The Fourth Dose of mRNA COVID-19 Vaccine Following 12 Different Three-Dose Regimens: Safety and Immunogenicity to Omicron BA.4/BA.5
Abstract
:1. Introduction
2. Materials and Methods
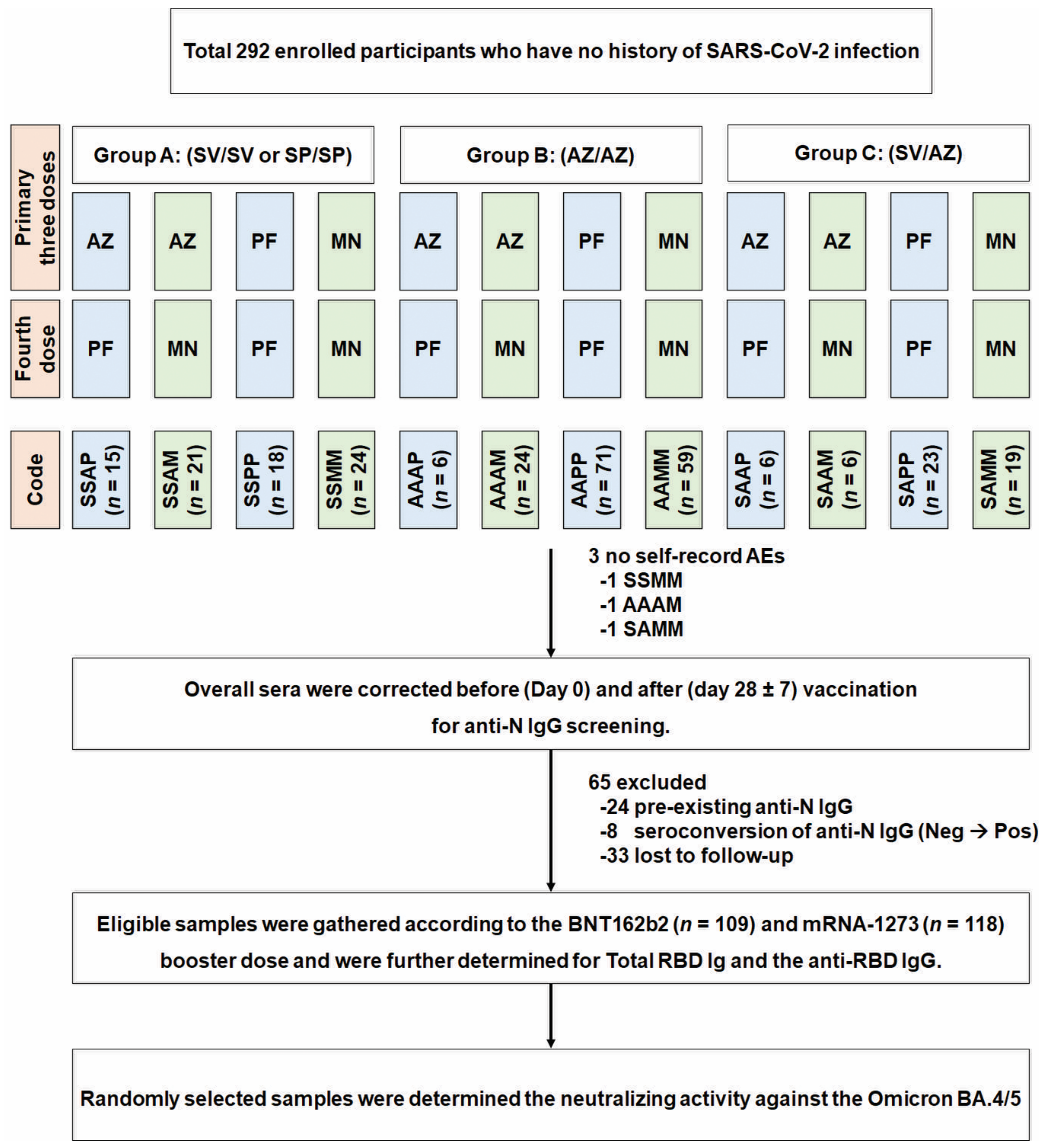
2.1. Study Designs and Participant Enrolment
2.2. Vaccines
2.3. Safety Assessments
2.4. Laboratory Assessments
2.5. Statistical Analysis
3. Results
3.1. Demographic Data and Baseline Characteristics
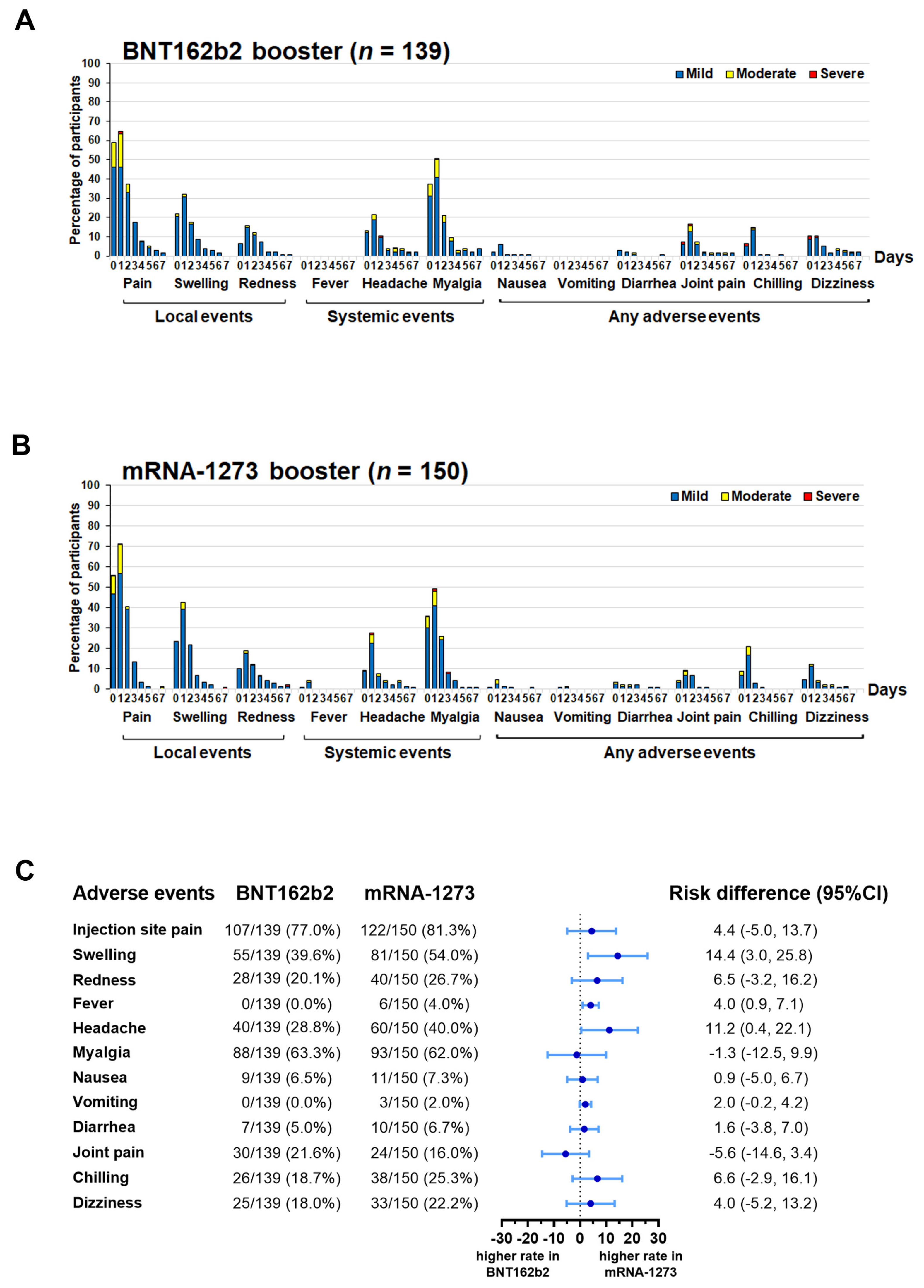
3.2. Local and Systemic Adverse Events Resolved after Receiving the mRNA-COVID-19 Vaccine as the Fourth Booster Dose
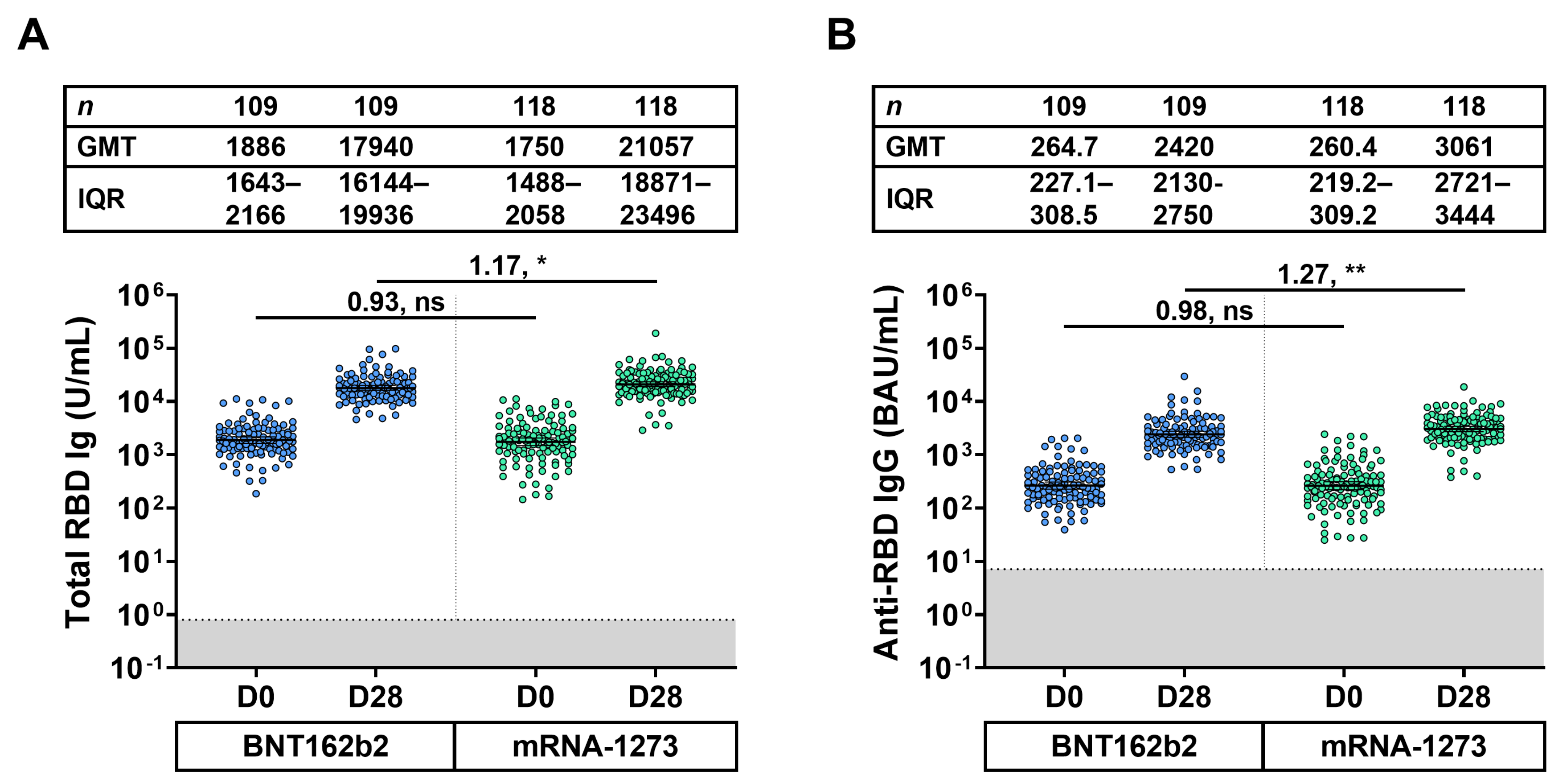
3.3. Total-RBD Ig and Anti-RBD IgG Responses of Eligible Participants
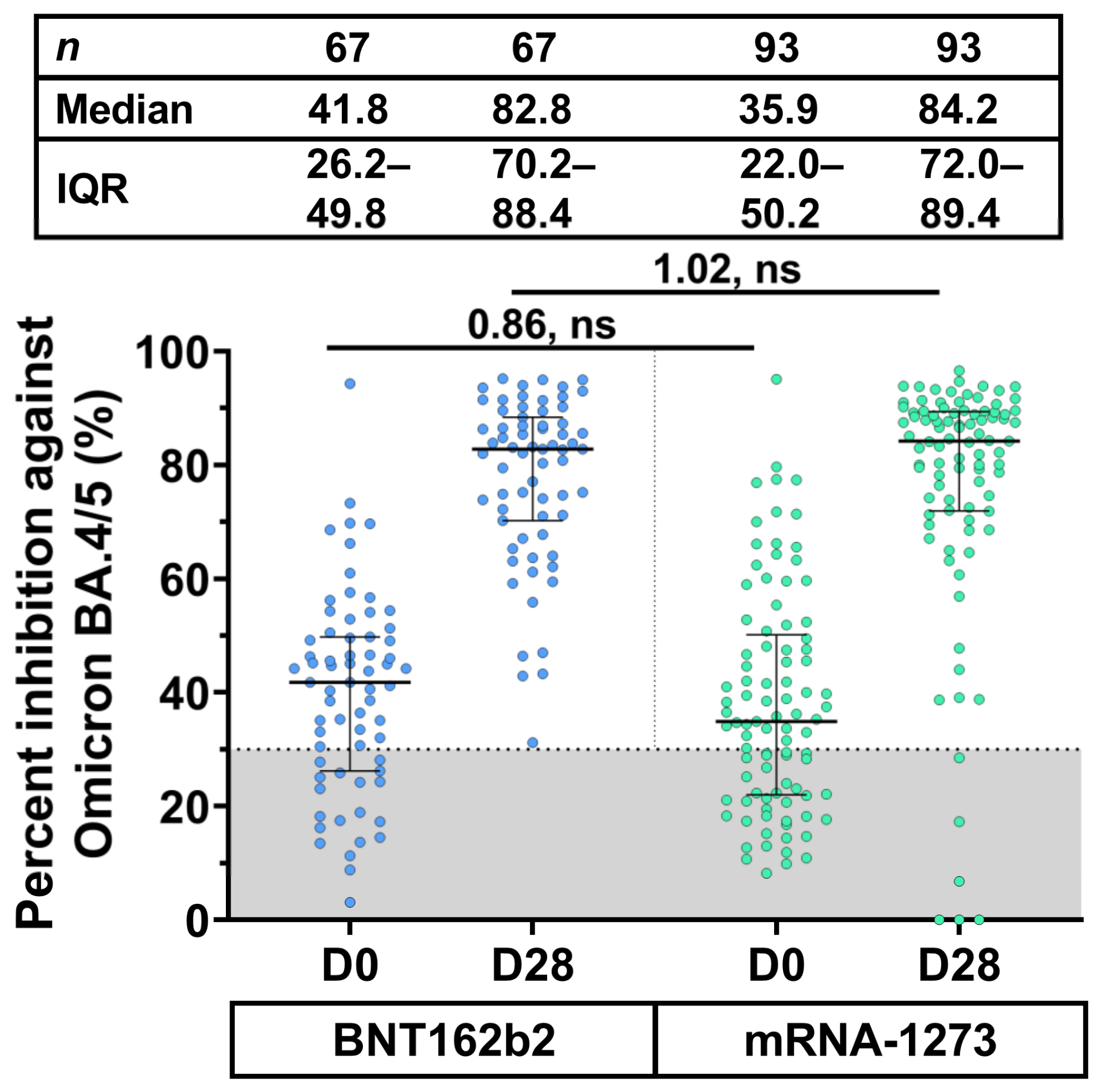
3.4. Neutralizing Activity against Omicron BA.4/5 Using sVNT50
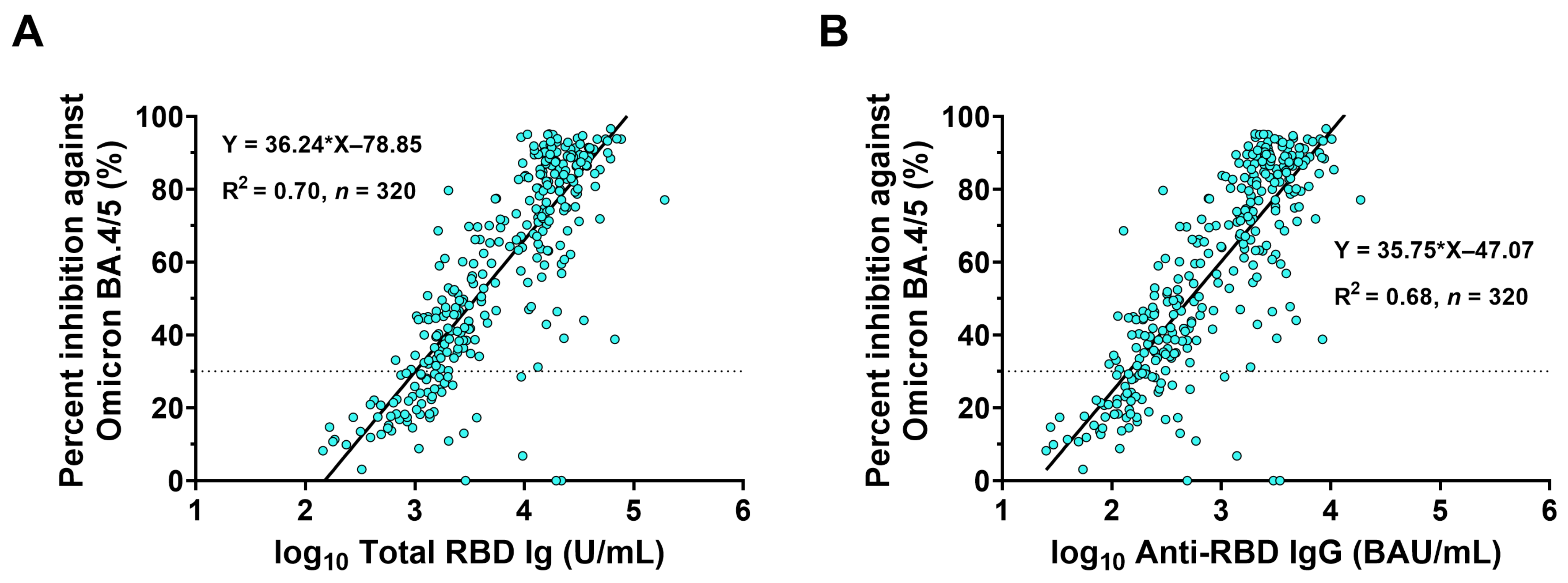
3.5. Correlations between Binding Antibody and Neutralizing Activity against Omicron BA.4/5
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shrestha, L.B.; Foster, C.; Rawlinson, W.; Tedla, N.; Bull, R.A. Evolution of the SARS-CoV-2 Omicron variants BA.1 to BA.5: Implications for immune escape and transmission. Rev. Med. Virol. 2022, 32, e2381. [Google Scholar] [CrossRef] [PubMed]
- Tegally, H.; Moir, M.; Everatt, J.; Giovanetti, M.; Scheepers, C.; Wilkinson, E.; Subramoney, K.; Makatini, Z.; Moyo, S.; Amoako, D.G.; et al. Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa. Nat. Med. 2022, 28, 1785–1790. [Google Scholar] [CrossRef]
- Worldmeter. COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/ (accessed on 9 January 2023).
- Magen, O.; Waxman, J.G.; Makov-Assif, M.; Vered, R.; Dicker, D.; Hernán, M.A.; Lipsitch, M.; Reis, B.Y.; Balicer, R.D.; Dagan, N. Fourth dose of BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N. Engl. J. Med. 2022, 386, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Thailand Department of Disease Control. COVID-19 Vaccine in Thailand. Available online: https://ddc.moph.go.th/vaccine-covid19/getFiles/7/1670402801471.jpg (accessed on 6 January 2023).
- Akaishi, T.; Kushimoto, S.; Katori, Y.; Sugawara, N.; Egusa, H.; Igarashi, K.; Fujita, M.; Kure, S.; Takayama, S.; Abe, M.; et al. Effectiveness of third vaccine dose for Coronavirus disease 2019 during the Omicron variant pandemic: A prospective observational study in Japan. Sci. Rep. 2022, 12, 13589. [Google Scholar] [CrossRef] [PubMed]
- Wan, E.Y.F.; Mok, A.H.Y.; Yan, V.K.C.; Wang, B.; Zhang, R.; Hong, S.N.; Chui, C.S.L.; Li, X.; Wong, C.K.H.; Lai, F.T.T.; et al. Vaccine effectiveness of BNT162b2 and CoronaVac against SARS-CoV-2 Omicron BA.2 infection, hospitalisation, severe complications, cardiovascular disease and mortality in patients with diabetes mellitus: A case control study. J. Infect. 2022, 85, e140–e144. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, E.K.; Britton, A.; Fleming-Dutra, K.E.; Smith, Z.R.; Shang, N.; Derado, G.; Miller, J.; Schrag, S.J.; Verani, J.R. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta variants. JAMA 2022, 327, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Favresse, J.; Gillot, C.; Bayart, J.L.; David, C.; Simon, G.; Wauthier, L.; Closset, M.; Dogné, J.M.; Douxfils, J. Vaccine-induced binding and neutralizing antibodies against Omicron 6 months after a homologous BNT162b2 booster. J. Med. Virol. 2022, 95, e28164. [Google Scholar] [CrossRef]
- Adams, K.; Rhoads, J.P.; Surie, D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Talbot, H.K.; Casey, J.D.; Zepeski, A.; Shapiro, N.I.; et al. Vaccine effectiveness of primary series and booster doses against COVID-19 associated hospital admissions in the United States: Living test negative design study. BMJ 2022, 379, e072065. [Google Scholar] [CrossRef]
- Assawakosri, S.; Kanokudom, S.; Chansaenroj, J.; Suntronwong, N.; Auphimai, C.; Nilyanimit, P.; Vichaiwattana, P.; Thongmee, T.; Duangchinda, T.; Chantima, W.; et al. Persistence of immunity against Omicron BA.1 and BA.2 following homologous and heterologous COVID-19 booster vaccines in healthy adults after a two-doses AZD1222 vaccination. Int. J. Infect. Dis. 2022, 122, 793–801. [Google Scholar] [CrossRef]
- Assawakosri, S.; Kanokudom, S.; Suntronwong, N.; Auphimai, C.; Nilyanimit, P.; Vichaiwattana, P.; Thongmee, T.; Duangchinda, T.; Chantima, W.; Pakchotanon, P.; et al. Neutralizing activities against the Omicron variant after a heterologous booster in healthy adults receiving two doses of CoronaVac vaccination. J. Infect. Dis. 2022, 226, 1372–1381. [Google Scholar] [CrossRef]
- Kanokudom, S.; Assawakosri, S.; Suntronwong, N.; Chansaenroj, J.; Auphimai, C.; Nilyanimit, P.; Vichaiwattana, P.; Thongmee, T.; Yorsaeng, R.; Duangchinda, T.; et al. Comparison of the reactogenicity and immunogenicity of a reduced and standard booster dose of the mRNA COVID-19 vaccine in healthy adults after two doses of inactivated vaccine. Vaccine 2022, 40, 5657–5663. [Google Scholar] [CrossRef] [PubMed]
- Kanokudom, S.; Chansaenroj, J.; Suntronwong, N.; Assawakosri, S.; Yorsaeng, R.; Nilyanimit, P.; Aeemjinda, R.; Khanarat, N.; Vichaiwattana, P.; Klinfueng, S.; et al. Safety and immunogenicity of a third dose of COVID-19 protein subunit vaccine (Covovax(TM)) after homologous and heterologous two-dose regimens. Int. J. Infect. Dis. 2022, 126, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Suntronwong, N.; Kanokudom, S.; Auphimai, C.; Assawakosri, S.; Thongmee, T.; Vichaiwattana, P.; Duangchinda, T.; Chantima, W.; Pakchotanon, P.; Chansaenroj, J.; et al. Effects of boosted mRNA and adenoviral-vectored vaccines on immune responses to omicron BA.1 and BA.2 following the heterologous CoronaVac/AZD1222 vaccination. J. Med. Virol. 2022, 94, 713–722. [Google Scholar] [CrossRef]
- Amanatidou, E.; Gkiouliava, A.; Pella, E.; Serafidi, M.; Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Dalamaga, M. Breakthrough infections after COVID-19 vaccination: Insights, perspectives and challenges. Metabol. Open. 2022, 14, 100180. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Amir, O.; Freedman, L.; Alroy-Preis, S.; Ash, N.; Huppert, A.; Milo, R. Protection by a fourth dose of BNT162b2 against Omicron in Israel. N. Engl. J. Med. 2022, 386, 1712–1720. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Gonen, T.; Gilboa, M.; Mandelboim, M.; Indenbaum, V.; Amit, S.; Meltzer, L.; Asraf, K.; Cohen, C.; Fluss, R.; et al. Efficacy of a fourth dose of COVID-19 mRNA vaccine against Omicron. N. Engl. J. Med. 2022, 386, 1377–1380. [Google Scholar] [CrossRef]
- Nordström, P.; Ballin, M.; Nordström, A. Effectiveness of a fourth dose of mRNA COVID-19 vaccine against all-cause mortality in long-term care facility residents and in the oldest old: A nationwide, retrospective cohort study in Sweden. Lancet Reg. Health Eur. 2022, 21, 100466. [Google Scholar] [CrossRef]
- Cohen, M.J.; Oster, Y.; Moses, A.E.; Spitzer, A.; Benenson, S. Israeli-Hospitals 4th Vaccine Working Group.; Association of receiving a fourth dose of the BNT162b vaccine with SARS-CoV-2 infection among health care workers in Israel. JAMA Netw. Open 2022, 5, e2224657. [Google Scholar] [CrossRef]
- Intawong, K.; Chariyalertsak, S.; Chalom, K.; Wonghirundecha, T.; Kowatcharakul, W.; Thongprachum, A.; Chotirosniramit, N.; Teacharak, W.; Khammawan, P.; Waneesorn, J.; et al. Effectiveness of heterologous third and fourth dose COVID-19 vaccine schedules for SARS-CoV-2 infection during delta and omicron predominance in Thailand: A test-negative, case-control study. Lancet Reg. Health Southeast Asia 2022, 2022, 100121. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA vaccine against SARS-CoV-2—Preliminary report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- The WHO Strategic Advisory Group of Experts on Immunization (SAGE). The Moderna COVID-19 (mRNA-1273) Vaccine: What You Need to Know. Available online: https://www.who.int/news-room/feature-stories/detail/the-moderna-covid-19-mrna-1273-vaccine-what-you-need-to-know (accessed on 18 August 2022).
- Wanlapakorn, N.; Suntronwong, N.; Kanokudom, S.; Assawakosri, S.; Nilyanimit, P.; Yorsaeng, R.; Chansaenroj, J.; Poovorawan, Y. Immunogenicity of the BNT162b2 COVID-19 vaccine as a third dose (booster) following two doses of different primary series regimens in Thailand. Pathog. Glob. Health 2022, 116, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCoV-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.P.S.; Feng, S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): A multicentre, blinded, phase 2, randomised trial. Lancet Infect. Dis. 2022, 22, 1131–1141. [Google Scholar]
- Suntronwong, N.; Assawakosri, S.; Kanokudom, S.; Yorsaeng, R.; Auphimai, C.; Thongmee, T.; Vichaiwattana, P.; Duangchinda, T.; Chantima, W.; Pakchotanon, P.; et al. Strong correlations between the binding antibodies against wild-type and neutralizing antibodies against Omicron BA.1 and BA.2 variants of SARS-CoV-2 in individuals following booster (third-dose) vaccination. Diagnostics 2022, 12, 1781. [Google Scholar] [CrossRef]
| Characteristic | BNT162b2 | mRNA-1273 |
|---|---|---|
| Number (n) | 109 | 118 |
| Sex, female n (%) | 72 (66.1) | 71 (60.2) |
| Age, years, mean [SD] (min–max) | 48.8 [11.5] (20.0–73.0) | 48.2 [11.8] (23.0–74.0) |
| No comorbidity (%) | 68 (62.4) | 86 (72.9) |
| Underlying diseases (%) | ||
| Allergy | 5 (4.6) | 5 (4.2) |
| Dyslipidemia | 12 (11.0) | 11 (9.3) |
| Diabetes mellitus | 4 (3.7) | 4 (3.4) |
| Hypertension | 11 (10.1) | 15 (12.7) |
| Thyroid | 2 (1.8) | 2 (1.7) |
| Others | 10 (9.2) | 4 (3.4) |
| Interval between the 3rd and 4th dose (days) | ||
| Median [IQR] (min–max) | 191.0 [163.0–223.0] (94.0–337.0) | 210.5 [177.0–224.3] (117.0–371.0) |
| Interval between the 4th dose and blood collection (days) | ||
| Median [IQR] (min–max) | 28.0 [28.0–29.0] (22.0–32.0) | 28.0 [28.0–32.0] (26.0–34.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanokudom, S.; Chansaenroj, J.; Suntronwong, N.; Assawakosri, S.; Yorsaeng, R.; Nilyanimit, P.; Aeemjinda, R.; Khanarat, N.; Vichaiwattana, P.; Klinfueng, S.; et al. The Fourth Dose of mRNA COVID-19 Vaccine Following 12 Different Three-Dose Regimens: Safety and Immunogenicity to Omicron BA.4/BA.5. Vaccines 2023, 11, 570. https://doi.org/10.3390/vaccines11030570
Kanokudom S, Chansaenroj J, Suntronwong N, Assawakosri S, Yorsaeng R, Nilyanimit P, Aeemjinda R, Khanarat N, Vichaiwattana P, Klinfueng S, et al. The Fourth Dose of mRNA COVID-19 Vaccine Following 12 Different Three-Dose Regimens: Safety and Immunogenicity to Omicron BA.4/BA.5. Vaccines. 2023; 11(3):570. https://doi.org/10.3390/vaccines11030570
Chicago/Turabian StyleKanokudom, Sitthichai, Jira Chansaenroj, Nungruthai Suntronwong, Suvichada Assawakosri, Ritthideach Yorsaeng, Pornjarim Nilyanimit, Ratchadawan Aeemjinda, Nongkanok Khanarat, Preeyaporn Vichaiwattana, Sirapa Klinfueng, and et al. 2023. "The Fourth Dose of mRNA COVID-19 Vaccine Following 12 Different Three-Dose Regimens: Safety and Immunogenicity to Omicron BA.4/BA.5" Vaccines 11, no. 3: 570. https://doi.org/10.3390/vaccines11030570
APA StyleKanokudom, S., Chansaenroj, J., Suntronwong, N., Assawakosri, S., Yorsaeng, R., Nilyanimit, P., Aeemjinda, R., Khanarat, N., Vichaiwattana, P., Klinfueng, S., Thongmee, T., Srimuan, D., Thatsanathorn, T., Sudhinaraset, N., Wanlapakorn, N., Honsawek, S., & Poovorawan, Y. (2023). The Fourth Dose of mRNA COVID-19 Vaccine Following 12 Different Three-Dose Regimens: Safety and Immunogenicity to Omicron BA.4/BA.5. Vaccines, 11(3), 570. https://doi.org/10.3390/vaccines11030570





