazil Following Immunization with 17DD" Vaccines 11, no. 2: 445. https://doi.org/10.3390/vaccines11020445
APA Stylede Andrade Gandolfi, F., Estofolete, C. F., Wakai, M. C., Negri, A. F., Barcelos, M. D., Vasilakis, N., & Nogueira, M. L. (2023). Yellow Fever Vaccine-Related Neurotropic Disease in Brazil Following Immunization with 17DD. Vaccines, 11(2), 445. https://doi.org/10.3390/vaccines11020445






