B-Cell Epitope Mapping of the Plasmodium falciparum Malaria Vaccine Candidate GMZ2.6c in a Naturally Exposed Population of the Brazilian Amazon
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Volunteers
2.2. Epidemiological Survey
2.3. Blood Sampling and Malaria Diagnosis
2.4. B-Cell Epitope Prediction of Pfs48/45
2.5. Synthetic Peptides
2.6. Enzyme-Linked Immunosorbent Assay
2.7. Statistical Analysis
3. Results
3.1. Population Characteristics
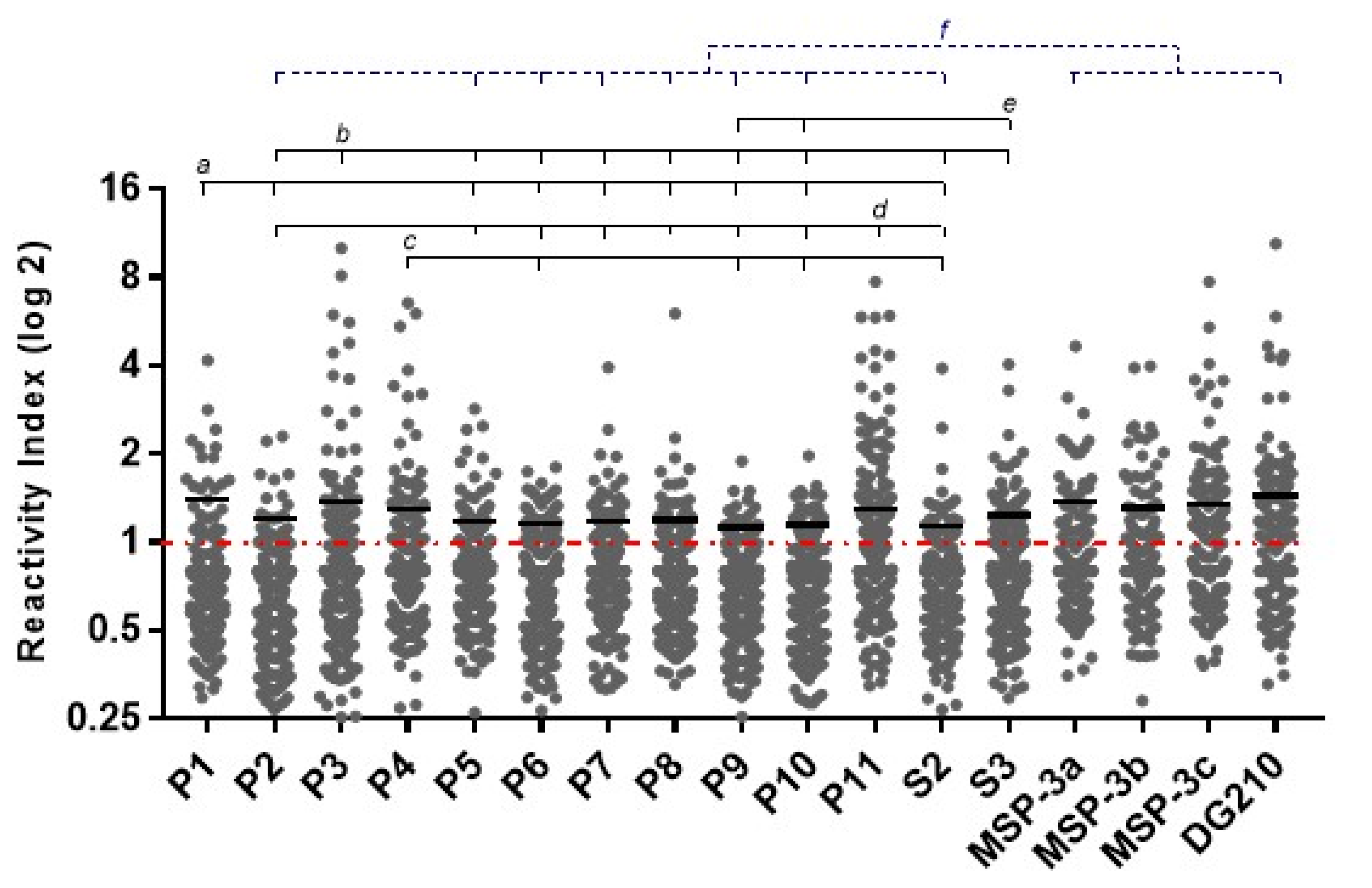
3.2. Frequencies and IgG Levels of Pre-Identified Linear B-Cell Epitopes of GLURP27–500 and MSP-3155–249
3.3. IgG Subclasses Distribution against the Immunodominant Epitopes of GLURP27–500 and MSP-3155–249
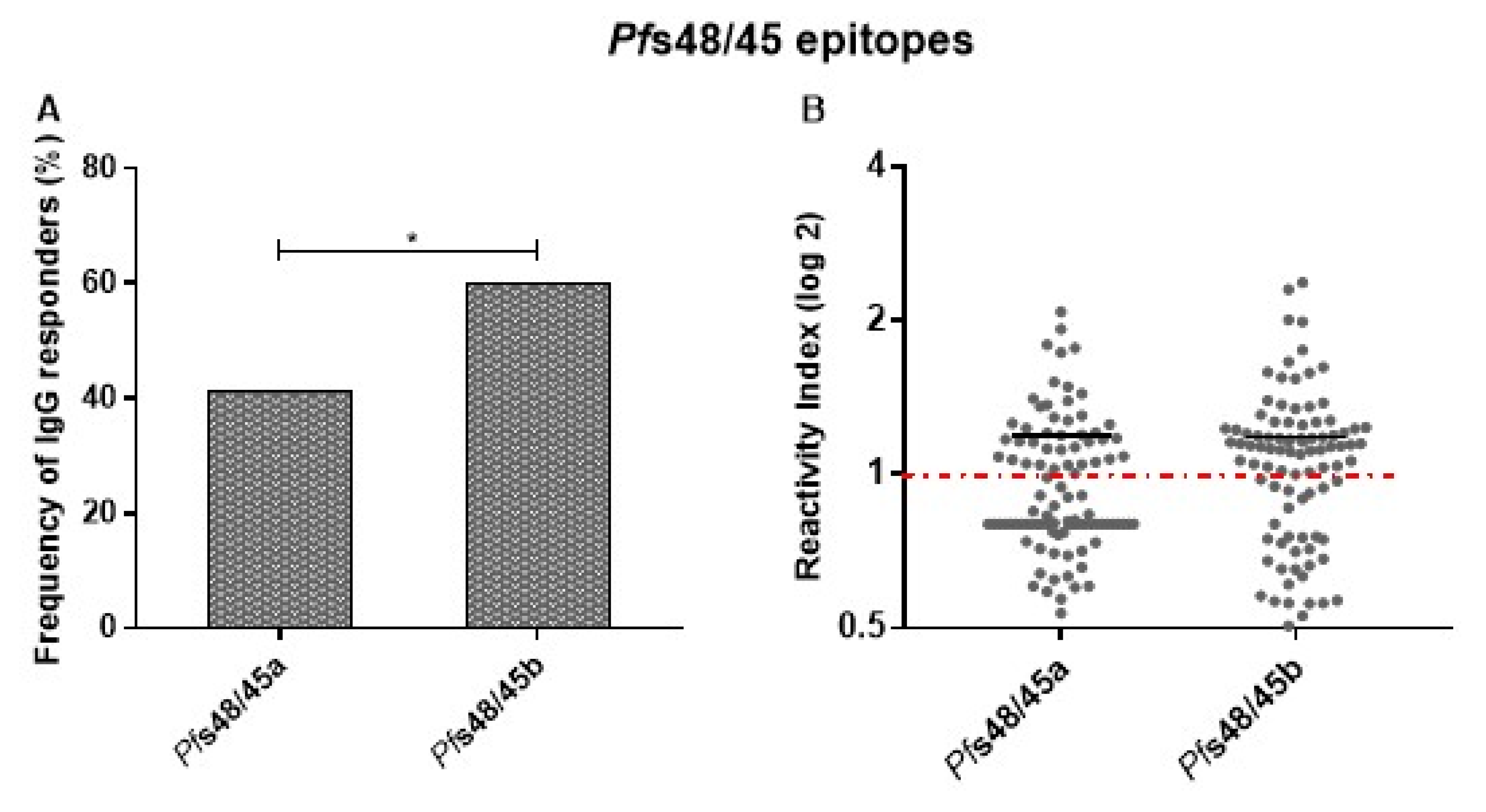
3.4. Experimental Validation of Predicted Linear B-Cell Epitopes of Pfs48/45291–428
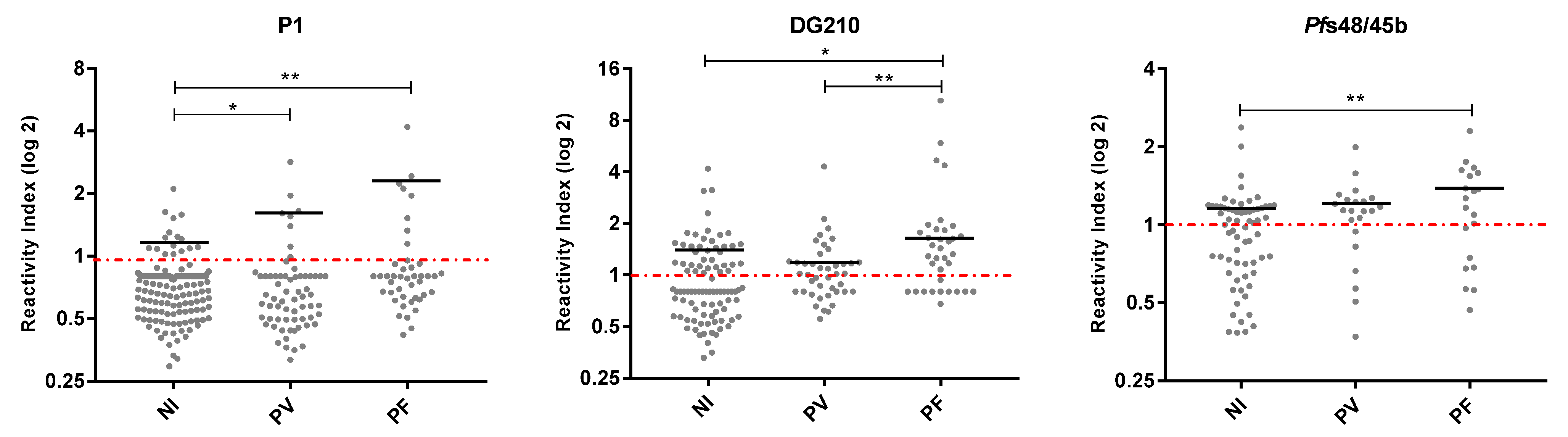
3.5. Frequencies and IgG Antibody Levels in Non-Infected and Infected by P. vivax and P. falciparum Individuals
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization World Malaria Report. Available online: https://www.malariaworld.org/blogs/world-malaria-report-2022 (accessed on 26 December 2022).
- Dimbu, P.R.; Horth, R.; Cândido, A.L.M.; Ferreira, C.M.; Caquece, F.; Garcia, L.E.A.; André, K.; Pembele, G.; Jandondo, D.; Bondo, B.J.; et al. Continued Low Efficacy of Artemether-Lumefantrine in Angola in 2019. Antimicrob. Agents Chemother. 2021, 65, e01949-20. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, L.C.; Cox, H.; Early, A.M.; Mok, S.; Lazrek, Y.; Paquet, J.C.; Ade, M.P.; Lucchi, N.W.; Grant, Q.; Udhayakumar, V.; et al. Local Emergence in Amazonia of Plasmodium falciparum K13 C580Y Mutants Associated with in Vitro Artemisinin Resistance. Elife 2020, 9, e51015. [Google Scholar] [CrossRef]
- Gansané, A.; Moriarty, L.F.; Ménard, D.; Yerbanga, I.; Ouedraogo, E.; Sondo, P.; Kinda, R.; Tarama, C.; Soulama, E.; Tapsoba, M.; et al. Anti-Malarial Efficacy and Resistance Monitoring of Artemether-Lumefantrine and Dihydroartemisinin-Piperaquine Shows Inadequate Efficacy in Children in Burkina Faso, 2017–2018. Malar. J. 2021, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Uwimana, A.; Umulisa, N.; Venkatesan, M.; Svigel, S.S.; Zhou, Z.; Munyaneza, T.; Habimana, R.M.; Rucogoza, A.; Moriarty, L.F.; Sandford, R.; et al. Association of Plasmodium falciparum Kelch13 R561H Genotypes with Delayed Parasite Clearance in Rwanda: An Open-Label, Single-Arm, Multicentre, Therapeutic Efficacy Study. Lancet Infect. Dis. 2021, 21, 1120–1128. [Google Scholar] [CrossRef]
- Das, S.; Kar, A.; Manna, S.; Mandal, S.; Mandal, S.; Das, S.; Saha, B.; Hati, A.K. Artemisinin Combination Therapy Fails Even in the Absence of Plasmodium falciparum Kelch13 Gene Polymorphism in Central India. Sci. Rep. 2021, 11, 9946. [Google Scholar] [CrossRef]
- Hancock, P.A.; Hendriks, C.J.M.; Tangena, J.A.; Gibson, H.; Hemingway, J.; Coleman, M.; Gething, P.W.; Cameron, E.; Bhatt, S.; Moyes, C.L. Mapping Trends in Insecticide Resistance Phenotypes in African Malaria Vectors. PLoS Biol. 2020, 18, e3000633. [Google Scholar] [CrossRef]
- Mugo, R.M.; Mwai, K.; Mwacharo, J.; Shee, F.M.; Musyoki, J.N.; Wambua, J.; Otieno, E.; Bejon, P.; Ndungu, F.M. Seven-Year Kinetics of RTS, S/AS01-Induced Anti-CSP Antibodies in Young Kenyan Children. Malar. J. 2021, 20, 452. [Google Scholar] [CrossRef]
- Baldwin, S.L.; Roeffen, W.; Singh, S.K.; Tiendrebeogo, R.W.; Christiansen, M.; Beebe, E.; Carter, D.; Fox, C.B.; Howard, R.F.; Reed, S.G.; et al. Synthetic TLR4 Agonists Enhance Functional Antibodies and CD4+ T-Cell Responses against the Plasmodium falciparum GMZ2.6C Multi-Stage Vaccine Antigen. Vaccine 2016, 34, 2207–2215. [Google Scholar] [CrossRef]
- Borre, M.B.; Dziegiel, M.; Høgh, B.; Petersen, E.; Rieneck, K.; Riley, E.; Meis, J.F.; Aikawa, M.; Nakamura, K.; Harada, M.; et al. Primary Structure and Localization of a Conserved Immunogenic Plasmodium falciparum Glutamate Rich Protein (GLURP) Expressed in Both the Preerythrocytic and Erythrocytic Stages of the Vertebrate Life Cycle. Mol. Biochem. Parasitol. 1991, 49, 119–131. [Google Scholar] [CrossRef] [PubMed]
- del Quintana, M.P.; Ch’ng, J.H.; Zandian, A.; Imam, M.; Hultenby, K.; Theisen, M.; Nilsson, P.; Qundos, U.; Moll, K.; Chan, S.; et al. SURGE Complex of Plasmodium falciparum in the Rhoptry-Neck (SURFIN4.2-RON4-GLURP) Contributes to Merozoite Invasion. PLoS ONE 2018, 13, e0201669. [Google Scholar] [CrossRef] [PubMed]
- McColl, D.J.; Anders, R.F. Conservation of Structural Motifs and Antigenic Diversity in the Plasmodium falciparum Merozoite Surface Protein-3 (MSP-3). Mol. Biochem. Parasitol. 1997, 90, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Imam, M.; Singh, S.; Kaushik, N.K.; Chauhan, V.S. Plasmodium falciparum Merozoite Surface Protein 3. J. Biol. Chem. 2014, 289, 3856–3868. [Google Scholar] [CrossRef]
- van Dijk, M.R.; Janse, C.J.; Thompson, J.; Waters, A.P.; Braks, J.A.M.; Dodemont, H.J.; Stunnenberg, H.G.; van Gemert, G.J.; Sauerwein, R.W.; Eling, W. A Central Role for P48/45 in Malaria Parasite Male Gamete Fertility. Cell 2001, 104, 153–164. [Google Scholar] [CrossRef]
- Kana, I.H.; Adu, B.; Tiendrebeogo, R.W.; Singh, S.K.; Dodoo, D.; Theisen, M. Naturally Acquired Antibodies Target the Glutamate-Rich Protein on Intact Merozoites and Predict Protection Against Febrile Malaria. J. Infect. Dis. 2017, 215, 623–630. [Google Scholar] [CrossRef]
- Dodoo, D.; Theisen, M.; Kurtzhals, J.A.L.; Akanmori, B.D.; Koram, K.A.; Jepsen, S.; Nkrumah, F.K.; Theander, T.G.; Hviid, L. Naturally Acquired Antibodies to the Glutamate-Rich Protein Are Associated with Protection against Plasmodium falciparum Malaria. J. Infect. Dis. 2000, 181, 1202–1205. [Google Scholar] [CrossRef]
- Soe, S.; Theisen, M.; Roussilhon, C.; Aye, K.S.; Druilhe, P. Association between Protection against Clinical Malaria and Antibodies to Merozoite Surface Antigens in an Area of Hyperendemicity in Myanmar: Complementarity between Responses to Merozoite Surface Protein 3 and the 220-Kilodalton Glutamate-Rich Protein. Infect. Immun. 2004, 72, 247–252. [Google Scholar] [CrossRef]
- Lusingu, J.P.A.; Vestergaard, L.S.; Alifrangis, M.; Mmbando, B.P.; Theisen, M.; Kitua, A.Y.; Lemnge, M.M.; Theander, T.G. Cytophilic Antibodies to Plasmodium falciparum Glutamate Rich Protein Are Associated with Malaria Protection in an Area of Holoendemic Transmission. Malar. J. 2005, 4, 48. [Google Scholar] [CrossRef]
- Bousema, J.T.; Drakeley, C.J.; Kihonda, J.; Hendriks, J.C.M.; Akim, N.I.J.; Roeffen, W.; Sauerwein, R.W. A Longitudinal Study of Immune Responses to Plasmodium falciparum Sexual Stage Antigens in Tanzanian Adults. Parasite Immunol. 2007, 29, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Baptista, B.O.; de Souza, A.B.L.; Riccio, E.K.P.; Bianco-Junior, C.; Totino, P.R.R.; Martins da Silva, J.H.; Theisen, M.; Singh, S.K.; Amoah, L.E.; Ribeiro-Alves, M.; et al. Naturally Acquired Antibody Response to a Plasmodium falciparum Chimeric Vaccine Candidate GMZ2.6c and Its Components (MSP-3, GLURP, and Pfs48/45) in Individuals Living in Brazilian Malaria-Endemic Areas. Malar. J. 2022, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health of Brazil. Epidemiological Situation of Malaria in Brazil. Available online: https://portalarquivos.saude.gov.br/images/pdf/2019/dezembro/03/3.%20a%20-%20Situa%C3%A7%C3%A3o%20Epidemiol%C3%B3gica%20da%20Mal%C3%A1ria_Brasil_CIT_28.11.2018.pdf (accessed on 15 December 2020).
- Shute, G.T. The Microscpopic Diagnosis of Malaria. In Malaria: Principles and Practice of Malariology; Wernsdorfer, W., McGregor, S., Eds.; Churchill Livingstone: New York, NY, USA, 1988; pp. 781–814. [Google Scholar]
- Snounou, G. Detection and Identification of the Four Malaria Parasite Species Infecting Humans by PCR Amplification. Methods Mol. Biol. 1996, 50, 263–291. [Google Scholar] [CrossRef]
- Ministry of Health of Brazil. Guide to Malaria Treatment in Brazil. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/guia_tratamento_malaria_brasil.pdf (accessed on 19 November 2020).
- Larsen, J.E.P.; Lund, O.; Nielsen, M. Improved Method for Predicting Linear B-Cell Epitopes. Immunome Res. 2006, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Oeuvray, C.; Bouharoun-Tayoun, H.; Gras-Masse, H.; Bottius, E.; Kaidoh, T.; Aikawa, M.; Filgueira, M.; Tartar, A.; Druilhe, P. Merozoite Surface Protein-3: A Malaria Protein Inducing Antibodies That Promote Plasmodium falciparum Killing by Cooperation with Blood Monocytes. Blood 1994, 84, 1594–1602. [Google Scholar] [CrossRef]
- Theisen, M.; Soe, S.; Jessing, S.G.; Okkels, L.M.; Danielsen, S.; Oeuvray, C.; Druilhe, P.; Jepsen, S. Identification of a Major B-Cell Epitope of the Plasmodium falciparum Glutamate-Rich Protein (GLURP), Targeted by Human Antibodies Mediating Parasite Killing. Vaccine 2000, 19, 204–212. [Google Scholar] [CrossRef]
- Beutling, U.; Frank, R. Epitope Analysis Using Synthetic Peptide Repertoires Prepared by SPOT Synthesis Technology. In Antibody Engineering; Springer: Berlin/Heidelberg, Germany, 2010; Volume 1, pp. 537–571. [Google Scholar]
- Ghattas, M.; Dwivedi, G.; Lavertu, M.; Alameh, M.G. Vaccine Technologies and Platforms for Infectious Diseases: Current Progress, Challenges, and Opportunities. Vaccines 2021, 9, 1490. [Google Scholar] [CrossRef] [PubMed]
- Noya, O.; Patarroyo, M.; Guzman, F.; de Noya, B. Immunodiagnosis of Parasitic Diseases with Synthetic Peptides. Curr. Protein Pept. Sci. 2003, 4, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Shirai, H.; Prades, C.; Vita, R.; Marcatili, P.; Popovic, B.; Xu, J.; Overington, J.P.; Hirayama, K.; Soga, S.; Tsunoyama, K.; et al. Antibody Informatics for Drug Discovery. Biochim. Biophys. Acta 2014, 1844, 2002–2015. [Google Scholar] [CrossRef]
- Doolan, D.L.; Dobaño, C.; Baird, J.K. Acquired Immunity to Malaria. Clin. Microbiol. Rev. 2009, 22, 13–36, Table of Contents. [Google Scholar] [CrossRef]
- Gonzales, S.J.; Reyes, R.A.; Braddom, A.E.; Batugedara, G.; Bol, S.; Bunnik, E.M. Naturally Acquired Humoral Immunity Against Plasmodium falciparum Malaria. Front. Immunol. 2020, 11, 594653. [Google Scholar] [CrossRef]
- Ministry of Health of Brazil. Boletim Epidemiológico. Available online: http://portalsaude.saude.gov.br (accessed on 11 November 2021).
- Oeuvray, C.; Bouharoun-Tayoun, H.; Grass-Masse, H.; Iepers, J.P.; Ralamboranto, L.; Tartar, A.; Druilhe, P. A Novel Merozoite Surface Antigen of Plasmodium falciparum (MSP-3) Identified by Cellular-Antibody Cooperative Mechanism Antigenicity and Biological Activity of Antibodies. Mem. Inst. Oswaldo Cruz 1994, 89, 77–80. [Google Scholar] [CrossRef]
- Theisen, M.; Soe, S.; Oeuvray, C.; Thomas, A.W.; Vuust, J.; Danielsen, S.; Jepsen, S.; Druilhe, P. The Glutamate-Rich Protein (GLURP) of Plasmodium falciparum Is a Target for Antibody-Dependent Monocyte-Mediated Inhibition of Parasite Growth In Vitro. Infect. Immun. 1998, 66, 11–17. [Google Scholar] [CrossRef]
- Nebie, I.; Diarra, A.; Ouedraogo, A.; Soulama, I.; Bougouma, E.C.; Tiono, A.B.; Konate, A.T.; Chilengi, R.; Theisen, M.; Dodoo, D.; et al. Humoral Responses to Plasmodium falciparum Blood-Stage Antigens and Association with Incidence of Clinical Malaria in Children Living in an Area of Seasonal Malaria Transmission in Burkina Faso, West Africa. Infect. Immun. 2008, 76, 759–766. [Google Scholar] [CrossRef]
- Osier, F.H.A.; Fegan, G.; Polley, S.D.; Murungi, L.; Verra, F.; Tetteh, K.K.A.; Lowe, B.; Mwangi, T.; Bull, P.C.; Thomas, A.W.; et al. Breadth and Magnitude of Antibody Responses to Multiple Plasmodium falciparum Merozoite Antigens Are Associated with Protection from Clinical Malaria. Infect. Immun. 2008, 76, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Courtin, D.; Oesterholt, M.; Huismans, H.; Kusi, K.; Milet, J.; Badaut, C.; Gaye, O.; Roeffen, W.; Remarque, E.J.; Sauerwein, R.; et al. The Quantity and Quality of African Children’s IgG Responses to Merozoite Surface Antigens Reflect Protection against Plasmodium falciparum Malaria. PLoS ONE 2009, 4, e7590. [Google Scholar] [CrossRef] [PubMed]
- Iriemenam, N.C.; Khirelsied, A.H.; Nasr, A.; ElGhazali, G.; Giha, H.A.; Elhassan A-Elgadir, T.M.; Agab-Aldour, A.A.; Montgomery, S.M.; Anders, R.F.; Theisen, M.; et al. Antibody Responses to a Panel of Plasmodium falciparum Malaria Blood-Stage Antigens in Relation to Clinical Disease Outcome in Sudan. Vaccine 2009, 27, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Dodoo, D.; Atuguba, F.; Bosomprah, S.; Ansah, N.A.; Ansah, P.; Lamptey, H.; Egyir, B.; Oduro, A.R.; Gyan, B.; Hodgson, A.; et al. Antibody Levels to Multiple Malaria Vaccine Candidate Antigens in Relation to Clinical Malaria Episodes in Children in the Kasena-Nankana District of Northern Ghana. Malar. J. 2011, 10, 108. [Google Scholar] [CrossRef]
- Guiyedi, V.; Bécavin, C.; Herbert, F.; Gray, J.; Cazenave, P.A.; Kombila, M.; Crisanti, A.; Fesel, C.; Pied, S. Asymptomatic Plasmodium falciparum Infection in Children Is Associated with Increased Auto-Antibody Production, High IL-10 Plasma Levels and Antibodies to Merozoite Surface Protein 3. Malar. J. 2015, 14, 162. [Google Scholar] [CrossRef]
- Pratt-Riccio, L.R.; Lima-Junior, J.C.; Carvalho, L.J.M.; Theisen, M.; Espíndola-Mendes, E.C.; Santos, F.; Oliveira-Ferreira, J.; Goldberg, A.C.; Daniel-Ribeiro, C.T.; Banic, D.M. Antibody Response Profiles Induced by Plasmodium falciparum Glutamate-Rich Protein in Naturally Exposed Individuals from a Brazilian Area Endemic for Malaria. Am. J. Trop. Med. Hyg. 2005, 73, 1096–1103. [Google Scholar] [CrossRef]
- Carvalho, L.J.M.; Alves, F.A.; Bianco, C.; Oliveira, S.G.; Zanini, G.M.; Soe, S.; Druilhe, P.; Theisen, M.; Muniz, J.A.P.C.; Daniel-Ribeiro, C.T. Immunization of Saimiri sciureus Monkeys with a Recombinant Hybrid Protein Derived from the Plasmodium falciparum Antigen Glutamate-Rich Protein and Merozoite Surface Protein 3 Can Induce Partial Protection with Freund and Montanide ISA720 Adjuvants. Clin. Diagn. Lab. Immunol. 2005, 12, 242–248. [Google Scholar] [CrossRef]
- Pratt-Riccio, L.R.; de Perce-da-Silva, D.S.; Lima-Junior, J.d.C.; Theisen, M.; Santos, F.; Daniel-Ribeiro, C.T.; de Oliveira-Ferreira, J.; Banic, D.M. Genetic Polymorphisms in the Glutamate-Rich Protein of Plasmodium falciparum Field Isolates from a Malaria-Endemic Area of Brazil. Mem. Inst. Oswaldo Cruz 2013, 108, 523–528. [Google Scholar] [CrossRef]
- Ambrosino, E.; Dumoulin, C.; Orlandi-Pradines, E.; Remoue, F.; Toure-Baldé, A.; Tall, A.; Sarr, J.B.; Poinsignon, A.; Sokhna, C.; Puget, K.; et al. A Multiplex Assay for the Simultaneous Detection of Antibodies against 15 Plasmodium falciparum and Anopheles gambiae Saliva Antigens. Malar. J. 2010, 9, 317. [Google Scholar] [CrossRef]
- Singh, S.; Soe, S.; Mejia, J.P.; Roussilhon, C.; Theisen, M.; Corradin, G.; Druilhe, P. Identification of a Conserved Region of Plasmodium falciparum MSP3 Targeted by Biologically Active Antibodies to Improve Vaccine Design. J. Infect. Dis. 2004, 190, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Druilhe, P.; Spertini, F.; Soesoe, D.; Corradin, G.; Mejia, P.; Singh, S.; Audran, R.; Bouzidi, A.; Oeuvray, C.; Roussilhon, C. A Malaria Vaccine That Elicits in Humans Antibodies Able to Kill Plasmodium falciparum. PLoS Med. 2005, 2, e344. [Google Scholar] [CrossRef] [PubMed]
- Nebie, I.; Diarra, A.; Ouedraogo, A.; Tiono, A.B.; Konate, A.T.; Gansane, A.; Soulama, I.; Cousens, S.; Leroy, O.; Sirima, S.B. Humoral and Cell-Mediated Immunity to MSP3 Peptides in Adults Immunized with MSP3 in Malaria Endemic Area, Burkina Faso. Parasite Immunol. 2009, 31, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Bang, G.; Tougan, T.; Palacpac, N.M.Q.; Arisue, N.; Aoshi, T.; Matsumoto, Y.; Ishii, K.J.; Egwang, T.G.; Druilhe, P.; et al. Protective Epitopes of the Plasmodium falciparum SERA5 Malaria Vaccine Reside in Intrinsically Unstructured N-Terminal Repetitive Sequences. PLoS ONE 2014, 9, e98460. [Google Scholar] [CrossRef]
- Tohmoto, T.; Takashima, E.; Takeo, S.; Morita, M.; Nagaoka, H.; Udomsangpetch, R.; Sattabongkot, J.; Ishino, T.; Torii, M.; Tsuboi, T. Anti-MSP11 IgG Inhibits Plasmodium falciparum Merozoite Invasion into Erythrocytes in Vitro. Parasitol. Int. 2019, 69, 25–29. [Google Scholar] [CrossRef]
- Hill, D.L.; Wilson, D.W.; Sampaio, N.G.; Eriksson, E.M.; Ryg-Cornejo, V.; Harrison, G.L.A.; Uboldi, A.D.; Robinson, L.J.; Beeson, J.G.; Siba, P.; et al. Merozoite Antigens of Plasmodium falciparum Elicit Strain-Transcending Opsonizing Immunity. Infect. Immun. 2016, 84, 2175–2184. [Google Scholar] [CrossRef]
- Kana, I.H.; Singh, S.K.; Garcia-Senosiain, A.; Dodoo, D.; Singh, S.; Adu, B.; Theisen, M. Breadth of Functional Antibodies Is Associated with Plasmodium falciparum Merozoite Phagocytosis and Protection Against Febrile Malaria. J. Infect. Dis. 2019, 220, 275–284. [Google Scholar] [CrossRef]
- Mbengue, B.; Fall, M.M.; Varela, M.L.; Loucoubar, C.; Joos, C.; Fall, B.; Niang, M.S.; Niang, B.; Mbow, M.; Dieye, A.; et al. Analysis of Antibody Responses to Selected Plasmodium falciparum Merozoite Surface Antigens in Mild and Cerebral Malaria and Associations with Clinical Outcomes. Clin. Exp. Immunol. 2019, 196, 86–96. [Google Scholar] [CrossRef]
- Feng, G.; Boyle, M.J.; Cross, N.; Chan, J.-A.; Reiling, L.; Osier, F.; Stanisic, D.I.; Mueller, I.; Anders, R.F.; McCarthy, J.S.; et al. Human Immunization with a Polymorphic Malaria Vaccine Candidate Induced Antibodies to Conserved Epitopes That Promote Functional Antibodies to Multiple Parasite Strains. J. Infect. Dis. 2018, 218, 35–43. [Google Scholar] [CrossRef]
- Reiling, L.; Boyle, M.J.; White, M.T.; Wilson, D.W.; Feng, G.; Weaver, R.; Opi, D.H.; Persson, K.E.M.; Richards, J.S.; Siba, P.M.; et al. Targets of Complement-Fixing Antibodies in Protective Immunity against Malaria in Children. Nat. Commun. 2019, 10, 610. [Google Scholar] [CrossRef]
- Dobaño, C.; Santano, R.; Vidal, M.; Jiménez, A.; Jairoce, C.; Ubillos, I.; Dosoo, D.; Aguilar, R.; Williams, N.A.; Díez-Padrisa, N.; et al. Differential Patterns of IgG Subclass Responses to Plasmodium falciparum Antigens in Relation to Malaria Protection and RTS,S Vaccination. Front. Immunol. 2019, 10, 439. [Google Scholar] [CrossRef]
- Theisen, M.; Dodoo, D.; Toure-Balde, A.; Soe, S.; Corradin, G.; Koram, K.K.; Kurtzhals, J.A.L.; Hviid, L.; Theander, T.; Akanmori, B.; et al. Selection of Glutamate-Rich Protein Long Synthetic Peptides for Vaccine Development: Antigenicity and Relationship with Clinical Protection and Immunogenicity. Infect. Immun. 2001, 69, 5223–5229. [Google Scholar] [CrossRef] [PubMed]
- Scopel, K.K.G.; Fontes, C.J.F.; Ferreira, M.U.; Braga, E.M. Factors Associated with Immunoglobulin G Subclass Polarization in Naturally Acquired Antibodies to Plasmodium falciparum Merozoite Surface Proteins: A Cross-Sectional Survey in Brazilian Amazonia. Clin. Vaccine Immunol. 2006, 13, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Tangye, S.G.; Ferguson, A.; Avery, D.T.; Ma, C.S.; Hodgkin, P.D. Isotype Switching by Human B Cells Is Division-Associated and Regulated by Cytokines. J. Immunol. 2002, 169, 4298–4306. [Google Scholar] [CrossRef]
- Cassiano, G.C.; Furini, A.A.C.; Capobianco, M.P.; Storti-Melo, L.M.; Cunha, M.G.; Kano, F.S.; Carvalho, L.H.; Soares, I.S.; Santos, S.E.; Póvoa, M.M.; et al. Polymorphisms in B Cell Co-Stimulatory Genes Are Associated with IgG Antibody Responses against Blood–Stage Proteins of Plasmodium vivax. PLoS ONE 2016, 11, e0149581. [Google Scholar] [CrossRef] [PubMed]
- Stone, W.J.R.; Campo, J.J.; Ouédraogo, A.L.; Meerstein-Kessel, L.; Morlais, I.; Da, D.; Cohuet, A.; Nsango, S.; Sutherland, C.J.; van de Vegte-Bolmer, M.; et al. Unravelling the Immune Signature of Plasmodium falciparum Transmission-Reducing Immunity. Nat. Commun. 2018, 9, 558. [Google Scholar] [CrossRef]
- Stone, W.J.R.; Dantzler, K.W.; Nilsson, S.K.; Drakeley, C.J.; Marti, M.; Bousema, T.; Rijpma, S.R. Naturally Acquired Immunity to Sexual Stage P. falciparum Parasites. Parasitology 2016, 143, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Roeffen, W.; Teelen, K.; van As, J.; vd Vegte-Bolmer, M.; Eling, W.; Sauerwein, R. Plasmodium falciparum: Production and Characterization of Rat Monoclonal Antibodies Specific for the Sexual-Stage Pfs48/45 Antigen. Exp. Parasitol. 2001, 97, 45–49. [Google Scholar] [CrossRef]
- de Jong, R.M.; Meerstein-Kessel, L.; Da, D.F.; Nsango, S.; Challenger, J.D.; van de Vegte-Bolmer, M.; van Gemert, G.J.; Duarte, E.; Teyssier, N.; Sauerwein, R.W.; et al. Monoclonal Antibodies Block Transmission of Genetically Diverse Plasmodium falciparum Strains to Mosquitoes. NPJ Vaccines 2021, 6, 101. [Google Scholar] [CrossRef]
- Singh, S.K.; Plieskatt, J.; Chourasia, B.K.; Fabra-García, A.; Garcia-Senosiain, A.; Singh, V.; Bengtsson, K.L.; Reimer, J.M.; Sauerwein, R.; Jore, M.M.; et al. A Reproducible and Scalable Process for Manufacturing a Pfs48/45 Based Plasmodium falciparum Transmission-Blocking Vaccine. Front. Immunol. 2021, 11, 606266. [Google Scholar] [CrossRef]
- Biswas, S.; Seth, R.K.; Tyagi, P.K.; Sharma, S.K.; Dash, A.P. Naturally Acquired Immunity and Reduced Susceptibility to falciparum Malaria in Two Subpopulations of Endemic Eastern India. Scand. J. Immunol. 2008, 67, 177–184. [Google Scholar] [CrossRef]
- Balam, S.; Olugbile, S.; Servis, C.; Diakité, M.; D’Alessandro, A.; Frank, G.; Moret, R.; Nebie, I.; Tanner, M.; Felger, I.; et al. Plasmodium falciparum Merozoite Surface Protein 2: Epitope Mapping and Fine Specificity of Human Antibody Response against Non-Polymorphic Domains. Malar. J. 2014, 13, 510. [Google Scholar] [CrossRef] [PubMed]
- Nixon, C.E.; Park, S.; Pond-Tor, S.; Raj, D.; Lambert, L.E.; Orr-Gonzalez, S.; Barnafo, E.K.; Rausch, K.M.; Friedman, J.F.; Fried, M.; et al. Identification of Protective B-Cell Epitopes within the Novel Malaria Vaccine Candidate Plasmodium falciparum Schizont Egress Antigen 1. Clin. Vaccine Immunol. 2017, 24, e00068-17. [Google Scholar] [CrossRef]
- Sanchez-Trincado, J.L.; Gomez-Perosanz, M.; Reche, P.A. Fundamentals and Methods for T- and B-Cell Epitope Prediction. J Immunol. Res. 2017, 2017, 2680160. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Hickey, J.M.; Miura, K.; Joshi, S.B.; Volkin, D.B.; King, C.R.; Plieskatt, J.L. A C-Terminal Pfs48/45 Malaria Transmission-Blocking Vaccine Candidate Produced in the Baculovirus Expression System. Sci. Rep. 2020, 10, 395. [Google Scholar] [CrossRef]
- Lennartz, F.; Brod, F.; Dabbs, R.; Miura, K.; Mekhaiel, D.; Marini, A.; Jore, M.M.; Søgaard, M.M.; Jørgensen, T.; de Jongh, W.A.; et al. Structural Basis for Recognition of the Malaria Vaccine Candidate Pfs48/45 by a Transmission Blocking Antibody. Nat. Commun. 2018, 9, 3822. [Google Scholar] [CrossRef] [PubMed]
- Kurtovic, L.; Boyle, M.J.; Opi, D.H.; Kennedy, A.T.; Tham, W.; Reiling, L.; Chan, J.; Beeson, J.G. Complement in Malaria Immunity and Vaccines. Immunol. Rev. 2020, 293, 38–56. [Google Scholar] [CrossRef]
- Ouédraogo, A.L.; Eckhoff, P.A.; Luty, A.J.F.; Roeffen, W.; Sauerwein, R.W.; Bousema, T.; Wenger, E.A. Modeling the Impact of Plasmodium falciparum Sexual Stage Immunity on the Composition and Dynamics of the Human Infectious Reservoir for Malaria in Natural Settings. PLoS Pathog. 2018, 14, e1007034. [Google Scholar] [CrossRef]
- Ayanful-Torgby, R.; Sarpong, E.; Abagna, H.B.; Donu, D.; Obboh, E.; Mensah, B.A.; Adjah, J.; Williamson, K.C.; Amoah, L.E. Persistent Plasmodium falciparum Infections Enhance Transmission-Reducing Immunity Development. Sci. Rep. 2021, 11, 21380. [Google Scholar] [CrossRef]



| Personal Data | n = 303 | |
| Sex | Male | 159/303 (52.5%) |
| Female | 144/303 (47.5%) | |
| Age (years) | 32 (28–34) | |
| Time of residence in malaria-endemic area (years) | 31 (28–34) | |
| Clinical And Epidemiological Data | ||
| Number of past malaria episodes | 8 (6–10) | |
| Time elapsed since the last malaria episode (months) | 12 (6–12) | |
| Time of symptoms (days) | 4 (2–4) | |
| Diagnosis | P. falciparum | 53 (17.5%) |
| P. vivax | 82 (27%) | |
| Parasitemia (parasites/µL of blood) | P. falciparum | 8000 (4000–12,000) |
| P. vivax | 20,000 (7000–32,000) |
| NI | PV | PF | ||
| P7 | Responders | 37/148 (25%) | 10/65 (15.4%) | 15/45 (33.3%) a |
| Non-responders | 111/148 (75%) | 55/65 (84.6%) | 30/45 (66.7%) | |
| P8 | Responders | 30/152 (19.7%) | 6/63 (9.5%) | 11/43 (25.6%) b |
| Non-responders | 122/152 (80.3%) | 57/63 (90.5%) | 32/43 (74.4%) | |
| S3 | Responders | 41/161 (25.5%) | 3/61 (4.9%) c | 11/36 (30.6%) |
| Non-responders | 120/161 (74.5%) | 58/61 (95.1%) | 25/36 (69.4%) | |
| MSP-3a | Responders | 15/91 (16.5%) | 6/43 (14%) | 20/36 (55.6%) d |
| Non-responders | 76/91 (83.5%) | 37/43 (86%) | 16/36 (44.4%) | |
| MSP-3b | Responders | 20/90 (22.2%) | 11/44 (25%) | 19/36 (52.8%) e |
| Non-responders | 70/90 (77.8%) | 33/44 (75%) | 17/36 (47.2%) | |
| MSP-3c | Responders | 42/94 (44.7%) | 16/42 (38.1%) | 24/34 (70.6%) f |
| Non-responders | 52/94 (55.3%) | 26/42 (61.9%) | 10/34 (29.4%) | |
| DG210 | Responders | 38/93 (40.9%) | 23/42 (54.8%) | 24/35 (68.6%) g |
| Non-responders | 55/93 (59.1%) | 19/42 (45.2%) | 11/35 (31.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baptista, B.d.O.; Souza, A.B.L.d.; Oliveira, L.S.d.; Souza, H.A.d.S.d.; Barros, J.P.d.; Queiroz, L.T.d.; Souza, R.M.d.; Amoah, L.E.; Singh, S.K.; Theisen, M.; et al. B-Cell Epitope Mapping of the Plasmodium falciparum Malaria Vaccine Candidate GMZ2.6c in a Naturally Exposed Population of the Brazilian Amazon. Vaccines 2023, 11, 446. https://doi.org/10.3390/vaccines11020446
Baptista BdO, Souza ABLd, Oliveira LSd, Souza HAdSd, Barros JPd, Queiroz LTd, Souza RMd, Amoah LE, Singh SK, Theisen M, et al. B-Cell Epitope Mapping of the Plasmodium falciparum Malaria Vaccine Candidate GMZ2.6c in a Naturally Exposed Population of the Brazilian Amazon. Vaccines. 2023; 11(2):446. https://doi.org/10.3390/vaccines11020446
Chicago/Turabian StyleBaptista, Barbara de Oliveira, Ana Beatriz Lopes de Souza, Luana Santos de Oliveira, Hugo Amorim dos Santos de Souza, Jenifer Peixoto de Barros, Lucas Tavares de Queiroz, Rodrigo Medeiros de Souza, Linda Eva Amoah, Susheel Kumar Singh, Michael Theisen, and et al. 2023. "B-Cell Epitope Mapping of the Plasmodium falciparum Malaria Vaccine Candidate GMZ2.6c in a Naturally Exposed Population of the Brazilian Amazon" Vaccines 11, no. 2: 446. https://doi.org/10.3390/vaccines11020446
APA StyleBaptista, B. d. O., Souza, A. B. L. d., Oliveira, L. S. d., Souza, H. A. d. S. d., Barros, J. P. d., Queiroz, L. T. d., Souza, R. M. d., Amoah, L. E., Singh, S. K., Theisen, M., Rodrigues-da-Silva, R. N., Riccio, E. K. P., Totino, P. R. R., Lima-Junior, J. d. C., Daniel-Ribeiro, C. T., & Pratt-Riccio, L. R. (2023). B-Cell Epitope Mapping of the Plasmodium falciparum Malaria Vaccine Candidate GMZ2.6c in a Naturally Exposed Population of the Brazilian Amazon. Vaccines, 11(2), 446. https://doi.org/10.3390/vaccines11020446







