Vaccines against Tuberculosis: Where Are We Now?
Abstract
1. Introduction
2. Description of Various Vaccine Candidates Designed against TB
2.1. Fusion Protein Candidates
2.1.1. M72/AS01E
2.1.2. H4:IC31
2.1.3. H56:IC31
2.1.4. ID93-GLA
2.1.5. GamTBvac
2.1.6. AEC/BC02
2.1.7. Recombinant BCG (rBCG) Vaccine
2.1.8. VPM1002
2.1.9. BCG + H107
2.2. Viral-Mediated Delivery of Mycobacterial Antigen
2.2.1. MVA85A
2.2.2. Adenovirus Based Vaccine Candidate
2.2.3. TB/FLU-01L
2.2.4. TB/FLU-04L
2.2.5. ChAdOx185A-MVA85A
2.2.6. RhCMV/TB
2.2.7. rLCMV-Based M.tb Vaccine
2.3. M.tb Auxotrophs, Mutants, Whole Cells or Heat-Inactivated Fragments as Vaccine Candidates
2.3.1. MTBVAC
2.3.2. DAR-901
2.3.3. IKEPLUS
2.3.4. M. vaccae Vaccine
2.3.5. RUTI®
2.3.6. Mycobacterium indicus pranii (MIP)
3. Newer Avenues in TB Vaccine Research
3.1. Multiepitope DNA-Based Dual Vaccine for HIV and TB
3.2. Aptamers-Based TB Vaccine
3.3. RNA/DNA and Peptide-Based Vaccines
3.4. PE/PPE Proteins as Vaccine Candidates
3.5. HIV–TB Co-Infection and Application of Vaccines
4. Challenges in TB Vaccine Research and Innovations—The Tip of the Iceberg
4.1. Intracellular Behaviour of M.tb Is Unclear
4.2. Different M.tb Strains, Different Patterns
4.3. Safety Concerns
4.4. Lost in Transition
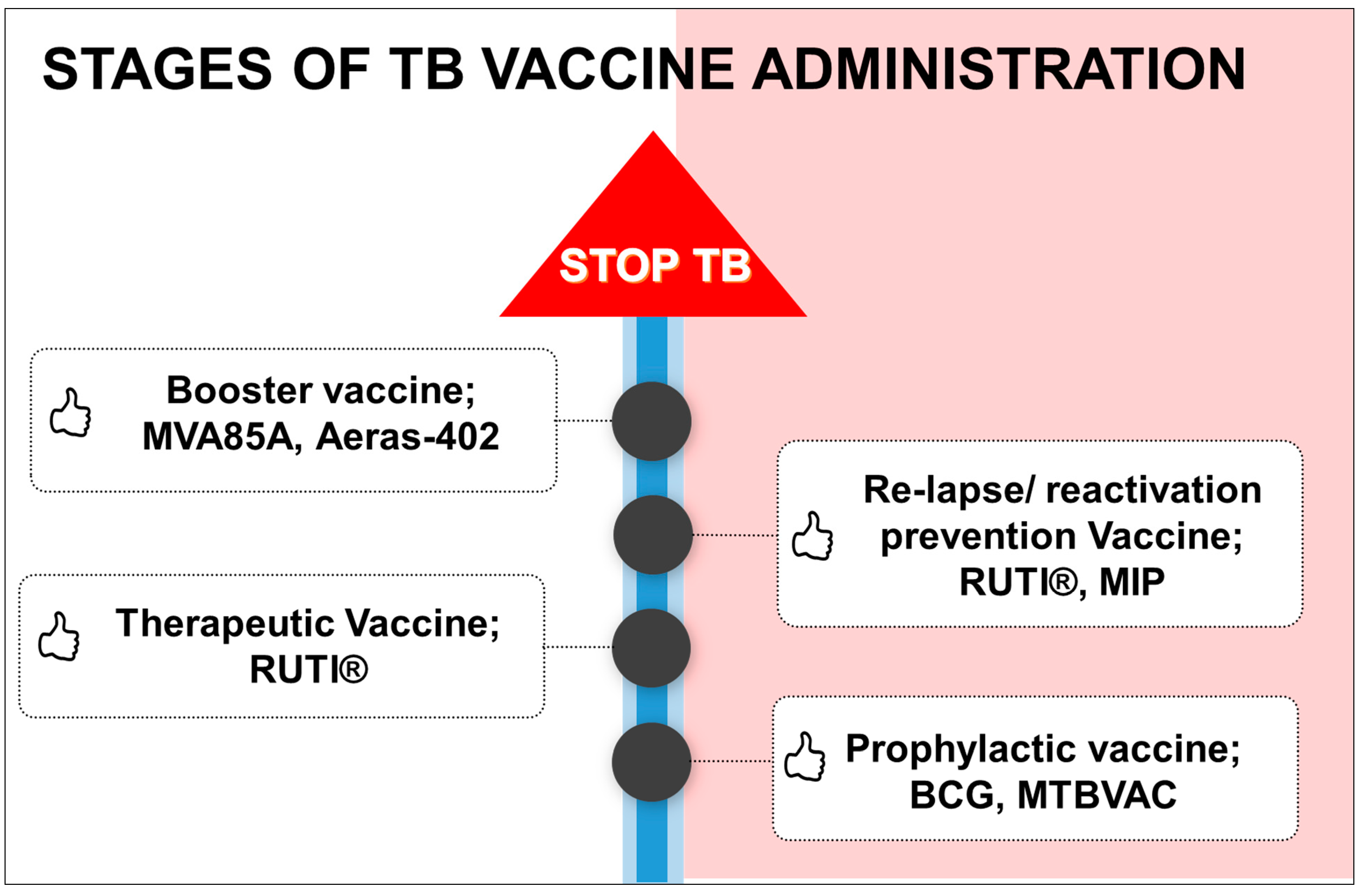
4.5. Stages of Vaccine Administration According to Need
4.6. Limitation in Using Appropriate Animal Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. 2022. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 (accessed on 27 March 2023).
- Kayser, V.; Ramzan, I. Vaccines and vaccination: History and emerging issues. Hum. Vaccines Immunother. 2021, 17, 5255–5268. [Google Scholar] [CrossRef]
- Fatima, S.; Kumari, A.; Das, G.; Dwivedi, V.P. Tuberculosis vaccine: A journey from BCG to present. Life Sci. 2020, 252, 117594. [Google Scholar] [CrossRef] [PubMed]
- Treatment Action Group. Available online: https://www.treatmentactiongroup.org/resources/pipeline-report/2022-pipeline-report/ (accessed on 9 March 2023).
- Wilkie, M.; Satti, I.; Minhinnick, A.; Harris, S.; Riste, M.; Ramon, R.L.; Sheehan, S.; Thomas, Z.M.; Wright, D.; Stockdale, L.; et al. A phase I trial evaluating the safety and immunogenicity of a candidate tuberculosis vaccination regimen, ChAdOx1 85A prime—MVA85A boost in healthy UK adults. Vaccine 2020, 38, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Saramago, S.; Magalhães, J.; Pinheiro, M. Tuberculosis Vaccines: An update of recent and ongoing clinical trials. Appl. Sci. 2021, 11, 9250. [Google Scholar] [CrossRef]
- Schrager, L.K.; Vekemens, J.; Drager, N.; Lewinsohn, D.M.; Olesen, O.F. The status of tuberculosis vaccine development. Lancet Infect Dis. 2020, 20, e28–e37. [Google Scholar] [CrossRef] [PubMed]
- Day, C.L.; Tameris, M.; Mansoor, N.; van Rooyen, M.; de Kock, M.; Geldenhuys, H.; Erasmus, M.; Makhethe, L.; Hughes, E.J.; Gelderbloem, S. Induction and regulation of T-cell immunity by the novel tuberculosis vaccine M72/AS01 in South African adults. Am. J. Respir. Crit. Care Med. 2013, 188, 492–502. [Google Scholar] [CrossRef]
- Kumarasamy, N.; Poongulali, S.; Beulah, F.E.; Akite, E.J.; Ayuk, L.N.; Bollaerts, A.; Demoitié, M.A.; Jongert, E.; Ofori-Anyinam, O.; Van Der Meeren, O. Long-term safety and immunogenicity of the M72/AS01E candidate tuberculosis vaccine in HIV-positive and -negative Indian adults: Results from a phase II randomized controlled trial. Medicine 2018, 97, e13120. [Google Scholar] [CrossRef]
- Geldenhuys, H.; Mearns, H.; Miles, D.J.; Tameris, M.; Hokey, D.; Shi, Z.; Bennett, S.; Andersen, P.; Kromann, I.; Hoff, S.T.; et al. The tuberculosis vaccine H4:IC31 is safe and induces a persistent polyfunctional CD4 T cell response in South African adults: A randomized controlled trial. Vaccine 2015, 33, 3592–3599. [Google Scholar] [CrossRef]
- Luabeya, A.K.; Kagina, B.M.; Tameris, M.D.; Geldenhuys, H.; Hoff, S.T.; Shi, Z.; Kromann, I.; Hatherill, M.; Mahomed, H.; Hanekom, W.A.; et al. First-in-human trial of the post-exposure tuberculosis vaccine H56:IC31 in Mycobacterium tuberculosis infected and non-infected healthy adults. Vaccine 2015, 33, 4130–4140. [Google Scholar] [CrossRef]
- Day, T.A.; Penn-Nicholson, A.; Luabeya, A.K.K.; Fiore-Gartland, A.; Du Plessis, N.; Loxton, A.G.; Vergara, J.; Rolf, T.A.; Reid, T.D.; Toefy, A.; et al. TBVPX-203 study team. Safety and immunogenicity of the adjunct therapeutic vaccine ID93 + GLA-SE in adults who have completed treatment for tuberculosis: A randomised, double-blind, placebo-controlled, phase 2a trial. Lancet Respir. Med. 2021, 9, 373–386. [Google Scholar] [CrossRef]
- Tkachuk, A.P.; Bykonia, E.N.; Popova, L.I.; Kleymenov, D.A.; Semashko, M.A.; Chulanov, V.P.; Fitilev, S.B.; Maksimov, S.L.; Smolyarchuk, E.A.; Manuylov, V.A.; et al. Safety and immunogenicity of the GamTBvac, the recombinant subunit tuberculosis vaccine candidate: A phase ii, multi-center, double-blind, randomized, placebo-controlled study. Vaccines 2020, 8, 652. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Guo, X.; Wang, C.; Du, W.; Shen, X.; Su, C.; Wu, Y.; Xu, M. Therapeutic effect of subunit vaccine AEC/BC02 on Mycobacterium tuberculosis post-chemotherapy relapse using a latent infection murine model. Vaccines 2022, 10, 825. [Google Scholar] [CrossRef]
- Sun, R.; Skeiky, Y.A.; Izzo, A.; Dheenadhayalan, V.; Imam, Z.; Penn, E.; Stagliano, K.; Haddock, S.; Mueller, S.; Fulkerson, J.; et al. Novel recombinant BCG expressing perfringolysin O and the over-expression of key immunodominant antigens; pre-clinical characterization, safety and protection against challenge with Mycobacterium tuberculosis. Vaccine 2009, 27, 4412–4423. [Google Scholar] [CrossRef] [PubMed]
- Nemes, E.; Geldenhuys, H.; Rozot, V.; Rutkowski, K.T.; Ratangee, F.; Bilek, N.; Mabwe, S.; Makhethe, L.; Erasmus, M.; Toefy, A.; et al. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N. Engl. J. Med. 2018, 379, 138–149. [Google Scholar] [CrossRef]
- Woodworth, J.S.; Clemmensen, H.S.; Battey, H.; Dijkman, K.; Lindenstrøm, T.; Laureano, R.S.; Taplitz, R.; Morgan, J.; Aagaard, C.; Rosenkrands, I. A Mycobacterium tuberculosis-specific subunit vaccine that provides synergistic immunity upon co-administration with Bacillus Calmette-Guérin. Nat. Comms. 2021, 12, 6658. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.; Cotton, M.F.; Eisele, B.; Gengenbacher, M.; Grode, L.; Hesseling, A.C.; Walzl, G. The BCG replacement vaccine VPM1002: From drawing board to clinical trial. Expert Rev. Vaccines 2014, 13, 619–630. [Google Scholar] [CrossRef]
- Riste, M.; Marshall, J.L.; Satti, I.; Harris, S.A.; Wilkie, M.; Ramon, R.L.; Wright, D.; Wittenberg, R.E.; Vermaak, S.; Doherty, R.P.; et al. Phase I trial evaluating the safety and immunogenicity of candidate TB vaccine MVA85A, delivered by aerosol to healthy M.tb-infected adults. Vaccines 2021, 9, 396. [Google Scholar] [CrossRef]
- Meyer, J.; Harris, S.A.; Satti, I.; Poulton, I.D.; Poyntz, H.C.; Tanner, R.; Rowland, R.; Griffiths, K.L.; Fletcher, H.A.; McShane, H. Comparing the safety and immunogenicity of a candidate TB vaccine MVA85A administered by intramuscular and intradermal delivery. Vaccine 2013, 31, 1026–1033. [Google Scholar] [CrossRef]
- Santosuosso, M.; Zhang, X.; McCormick, S.; Wang, J.; Hitt, M.; Xing, Z. Mechanisms of mucosal and parenteral tuberculosis vaccinations: Adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. J. Immunol. 2005, 174, 7986–7994. [Google Scholar] [CrossRef]
- Xing, Z.; McFarland, C.T.; Sallenave, J.M.; Izzo, A.; Wang, J.; McMurray, D.N. Intranasal mucosal boosting with an adenovirus-vectored vaccine markedly enhances the protection of BCG-primed guinea pigs against pulmonary tuberculosis. PLoS ONE 2009, 4, e5856. [Google Scholar] [CrossRef]
- Sivakumaran, D.; Blatner, G.; Bakken, R.; Hokey, D.; Ritz, C.; Jenum, S.; Grewal, H.M.S. A 2-Dose AERAS-402 regimen boosts CD8+ polyfunctionality in HIV-Negative, BCG-vaccinated recipients. Front. Immunol. 2021, 12, 673532. [Google Scholar] [CrossRef] [PubMed]
- Stukova, M. Randomized Open Label Phase 1 Clinical Trial of TB/FLU-01L Tuberculosis Vaccine Administered Intranasally or Sublingual in BCG-Vaccinated Healthy Adults. Global Forum on TB Vaccines, New Delhi, India. February 2018. Available online: https://tbvaccinesforum.org/wp-content/uploads/2018/03/5GF-Breakout-2-Stukova.pdf (accessed on 10 May 2023).
- Hansen, S.G.; Zak, D.E.; Xu, G.; Ford, J.C.; Marshall, E.E.; Malouli, D.; Gilbride, R.M.; Hughes, C.M.; Ventura, A.B.; Ainslie, E. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat. Med. 2018, 24, 130–143. [Google Scholar] [CrossRef]
- Belnoue, E.; Vogelzang, A.; Nieuwenhuizen, N.E.; Krzyzaniak, M.A.; Darbre, S.; Kreutzfeldt, M.; Wagner, I.; Merkler, D.; Lambert, P.H.; Kaufmann, S.H.E.; et al. Replication-deficient lymphocytic choriomeningitis virus-vectored vaccine candidate for the induction of t cell immunity against Mycobacterium tuberculosis. Int. J. Mol. Sci. 2022, 23, 2700. [Google Scholar] [CrossRef] [PubMed]
- Tameris, M.; Mearns, H.; Penn-Nicholson, A.; Gregg, Y.; Bilek, N.; Mabwe, S.; Geldenhuys, H.; Shenje, J.; Luabeya, A.K.K.; Murillo, I.; et al. MTBVAC Clinical Trial Team. Live-attenuated Mycobacterium tuberculosis vaccine MTBVAC versus BCG in adults and neonates: A almetted controlled, double-blind dose-escalation trial. Lancet Respir. Med. 2019, 7, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Munseri, P.; Said, J.; Amour, M.; Magohe, A.; Matee, M.; Rees, C.A.; Mackenzie, T.; Tvaroha, S.; Bailey-Kellogg, C.; Maro, I.; et al. DAR-901 vaccine for the prevention of infection with Mycobacterium tuberculosis among BCG-immunized adolescents in Tanzania: A randomized controlled, double-blind phase 2b trial. Vaccine 2020, 38, 7239–7245. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, K.A.; Dao, D.N.; Goldberg, M.F.; Hsu, T.; Venkataswamy, M.M.; Henao-Tamayo, M.; Ordway, D.; Sellers, R.S.; Jain, P.; Chen, B.; et al. A recombinant Mycobacterium smegmatis induces potent bactericidal immunity against Mycobacterium tuberculosis. Nat Med. 2011, 17, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Bourinbaiar, A.S.; Batbold, U.; Efremenko, Y.; Sanjagdorj, M.; Butov, D.; Damdinpurev, N.; Grinishina, E.; Mijiddorj, O.; Kovolev, M.; Baasanjav, K. Phase III, placebo-controlled, randomized, double-blind trial of tableted, therapeutic TB vaccine (V7) containing heat-killed M. vaccae administered daily for one month. J. Clin. Tuberc. Other Mycobact. Dis. 2019, 18, 100141. [Google Scholar] [CrossRef]
- Nell, A.S.; D’Lom, E.; Bouic, P.; Sabate, M.; Bosser, R.; Picas, J.; Amat, M.; Churchyard, G.; Cardona, P.J. Safety, tolerability, and immunogenicity of the novel antituberculous vaccine RUTI: Randomized, placebo-controlled phase II clinical trial in patients with latent tuberculosis infection. PLoS ONE 2014, 9, e89612. [Google Scholar] [CrossRef]
- Sharma, S.K.; Katoch, K.; Sarin, R.; Balambal, R.; Kumar Jain, N.; Patel, N.; Murthy, K.J.R.; Singla, N.; Saha, P.K.; Khanna, A.; et al. Efficacy and Safety of Mycobacterium indicus pranii as an adjunct therapy in Category II pulmonary tuberculosis in a randomized trial. Sci. Rep. 2017, 7, 3354. [Google Scholar] [CrossRef]
- Li, X.; Xu, W.; Xiong, S. A novel tuberculosis DNA vaccine in an HIV-1 p24 protein backbone confers protection against Mycobacterium tuberculosis and simultaneously elicits robust humoral and cellular responses to HIV-1. Clin. Vaccine Immunol. 2012, 19, 723–730. [Google Scholar] [CrossRef]
- Van Der Meeren, O.; Hatherill, M.; Nduba, V.; Wilkinson, R.J.; Muyoyeta, M.; Van Brakel, E.; Ayles, H.M.; Henostroza, G.; Thienemann, F.; Scriba, T.J.; et al. Phase 2b controlled trial of M72/AS01E vaccine to prevent tuberculosis. N. Engl. J. Med. 2018, 379, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Bekker, L.G.; Dintwe, O.; Fiore-Gartland, A.; Middelkoop, K.; Hutter, J.; Williams, A.; Randhawa, A.K.; Ruhwald, M.; Kromann, I.; Andersen, P.L. HVTN 602/Aeras A-042 Protocol Team. A phase 1b randomized study of the safety and immunological responses to vaccination with H4:IC31, H56:IC31, and BCG revaccination in Mycobacterium tuberculosis-uninfected adolescents in Cape Town, South Africa. eClinicalMedicine 2020, 21, 100313. [Google Scholar] [CrossRef] [PubMed]
- Coler, R.N.; Day, T.A.; Ellis, R.; Piazza, F.M.; Beckmann, A.M.; Vergara, J.; Rolf, T.; Lu, L.; Alter, G.; Hokey, D.; et al. The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: First-in-human trial. NPJ Vaccines 2018, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.B.; Chen, B.W.; Wang, G.Z.; Fu, L.L.; Shen, X.B.; Su, C.; Du, W.X.; Yang, L.; Xu, M. Recombinant tuberculosis vaccine AEC/BC02 induces antigen-specific cellular responses in mice and protects guinea pigs in a model of latent infection. J. Microbiol. Immunol. Infect. 2015, 48, 597–603. [Google Scholar] [CrossRef]
- Working Group on New TB Vaccines (WGNV), Stop TB Partnership. Available online: https://newtbvaccines.org/vaccine/aec-bc02/ (accessed on 15 September 2022).
- Da Costa, A.C.; Nogueira, S.V.; Kipnis, A.; Junqueira-Kipnis, A.P. Recombinant BCG: Innovations on an old vaccine. Scope of BCG strains and strategies to improve long-lasting memory. Front. Immunol. 2014, 5, 152. [Google Scholar] [CrossRef]
- Da Costa, A.C.; Costa-Júnior, A.D.O.; de Oliveira, F.M.; Nogueira, S.V.; Rosa, J.D.; Resende, D.P.; Kipnis, A.; Junqueira-Kipnis, A.P. A new recombinant BCG vaccine induces specific Th17 and Th1 effector cells with higher protective efficacy against tuberculosis. PLoS ONE 2014, 9, e112848. [Google Scholar] [CrossRef]
- Deng, Y.H.; He, H.Y.; Zhang, B.S. Evaluation of protective efficacy conferred by a recombinant Mycobacterium bovis BCG expressing a fusion protein of Ag85A-ESAT-6. J. Microbiol. Immunol. Infect. 2014, 47, 48–56. [Google Scholar] [CrossRef]
- Gengenbacher, M.; Nieuwenhuizen, N.; Vogelzang, A.; Liu, H.; Kaiser, P.; Schuerer, S.; Lazar, D.; Wagner, I.; Mollenkopf, H.J.; Kaufmann, S.H. Deletion of nuoG from the vaccine candidate Mycobacterium bovis BCG ΔureC::hly improves protection against tuberculosis. mBio 2016, 7, e00679-16. [Google Scholar] [CrossRef]
- Dhar, N.; Rao, V.; Tyagi, A.K. Skewing of the Th1/Th2 responses in mice due to variation in the level of expression of an antigen in a recombinant BCG system. Immunol. Lett. 2003, 88, 175–184. [Google Scholar] [CrossRef]
- Bao, L.; Chen, W.; Zhang, H.; Wang, X. Virulence, immunogenicity, and protective efficacy of two recombinant Mycobacterium bovis bacillus Calmette-Guérin strains expressing the antigen ESAT-6 from Mycobacterium tuberculosis. Infect. Immun. 2003, 71, 1656–1661. [Google Scholar] [CrossRef]
- Silver, R.F.; Wallis, R.S.; Ellner, J.J. Mapping of T cell epitopes of the 30-kDa alpha antigen of Mycobacterium bovis strain bacillus Calmette-Guerin in purified protein derivative (PPD)-positive individuals. J. Immunol. 1995, 154, 4665–4674. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.; Kaufmann, S.H.E. Vaccination strategies against intracellular microbes. FEMS Microbiol. Immunol. 1993, 7, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Das, A.; Mukhopadhyay, S. Immunoregulatory functions and expression patterns of PE/PPE family members: Roles in pathogenicity and impact on anti-tuberculosis vaccine and drug design. IUBMB Life 2015, 67, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Pym, A.S.; Brodin, P.; Majlessi, L.; Brosch, R.; Demangel, C.; Williams, A.; Griffiths, K.E.; Marchal, G.; Leclerc, C.; Cole, S.T. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat. Med. 2003, 9, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, M.A.; Harth, G.; Dillon, B.J.; Maslesa-Galic, S. Recombinant bacillus Calmette-Guérin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc. Natl. Acad. Sci. USA 2000, 97, 13853–13858. [Google Scholar] [CrossRef] [PubMed]
- Dhar, N.; Rao, V.; Tyagi, A.K. Immunogenicity of recombinant BCG vaccine strains overexpressing components of the antigen 85 complex of Mycobacterium tuberculosis. Med. Microbiol. Immunol. 2004, 193, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Yamada, H.; Shibata, K.; Maeda, N.; Yoshida, S.; Wajjwalku, W.; Ohara, N.; Yamada, T.; Kinoshita, T.; Yoshikai, Y. Efficacy of recombinant bacille Calmette-Guérin vaccine secreting interleukin-15/antigen 85B fusion protein in providing protection against Mycobacterium tuberculosis. J. Infect. Dis. 2008, 197, 1263–1274. [Google Scholar] [CrossRef]
- Jain, R.; Dey, B.; Dhar, N.; Rao, V.; Singh, R.; Gupta, U.D.; Katoch, V.M.; Ramanathan, V.D.; Tyagi, A.K. Enhanced and enduring protection against tuberculosis by recombinant BCG-Ag85C and its association with modulation of cytokine profile in lung. PLoS ONE 2008, 3, e3869. [Google Scholar] [CrossRef]
- Li, Q.; Yu, H.; Zhang, Y.; Wang, B.; Jiang, W.; Da, Z.; Xian, Q.; Wang, Y.; Liu, X.; Zhu, B. Immunogenicity and protective efficacy of a fusion protein vaccine consisting of antigen Ag85B and HspX against Mycobacterium tuberculosis infection in mice. Scand. J. Immunol. 2011, 73, 568–576. [Google Scholar] [CrossRef]
- Shi, C.; Chen, L.; Chen, Z.; Zhang, Y.; Zhou, Z.; Lu, J.; Fu, R.; Wang, C.; Fang, Z.; Fan, X. Enhanced protection against tuberculosis by vaccination with recombinant BCG over-expressing HspX protein. Vaccine 2010, 28, 5237–5244. [Google Scholar] [CrossRef]
- Chauhan, P.; Jain, R.; Dey, B.; Tyagi, A.K. Adjunctive immunotherapy with α-crystallin based DNA vaccination reduces tuberculosis chemotherapy period in chronically infected mice. Sci. Rep. 2013, 3, 1821. [Google Scholar] [CrossRef] [PubMed]
- Dey, B.; Jain, R.; Khera, A.; Gupta, U.D.; Katoch, V.M.; Ramanathan, V.D.; Tyagi, A.K. Latency antigen α-crystallin based vaccination imparts a robust protection against TB by modulating the dynamics of pulmonary cytokines. PLoS ONE 2011, 6, e18773. [Google Scholar] [CrossRef] [PubMed]
- Cobelens, F.; Suri, R.K.; Helinski, M.; Makanga, M.; Weinberg, A.L.; Schaffmeister, B.; Deege, F.; Hatherill, M. TB Vaccine Roadmap Stakeholder Group. Accelerating research and development of new vaccines against tuberculosis: A global roadmap. Lancet Infect. Dis. 2022, 22, e108–e120. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, N.E.; Kulkarni, P.S.; Shaligram, U.; Cotton, M.F.; Rentsch, C.A.; Eisele, B.; Grode, L.; Kaufmann, S.H.E. The recombinant Bacille Calmette-Guérin Vaccine VPM1002: Ready for clinical efficacy testing. Front. Immunol. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Grode, L.; Seiler, P.; Baumann, S.; Hess, J.; Brinkmann, V.; Nasser-Eddine, A.; Mann, P.; Goosmann, C.; Bandermann, S.; Smith, D.; et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis almett Calmette-Guérin mutants that secrete listeriolysin. J. Clin. Investig. 2005, 115, 2472–2479. [Google Scholar] [CrossRef]
- Ibanga, H.B.; Brookes, R.H.; Hill, P.C.; Owiafe, P.K.; Fletcher, H.A.; Lienhardt, C.; Hill, A.V.; Adegbola, R.A.; McShane, H. Early clinical trials with a new tuberculosis vaccine, MVA85A, in tuberculosis-endemic countries: Issues in study design. Lancet Infect Dis. 2006, 6, 522–528. [Google Scholar] [CrossRef]
- Minassian, A.M.; Rowland, R.; Beveridge, N.E.; Poulton, I.D.; Satti, I.; Harris, S.; Poyntz, H.; Hamill, M.; Griffiths, K.; Sander, C.R.; et al. A Phase I study evaluating the safety and immunogenicity of MVA85A, a candidate TB vaccine, in HIV-infected adults. BMJ Open 2011, 1, e000223. [Google Scholar] [CrossRef]
- Tameris, M.D.; Hatherill, M.; Landry, B.S.; Scriba, T.J.; Snowden, M.A.; Lockhart, S.; Shea, J.E.; McClain, J.B.; Hussey, G.D.; Hanekom, W.A.; et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: A randomised, placebo-controlled phase 2b trial. Lancet 2013, 381, 1021–1028. [Google Scholar] [CrossRef]
- Macleod, M. Learning lessons from MVA85A, a failed booster vaccine for BCG. BMJ 2018, 360, k66. [Google Scholar] [CrossRef]
- Majhen, D.; Calderon, H.; Chandra, N.; Fajardo, C.A.; Rajan, A.; Alemany, R.; Custers, J. Adenovirus-based vaccines for fighting infectious diseases and cancer: Progress in the field. Hum. Gene Ther. 2014, 25, 301–317. [Google Scholar] [CrossRef]
- Smaill, F.; Xing, Z. Human type 5 adenovirus-based tuberculosis vaccine: Is the respiratory route of delivery the future? Expert Rev. Vaccines 2014, 13, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Whelan, A.; Court, P.; Xing, Z.; Clifford, D.; Hogarth, P.J.; Vordermeier, M.; Villarreal-Ramos, B. Immunogenicity comparison of the intradermal or endobronchial boosting of BCG vaccinates with Ad5-85A. Vaccine 2012, 30, 6294–6300. [Google Scholar] [CrossRef] [PubMed]
- Smaill, F.; Jeyanathan, M.; Smieja, M.; Medina, M.F.; Thanthrige-Don, N.; Zganiacz, A.; Yin, C.; Heriazon, A.; Damjanovic, D.; Puri, L.; et al. A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Sci. Transl. Med. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- De Val, B.P.; Villarreal-Ramos, B.; Nofrarías, M.; López-Soria, S.; Romera, N.; Singh, M.; Abad, F.X.; Xing, Z.; Vordermeier, H.M.; Domingo, M. Goats primed with Mycobacterium bovis BCG and boosted with a recombinant adenovirus expressing Ag85A show enhanced protection against tuberculosis. Clin. Vaccine Immunol. 2012, 19, 1339–1347. [Google Scholar] [CrossRef]
- Kagina, B.M.; Tameris, M.D.; Geldenhuys, H.; Hatherill, M.; Abel, B.; Hussey, G.D.; Scriba, T.J.; Mahomed, H.; Sadoff, J.C.; Hanekom, W.A. The novel tuberculosis vaccine, AERAS-402, is safe in healthy infants previously vaccinated with BCG, and induces dose-dependent CD4 and CD8 T cell responses. Vaccine 2014, 32, 5908–5917. [Google Scholar] [CrossRef]
- Richardson, J.S.; Abou, M.C.; Tran, K.N.; Kumar, A.; Sahai, B.M.; Kobinger, G.P. Impact of systemic or mucosal immunity to adenovirus on Ad-based Ebola virus vaccine efficacy in guinea pigs. J. Infect. Dis. 2011, 204, 1032–1042. [Google Scholar] [CrossRef]
- Shurygina, A.P.; Zabolotnykh, N.; Vinogradova, T.; Khairullin, B.; Kassenov, M.; Nurpeisova, A.; Sarsenbayeva, G.; Sansyzbay, A.; Vasilyev, K.; Buzitskaya, J.; et al. Preclinical evaluation of TB/FLU-04L—An intranasal influenza vector-based boost vaccine against tuberculosis. Int. J. Mol. Sci. 2023, 24, 7439. [Google Scholar] [CrossRef]
- Stylianou, E.; Griffiths, K.L.; Poyntz, H.C.; Harrington-Kandt, R.; Dicks, M.D.; Stockdale, L.; Betts, G.; McShane, H. Improvement of BCG protective efficacy with a novel chimpanzee adenovirus and a modified vaccinia Ankara virus both expressing Ag85A. Vaccine 2015, 33, 6800–6808. [Google Scholar] [CrossRef]
- Satti, I.; Meyer, J.; Harris, S.A.; Manjaly Thomas, Z.R.; Griffiths, K.; Antrobus, R.D.; Rowland, R.; Ramon, R.L.; Smith, M.; Sheehan, S.; et al. Safety and immunogenicity of a candidate tuberculosis vaccine MVA85A delivered by aerosol in BCG-vaccinated healthy adults: A phase 1, double-blind, almetted controlled trial. Lancet Infect. Dis. 2014, 14, 9392020946. [Google Scholar] [CrossRef]
- Martín, C.; Marinova, D.; Aguiló, N.; Gonzalo-Asensio, J. MTBVAC, a live TB vaccine poised to initiate efficacy trials 100 years after BCG. Vaccine 2021, 39, 7277–7285. [Google Scholar] [CrossRef]
- Walker, K.B.; Brennan, M.J.; Ho, M.M.; Eskola, J.; Thiry, G.; Sadoff, J.; Dobbelaer, R.; Grode, L.; Liu, M.A.; Fruth, U. The second Geneva consensus: Recommendations for novel live TB vaccines. Vaccine 2010, 28, 2259–2270. [Google Scholar] [CrossRef] [PubMed]
- Hatherill, M.; White, R.G.; Hawn, T.R. Clinical development of New TB vaccines: Recent advances and next steps. Front. Microbiol. 2020, 10, 3154. [Google Scholar] [CrossRef] [PubMed]
- von Reyn, C.F.; Lahey, T.; Arbeit, R.D.; Landry, B.; Kailani, L.; Adams, L.V.; Haynes, B.C.; Mackenzie, T.; Wieland-Alter, W.; Connor, R.I. Safety and immunogenicity of an inactivated whole cell tuberculosis vaccine booster in adults primed with BCG: A randomized, controlled trial of DAR-901. PLoS ONE 2017, 12, e0175215. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.C.; Johnson, A.J.; Bharrhan, S.; Lindestam-Arlehamn, C.S.; Xu, J.; Garforth, S.J.; Chan, J.; Jacobs, W.R., Jr.; Sette, A.; Almo, S.C. Identification of mycobacterial ribosomal proteins as targets for CD4+ T Cells that enhance protective immunity in tuberculosis. Infect. Immun. 2018, 86, e00009-18. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Kennedy, S.C.; Lindestam Arlehamn, C.S.; Goldberg, M.F.; Saini, N.K.; Xu, J.; Paul, S.; Hegde, S.S.; Blanchard, J.S.; Chan, J. Identification of mycobacterial RplJ/L10 and RpsA/S1 proteins as novel targets for CD4+ T cells. Infect. Immun. 2017, 85, e01023-16. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05680415 (accessed on 3 March 2023).
- Wu, J.; Hu, Z.; Lu, S.H.; Fan, X.Y. Heterologous prime-boost BCG with DNA vaccine expressing fusion antigens Rv2299c and Ag85A improves protective efficacy against Mycobacterium tuberculosis in mice. Front. Microbiol. 2022, 13, 927031. [Google Scholar] [CrossRef]
- Bruffaerts, N.; Huygen, K.; Romano, M. DNA vaccines against tuberculosis. Expert Opin. Biol. Ther. 2014, 14, 1801–1813. [Google Scholar] [CrossRef]
- Srivastava, S.; Abraham, P.R.; Mukhopadhyay, S. Aptamers: An emerging tool for diagnosis and therapeutics in tuberculosis. Front. Cell Infect. Microbiol. 2021, 11, 656421. [Google Scholar] [CrossRef]
- Davydova, A.; Vorobjeva, M.; Pyshnyi, D.; Altman, S.; Vlassov, V.; Venyaminova, A. Aptamers against pathogenic microorganisms. Crit. Rev. Microbiol. 2016, 42, 847–865. [Google Scholar] [CrossRef]
- Pan, Q.; Wang, Q.; Sun, X.; Xia, X.; Wu, S.; Luo, F.; Zhang, X.L. Aptamer against mannose-capped lipoarabinomannan inhibits virulent Mycobacterium tuberculosis Infection in mice and rhesus monkeys. Mol. Ther. 2014, 22, 940–951. [Google Scholar] [CrossRef]
- Sun, X.; Pan, Q.; Yuan, C.; Wang, Q.; Tang, X.L.; Ding, K.; Zhou, X.; Zhang, X.L. A single ssDNA aptamer binding to mannose-capped lipoarabinomannan of Bacillus Calmette-Guérin in enhances immunoprotective effect against tuberculosis. J. Am. Chem. Soc. 2016, 138, 11680–11689. [Google Scholar] [CrossRef] [PubMed]
- Baig, I.A.; Moon, J.Y.; Lee, S.C.; Ryoo, S.W.; Yoon, M.Y. Development of ssDNA aptamers as potent inhibitors of Mycobacterium tuberculosis acetohydroxyacid synthase. Biochim. Biophys. Acta 2015, 1854, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Al Tbeishat, H. Novel in silico mRNA vaccine design exploiting proteins of M. tuberculosis that modulates host immune responses by inducing epigenetic modifications. Sci. Rep. 2022, 12, 4645. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Stavropoulos, E.; Yang, M.; Ragno, S.; Vordermeier, M.; Chambers, M.; Hewinson, G.; Lowrie, D.B.; Colston, M.J.; Tascon, R.E. RNA encoding the MPT83 antigen induces protective immune responses against Mycobacterium tuberculosis infection. Infect. Immun. 2004, 72, 6324–6329. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.A. A Comparison of Plasmid DNA and mRNA as Vaccine Technologies. Vaccines 2019, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Pan, C.; Cheng, P.; Wang, J.; Zhao, G.; Wu, X. Peptide-based vaccines for Tuberculosis. Front. Immunol. 2022, 13, 104. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Balaji, K.N. The PE and PPE proteins of Mycobacterium tuberculosis. Tuberculosis 2011, 91, 441–447. [Google Scholar] [CrossRef]
- Ates, L.S. New insights into the mycobacterial PE and PPE proteins provide a framework for future research. Mol. Microbiol. 2020, 113, 4–21. [Google Scholar] [CrossRef]
- Delogu, G.; Brennan, M.J. Comparative immune response to PE and PE_PGRS antigens of Mycobacterium tuberculosis. Infect. Immun. 2001, 69, 5606–5611. [Google Scholar] [CrossRef]
- Singh, S.K.; Tripathi, D.K.; Singh, P.K.; Sharma, S.; Srivastava, K.K. Protective and survival efficacies of Rv0160c protein in murine model of Mycobacterium tuberculosis. Appl. Microbiol. Biotechnol. 2013, 97, 5825–5837. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, E.; Huang, Q.; Ni, W.; Kong, C.; Liu, G.; Li, G.; Su, H.; Wang, H. PPE57 induces activation of macrophages and drives Th1-type immune responses through TLR2. J. Mol. Med. 2015, 93, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Kumari, R.; Singh, D.K.; Tiwari, S.; Singh, P.K.; Sharma, S.; Srivastava, K.K. Putative roles of a proline-glutamic acid-rich protein (PE3) in intracellular survival and as a candidate for subunit vaccine against Mycobacterium tuberculosis. Med. Microbiol. Immunol. 2013, 202, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Skeiky, Y.A.; Alderson, M.R.; Ovendale, P.J.; Lobet, Y.; Dalemans, W.; Orme, I.M.; Reed, S.G.; Campos-Neto, A. Protection of mice and guinea pigs against tuberculosis induced by immunization with a single Mycobacterium tuberculosis recombinant antigen, MTB41. Vaccine 2005, 23, 3937–3945. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, E.; Harrington-Kandt, R.; Beglov, J.; Bull, N.; Pinpathomrat, N.; Swarbrick, G.M.; Lewinsohn, D.A.; Lewinsohn, D.M.; McShane, H. Identification and evaluation of novel protective antigens for the development of a candidate tuberculosis subunit vaccine. Infect. Immun. 2018, 86, e00014-18. [Google Scholar] [CrossRef] [PubMed]
- Sali, M.; Di Sante, G.; Cascioferro, A.; Zumbo, A.; Nicolò, C.; Donà, V.; Rocca, S.; Procoli, A.; Morandi, M.; Ria, F.; et al. Surface expression of MPT64 as a fusion with the PE domain of PE_PGRS33 enhances Mycobacterium bovis BCG protective activity against Mycobacterium tuberculosis in mice. Infect. Immun. 2010, 78, 5202–5213. [Google Scholar] [CrossRef]
- TB Factsheet. Available online: https://www.unaids.org/sites/default/files/media_asset/20220324_TB_FactSheet_en.pdf (accessed on 4 May 2023).
- Sharan, R.; Kaushal, D. Vaccine strategies for the Mtb/HIV copandemic. NPJ Vaccines 2020, 5, 95. [Google Scholar] [CrossRef]
- Souriant, S.; Balboa, L.; Dupont, M.; Pingris, K.; Kviatcovsky, D.; Cougoule, C.; Lastrucci, C.; Bah, A.; Gasser, R.; Poincloux, R.; et al. Tuberculosis exacerbates HIV-1 infection through IL-10/STAT3-dependent tunneling nanotube formation in macrophages. Cell Rep. 2019, 26, 3586–3599. [Google Scholar] [CrossRef] [PubMed]
- Sotgiu, G.; Centis, R.; D’ambrosio, L.; Migliori, G.B. Tuberculosis treatment and drug regimens. Cold Spring Harb Perspect. Med. 2015, 5, 1–13. [Google Scholar] [CrossRef]
- Bruchfeld, J.; Correia-Neves, M.; Källenius, G. Tuberculosis and HIV Coinfection. Cold Spring Harb. Perspect. Med. 2015, 5, 1–16. [Google Scholar] [CrossRef]
- Shepelkova, G.S.; Evstifeev, V.V.; Tarasov, R.V.; Ergeshova, A.E.; Bagirov, M.A.; Yeremeev, V.V. MicroRNAs as biomarkers of active pulmonary tb course. Microorganisms 2023, 11, 626. [Google Scholar] [CrossRef]
- Saunders, N.J.; Trivedi, U.H.; Thomson, M.L.; Doig, C.; Laurenson, I.F.; Blaxter, M.L. Deep resequencing of serial sputum isolates of Mycobacterium tuberculosis during therapeutic failure due to poor compliance reveals stepwise mutation of key resistance genes on an otherwise stable genetic background. J. Infect. 2011, 62, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Prentice, R.L. Surrogate endpoints in clinical trials: Definition and operational criteria. Stat. Med. 1989, 8, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Irvine, E.B.; O’Neil, A.; Darrah, P.A.; Shin, S.; Choudhary, A.; Li, W.; Honnen, W.; Mehra, S.; Kaushal, D.; Gideon, H.P.; et al. Robust IgM responses following intravenous vaccination with Bacille Calmette-Guérin associate with prevention of Mycobacterium tuberculosis infection in macaques. Nat. Immunol. 2021, 22, 1515–1523. [Google Scholar] [CrossRef]
- Carmona, J.; Cruz, A.; Moreira-Teixeira, L.; Sousa, C.; Sousa, J.; Osorio, N.S.; Saraiva, A.L.; Svenson, S.; Kallenius, G.; Pedrosa, J.; et al. Mycobacterium tuberculosis strains are differentially recognized by TLRs with an impact on the immune response. PLoS ONE 2013, 8, e67277. [Google Scholar] [CrossRef]
- Merker, M.; Barbier, M.; Cox, H.; Rasigade, J.P.; Feuerriegel, S.; Kohl, T.A.; Diel, R.; Borrell, S.; Gagneux, S.; Nikolayevskyy, V.; et al. Compensatory evolution drives multidrug-resistant tuberculosis in central Asia. Elife 2018, 7, e18103. [Google Scholar] [CrossRef]
- Fursov, M.V.; Shitikov, E.A.; Lagutkin, D.A.; Fursova, A.D.; Ganina, E.A.; Kombarova, T.I.; Grishenko, N.S.; Rudnitskaya, T.I.; Bespiatykh, D.A.; Kolupaeva, N.V.; et al. MDR and Pre-XDR clinical Mycobacterium tuberculosis beijing strains: Assessment of virulence and host cytokine response in mice infectious model. Microorganisms 2021, 9, 1792. [Google Scholar] [CrossRef]
- Pu, W.; Zhao, C.; Wazir, J.; Su, Z.; Niu, M.; Song, S.; Wei, L.; Li, L.; Zhang, X.; Shi, X.; et al. Comparative transcriptomic analysis of THP-1-derived macrophages infected with Mycobacterium tuberculosis H37Rv, H37Ra and BCG. J. Cell Mol. Med. 2021, 25, 10504–10520. [Google Scholar] [CrossRef]
- Lagutkin, D.; Panova, A.; Vinokurov, A.; Gracheva, A.; Samoilova, A.; Vasilyeva, I. Genome-Wide study of drug resistant Mycobacterium tuberculosis and its intra-host evolution during treatment. Microorganisms 2022, 10, 1440. [Google Scholar] [CrossRef]
- Nadolinskaia, N.I.; Kotliarova, M.S.; Goncharenko, A.V. Fighting Tuberculosis: In Search of a BCG replacement. Microorganisms 2023, 11, 51. [Google Scholar] [CrossRef]
- Orme, I.M. Tuberculosis vaccine types and timings. Clin. Vaccine Immunol. 2015, 22, 249–257. [Google Scholar] [CrossRef]
- Dharmadhikari, A.S.; Nardell, E.A. What animal models teach humans about tuberculosis. Am. J. Respir. Cell Mol. Biol. 2008, 39, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Flores-Valdez, M.A.; Kupz, A.; Subbian, S. Recent developments in mycobacteria-based live attenuated vaccine candidates for tuberculosis. Biomedicines 2022, 10, 2749. [Google Scholar] [CrossRef] [PubMed]
- Curtis, N.; Sparrow, A.; Ghebreyesus, T.; Netea, M. Considering BCG vaccination to reduce the impact of COVID-19. Lancet 2020, 395, 1545–1546. [Google Scholar] [CrossRef] [PubMed]


| S. No | Vaccine Candidate | Antigen, Vector, and Formulation | Remark | References |
|---|---|---|---|---|
| Protein subunit vaccine | ||||
| 1. | M72/AS01E | Fusion proteins containing M.tb 32A and M.tb 39A + adjuvant AS01E. | The vaccine was found to be clinically tolerated in M.tb-infected and M.tb-uninfected adults, and it is highly immunogenic. It induced multifunctional mycobacteria-specific T cell populations after vaccination, as well as boosted distinct T cells primed by natural M.tb infection. It provides protection against active pulmonary tuberculosis disease. | [4,8,9] |
| 2. | H4:IC31 | Fusion protein comprising M.tb Ag85B and TB10.4, formulated in IC31 adjuvant. | H4:IC31 was found to be an immunogenic and safe vaccine. In BCG-vaccinated adults, antigen-specific, long-lasting strong CD4+ T cells were observed. | [4,10] |
| 3. | H56:IC31 | Fusion protein comprising M.tb Ag85B, ESAT-6, Rv2660 formulated in IC31 adjuvant. | H56:IC31 is a safe vaccine. It induces antigen-specific IgG and Th1-type CD4+ T cells in healthy adults without or with M.tb infection. All recruited adults were HIV-negative and BCG-vaccinated. | [4,11] |
| 4. | ID93-GLA | ID93 consists of M.tb antigens Rv2608, Rv3619c, Rv3620c, and Rv1813c formulated with GLA-SE adjuvant. | ID93 vaccine was studied in BCG-vaccinated, HIV-uninfected, drug-sensitive pulmonary tuberculosis patients. No adverse effect was observed. The vaccine was found to be immunogenic as it stimulated long-lasting, vaccine antigen-specific polyfunctional CD4+ T-cells and robust antibody production. Possible adjunctive therapeutic vaccine. | [12] |
| 5. | GamTBvac | The vaccine contains Ag85A and ESAT-6:CFP-10 fusion plus adjuvant DEAE-dextran core and CpG oligodeoxynucleotides. | The vaccine was found to be safe and immunogenic, inducing Th1-type CD4+ T cells and IgG antibodies in BCG-vaccinated, M.tb-uninfected adults. | [13] |
| 6. | AEC/BC02 | The vaccine contains M.tb protein subunits Ag85B and ESAT-6: CFP-10 along with Bacillus Calmette–Guérin CpG plus aluminum adjuvant system. | AEC/BC02 enhanced the potential of chemotherapy for latent TB infection in mice model. The vaccine induced Th1-type immune responses and decreased bacillary load in latently infected mice. | [14] |
| Recombinant BCG (rBCG) | ||||
| 7. | AFRO-1 | AFRO-1 is a rBCG overexpressing M.tb antigens Ag85A, Ag85B, 10.4 and Perfringolysin O from Clostridium perfringens. | The vaccine was found to be safe and well-tolerated by immunocompromised SCID mice. Mice vaccinated with rBCG (AFRO-1) showed enhanced cellular immune response against M.tb (strain HN878) infection. AFRO-1-vaccinated mice had better survival rates than BCG-vaccinated mice challenged with M.tb. Enhanced immune response was also observed in guinea pigs. | [15] |
| 8. | BCG- Revaccination | Whole-cell M. bovis. | (i) In BCG-vaccinated individuals, BCG-specific CD4+ T-cell responses were improved post BCG revaccination. | [16] |
| 9. | BCG + H107 | BCG plus H107 fusion protein (Rv3863, ESAT-6, EspI, EspC, EspA, MPT64, MPT70 and MPT83). | This vaccine stimulated the development of multifunctional T cell populations, induced Th17 response, and provided robust protection against pulmonary M.tb infection in mice. | [17] |
| 10. | VPM1002 | rBCG Prague strain overexpressing listeriolysin molecule from Listeria with inactivated urease subunit C. | VPM1002 vaccine is safe and has superior efficacy compared to BCG against M.tb infection in mice. The vaccine was found to be safe in various animal models, such as immune-deficient mice, guinea pigs, rabbits, and non-human primates. Phase I clinical trials have indicated safety. | [4,18] |
| Viral-mediated delivery of mycobacterial antigens | ||||
| 11. | MVA85A | Vaccinia virus expressing M.tb antigen Ag85A. | MVA85A was found to be safe and well-tolerated in BCG-vaccinated adults. The vaccine induced robust and durable cellular immune responses when delivered intramuscularly or by aerosol route. | [19,20] |
| 12. | AdHu5Ag85A | Replication-deficient adenoviral TB vaccine expressing M.tb Ag85A. | The vaccine-induced protection was observed against mycobacterial infections (M. bovis, M.tb) in animal models, such as mice, guinea pigs. | [21,22] |
| 13. | AERAS-402 | AERAS-402 is a replication-deficient Ad35 vaccine encoding the fusion protein of M.tb antigens 85A, 85B, and TB10.4. | The vaccine was safe, well-tolerated, and induced CD8+ T cell response in HIV-negative and BCG-vaccinated adults. | [23] |
| 14. | TB/FLU-01L | Attenuated influenza strain Flu NS106 expressing M.tb antigen ESAT-6. | The vaccine was safe and showed immunotherapeutic effect in mice. The vaccine was found to be safe and immunogenic in BCG-vaccinated adults. | [24] |
| 15. | TB/FLU-04L | Modified influenza vector with a truncated NS1 protein expressing M.tb antigens ESAT-6 and Ag85A. | TB/FLU-04L was found to be safe and induced protective immune response against M.tb infection in BCG-vaccinated adults. | [6] |
| 16. | Vaccine regimen ChAdOx185A- MVA85A | Replication-deficient chimpanzee adenovirus vector expressing M.tb antigen 85A (Ag85A) + recombinant, replication-deficient modified vaccinia virus expressing M.tb antigen Ag85A. | Vaccine was safe in BCG-vaccinated adults. | [5] |
| 17. | RhCMV/TB | A rhesus cytomegalovirus vector expressing 9 M.tb antigens such as Ag85A, Ag85B, ESAT-6 (acute phase), Rv3407, Rv1733, Rv2626 (latency), Rpf A, Rpf C, Rpf D (resuscitation). | The vaccine generated stronger, effector-differentiated CD4+ and CD8+ memory T cell responses, and induced protection against M.tb (Erdman strain) infection in rhesus macaques. | [25] |
| 18. | rLCMV-based M.tb vaccine | Replication-deficient lymphocytic choriomeningitis virus (rLCMV) expressing M.tb antigens Ag85B and TB10.4. | The vaccine generated polyfunctional M.tb-specific CD4+ and CD8+ T cell populations in mice and reduced lung infection burden during challenge with M.tb. | [26] |
| M.tb mutants or inactivated or fragmented M.tb strains as vaccine candidates | ||||
| 19. | MTBVAC | Live, attenuated M.tb clinical isolates—lineage 4 with deletion genes (phoP and fadD26). | MTBVAC is the only live attenuated vaccine, used in clinical trials as a preventive vaccine in newborns. MTBVAC is safe and immunogenic in BCG-vaccinated and HIV-negative adults. | [27] |
| 20. | DAR-901 | Inactivated whole-cell M. obuense. | DAR-901 was safe and tolerable in IGRA-negative, BCG-immunized adults in Tanzania. DAR-901 failed to prevent TB infection. | [4,28] |
| 21. | IKEPLUS | M. smegmatis Δesx-3 locus (IKE) + M.tb esx-3 genes. | IKEPLUS induces protective immunity to M.tb in mice. | [29] |
| 22. | M. vaccae based vaccine | Heat-killed non-tuberculous bacterium M. vaccae. | Improved bacterial clearance was observed in multidrug-resistant and drug-sensitive TB patients. It can prevent chemotherapy-induced hepatic damage and overcome weight loss and inflammation observed during TB infection. | [30] |
| 23. | RUTI® | The vaccine contains fragmented, purified, and heat-inactivated M.tb bacilli in liposomes. | Reasonably safe and provides potent stimulation of the immune response against tuberculosis. RUTI® showed polyfunctional T cell response and enhanced secretion of IFN-γ after vaccination, attempted to control MDR condition. | [31] |
| 24. | Mycobacterium indicus pranii (MIP) | Inactivated whole-cell M. indicus pranii. | MIP was safe with no adverse effects. A role of MIP in clearance of the M.tb bacilli was also suggested. | [32] |
| 25. | DNA-based Dual vaccine (pP24-M.tb DNA vaccine) | M.tb antigens, MPT64, Ag85A, Ag85B, and TB10.4 + a HIV protein, p24 as a backbone | pP24-M.tb vaccine was immunogenic, provided protection against M. bovis BCG, and reduced infection-related lung inflammation and injury in mice. Elicited robust immune responses to HIV-1. Promising vaccine to prevent dual infections with M.tb and HIV. | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, S.; Dey, S.; Mukhopadhyay, S. Vaccines against Tuberculosis: Where Are We Now? Vaccines 2023, 11, 1013. https://doi.org/10.3390/vaccines11051013
Srivastava S, Dey S, Mukhopadhyay S. Vaccines against Tuberculosis: Where Are We Now? Vaccines. 2023; 11(5):1013. https://doi.org/10.3390/vaccines11051013
Chicago/Turabian StyleSrivastava, Shruti, Sajal Dey, and Sangita Mukhopadhyay. 2023. "Vaccines against Tuberculosis: Where Are We Now?" Vaccines 11, no. 5: 1013. https://doi.org/10.3390/vaccines11051013
APA StyleSrivastava, S., Dey, S., & Mukhopadhyay, S. (2023). Vaccines against Tuberculosis: Where Are We Now? Vaccines, 11(5), 1013. https://doi.org/10.3390/vaccines11051013





