You Shall Not Pass: MX2 Proteins Are Versatile Viral Inhibitors
Abstract
:1. Introduction
2. Breadth of MX2 Antiviral Activity
3. MX2 Structure
4. Role of MX2 NTD
5. MX2 Oligomerization
6. G domain and GTPase Activity
7. Subcellular Localization
8. Role of Other Cellular Proteins
9. Post-Translational Modification of hMX2
10. Other hMX2 Activities
11. Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schoggins, J.W. Interferon-Stimulated Genes: What Do They All Do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef] [PubMed]
- McDougal, M.B.; Boys, I.N.; De La Cruz-Rivera, P.; Schoggins, J.W. Evolution of the interferon response: Lessons from ISGs of diverse mammals. Curr. Opin. Virol. 2022, 53, 101202. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.; Goujon, C.; Malim, M.H. HIV-1 and interferons: Who’s interfering with whom? Nat. Rev. Microbiol. 2015, 13, 403–413. [Google Scholar] [CrossRef]
- Tommila, V. Treatment of Dendritic Keratitis with Interferon. Acta Ophthalmol. 1963, 41, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Jordan, G.W.; Fried, R.P.; Merigan, T.C. Administration of human leukocyte interferon in herpes zoster. I. Safety, circulating, antiviral activity, and host responses to infection. J. Infect. Dis. 1974, 130, 56–62. [Google Scholar] [CrossRef]
- Meylan, P.R.; Guatelli, J.C.; Munis, J.R.; Richman, D.D.; Kornbluth, R.S. Mechanisms for the inhibition of HIV replication by interferons-alpha, -beta, and -gamma in primary human macrophages. Virology 1993, 193, 138–148. [Google Scholar] [CrossRef]
- Baca-Regen, L.; Heinzinger, N.; Stevenson, M.; Gendelman, H.E. Alpha interferon-induced antiretroviral activities: Restriction of viral nucleic acid synthesis and progeny virion production in human immunodeficiency virus type 1-infected monocytes. J. Virol. 1994, 68, 7559–7565. [Google Scholar] [CrossRef]
- Schoggins, J.W.; Rice, C.M. Interferon-stimulated genes and their antiviral effector functions. Curr. Opin. Virol. 2011, 1, 519–525. [Google Scholar] [CrossRef]
- Haller, O.; Frese, M.; Rost, D.; Nuttall, P.A.; Kochs, G. Tick-borne thogoto virus infection in mice is inhibited by the orthomyxovirus resistance gene product Mx1. J. Virol. 1995, 69, 2596–2601. [Google Scholar] [CrossRef]
- Pavlovic, J.; Haller, O.; Staeheli, P. Human and mouse Mx proteins inhibit different steps of the influenza virus multiplication cycle. J. Virol. 1992, 66, 2564–2569. [Google Scholar] [CrossRef]
- Frese, M.; Kochs, G.; Feldmann, H.; Hertkorn, C.; Haller, O. Inhibition of bunyaviruses, phleboviruses, and hantaviruses by human MxA protein. J. Virol. 1996, 70, 915–923. [Google Scholar] [CrossRef]
- Gordien, E.; Rosmorduc, O.; Peltekian, C.; Garreau, F.; Brechot, C.; Kremsdorf, D. Inhibition of hepatitis B virus replication by the interferon-inducible MxA protein. J. Virol. 2001, 75, 2684–2691. [Google Scholar] [CrossRef]
- Kochs, G.; Janzen, C.; Hohenberg, H.; Haller, O. Antivirally active MxA protein sequesters La Crosse virus nucleocapsid protein into perinuclear complexes. Proc. Natl. Acad. Sci. USA 2002, 99, 3153–3158. [Google Scholar] [CrossRef]
- Haller, O.; Staeheli, P.; Schwemmle, M.; Kochs, G. Mx GTPases: Dynamin-like antiviral machines of innate immunity. Trends Microbiol. 2015, 23, 154–163. [Google Scholar] [CrossRef]
- Lane, H.C.; Davey, V.; Kovacs, J.A.; Feinberg, J.; Metcalf, J.A.; Herpin, B.; Walker, R.; Deyton, L.; Davey, R.T., Jr.; Falloon, J.; et al. Interferon-alpha in patients with asymptomatic human immunodeficiency virus (HIV) infection. A randomized, placebo-controlled trial. Ann. Intern. Med. 1990, 112, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Verhelst, J.; Hulpiau, P.; Saelens, X. Mx proteins: Antiviral gatekeepers that restrain the uninvited. Microbiol. Mol. Biol. Rev. 2013, 77, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mariscal, J.A.; Gallardo-Galvez, J.B.; Mendez, T.; Alvarez, M.C.; Bejar, J. Cloning and characterization of the Mx1, Mx2 and Mx3 promoters from gilthead seabream (Sparus aurata). Fish Shellfish. Immunol. 2014, 38, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Trobridge, G.D.; Chiou, P.P.; Leong, J.A. Cloning of the rainbow trout (Oncorhynchus mykiss) Mx2 and Mx3 cDNAs and characterization of trout Mx protein expression in salmon cells. J. Virol. 1997, 71, 5304–5311. [Google Scholar] [CrossRef]
- Wu, Y.C.; Chi, S.C. Cloning and analysis of antiviral activity of a barramundi (Lates calcarifer) Mx gene. Fish Shellfish. Immunol. 2007, 23, 97–108. [Google Scholar] [CrossRef]
- Meier, E.; Kunz, G.; Haller, O.; Arnheiter, H. Activity of rat Mx proteins against a rhabdovirus. J. Virol. 1990, 64, 6263–6269. [Google Scholar] [CrossRef]
- Fernandez-Trujillo, M.A.; Garcia-Rosado, E.; Alonso, M.C.; Borrego, J.J.; Alvarez, M.C.; Bejar, J. Differential antiviral activity of Mx1, Mx2 and Mx3 proteins from gilthead seabream (Sparus aurata) against Infectious Pancreatic Necrosis Virus (IPNV). Mol. Immunol. 2011, 49, 107–114. [Google Scholar] [CrossRef]
- Chen, Y.M.; Su, Y.L.; Shie, P.S.; Huang, S.L.; Yang, H.L.; Chen, T.Y. Grouper Mx confers resistance to nodavirus and interacts with coat protein. Dev. Comp. Immunol. 2008, 32, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.K.; Takada, A.; Kon, Y.; Haller, O.; Watanabe, T. Identification of the murine Mx2 gene: Interferon-induced expression of the Mx2 protein from the feral mouse gene confers resistance to vesicular stomatitis virus. J. Virol. 1999, 73, 4925–4930. [Google Scholar] [CrossRef] [PubMed]
- Caipang, C.M.; Hirono, I.; Aoki, T. In vitro inhibition of fish rhabdoviruses by Japanese flounder, Paralichthys olivaceus Mx. Virology 2003, 317, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Trujillo, M.A.; Garcia-Rosado, E.; Alonso, M.C.; Borrego, J.J.; Alvarez, M.C.; Bejar, J. In vitro inhibition of sole aquabirnavirus by Senegalese sole Mx. Fish. Shellfish. Immunol. 2008, 24, 187–193. [Google Scholar] [CrossRef]
- Lin, C.H.; Christopher John, J.A.; Lin, C.H.; Chang, C.Y. Inhibition of nervous necrosis virus propagation by fish Mx proteins. Biochem. Biophys. Res. Commun. 2006, 351, 534–539. [Google Scholar] [CrossRef]
- Jin, H.K.; Yoshimatsu, K.; Takada, A.; Ogino, M.; Asano, A.; Arikawa, J.; Watanabe, T. Mouse Mx2 protein inhibits hantavirus but not influenza virus replication. Arch. Virol. 2001, 146, 41–49. [Google Scholar] [CrossRef]
- Fernandez-Trujillo, M.A.; Garcia-Rosado, E.; Alonso, M.C.; Castro, D.; Alvarez, M.C.; Bejar, J. Mx1, Mx2 and Mx3 proteins from the gilthead seabream (Sparus aurata) show in vitro antiviral activity against RNA and DNA viruses. Mol. Immunol. 2013, 56, 630–636. [Google Scholar] [CrossRef]
- Aebi, M.; Fah, J.; Hurt, N.; Samuel, C.E.; Thomis, D.; Bazzigher, L.; Pavlovic, J.; Haller, O.; Staeheli, P. cDNA structures and regulation of two interferon-induced human Mx proteins. Mol. Cell. Biol. 1989, 9, 5062–5072. [Google Scholar]
- Pavlovic, J.; Zurcher, T.; Haller, O.; Staeheli, P. Resistance to influenza virus and vesicular stomatitis virus conferred by expression of human MxA protein. J. Virol. 1990, 64, 3370–3375. [Google Scholar] [CrossRef]
- Goujon, C.; Moncorge, O.; Bauby, H.; Doyle, T.; Ward, C.C.; Schaller, T.; Hue, S.; Barclay, W.S.; Schulz, R.; Malim, M.H. Human MX2 is an interferon-induced post-entry inhibitor of HIV-1 infection. Nature 2013, 502, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.; Yadav, S.S.; Bitzegeio, J.; Kutluay, S.B.; Zang, T.; Wilson, S.J.; Schoggins, J.W.; Rice, C.M.; Yamashita, M.; Hatziioannou, T.; et al. MX2 is an interferon-induced inhibitor of HIV-1 infection. Nature 2013, 502, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Pan, Q.; Ding, S.; Qian, J.; Xu, F.; Zhou, J.; Cen, S.; Guo, F.; Liang, C. The interferon-inducible MxB protein inhibits HIV-1 infection. Cell Host Microbe 2013, 14, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Haller, O. Dynamins are forever: MxB inhibits HIV-1. Cell Host Microbe 2013, 14, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Fribourgh, J.L.; Nguyen, H.C.; Matreyek, K.A.; Alvarez, F.J.D.; Summers, B.J.; Dewdney, T.G.; Aiken, C.; Zhang, P.; Engelman, A.; Xiong, Y. Structural insight into HIV-1 restriction by MxB. Cell Host Microbe 2014, 16, 627–638. [Google Scholar] [CrossRef]
- Fricke, T.; White, T.E.; Schulte, B.; de Souza Aranha Vieira, D.A.; Dharan, A.; Campbell, E.M.; Brandariz-Nunez, A.; Diaz-Griffero, F. MxB binds to the HIV-1 core and prevents the uncoating process of HIV-1. Retrovirology 2014, 11, 68. [Google Scholar] [CrossRef]
- Betancor, G.; Dicks, M.D.J.; Jimenez-Guardeno, J.M.; Ali, N.H.; Apolonia, L.; Malim, M.H. The GTPase Domain of MX2 Interacts with the HIV-1 Capsid, Enabling Its Short Isoform to Moderate Antiviral Restriction. Cell. Rep. 2019, 29, 1923–1933.e3. [Google Scholar] [CrossRef]
- Yi, D.R.; An, N.; Liu, Z.L.; Xu, F.W.; Raniga, K.; Li, Q.J.; Zhou, R.; Wang, J.; Zhang, Y.X.; Zhou, J.M.; et al. Human MxB Inhibits the Replication of Hepatitis C Virus. J. Virol. 2019, 93, e01285-18. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, M.; Wu, G.; Ji, D.; Zhou, G.G.; Ren, P.G.; Fu, W. High accumulation of Mx2 renders limited multiplication of oncolytic herpes simplex virus-1 in human tumor cells. Sci. Rep. 2021, 11, 21227. [Google Scholar] [CrossRef]
- Serrero, M.C.; Girault, V.; Weigang, S.; Greco, T.M.; Ramos-Nascimento, A.; Anderson, F.; Piras, A.; Hickford Martinez, A.; Hertzog, J.; Binz, A.; et al. The interferon-inducible GTPase MxB promotes capsid disassembly and genome release of herpesviruses. Elife 2022, 11, e76804. [Google Scholar] [CrossRef]
- Wang, Y.X.; Niklasch, M.; Liu, T.; Wang, Y.; Shi, B.; Yuan, W.; Baumert, T.F.; Yuan, Z.; Tong, S.; Nassal, M.; et al. Interferon-inducible MX2 is a host restriction factor of hepatitis B virus replication. J. Hepatol. 2020, 72, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Jaguva Vasudevan, A.A.; Bahr, A.; Grothmann, R.; Singer, A.; Haussinger, D.; Zimmermann, A.; Munk, C. MXB inhibits murine cytomegalovirus. Virology 2018, 522, 158–167. [Google Scholar] [CrossRef]
- Matreyek, K.A.; Wang, W.; Serrao, E.; Singh, P.K.; Levin, H.L.; Engelman, A. Host and viral determinants for MxB restriction of HIV-1 infection. Retrovirology 2014, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Luo, F.; Chen, Q.; Zhu, N.; Wang, H.; Xie, L.; Xiong, H.; Yue, M.; Zhang, Y.; Feng, Y.; et al. IFN-lambdas inhibit Hantaan virus infection through the JAK-STAT pathway and expression of Mx2 protein. Genes. Immun. 2019, 20, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Crameri, M.; Bauer, M.; Caduff, N.; Walker, R.; Steiner, F.; Franzoso, F.D.; Gujer, C.; Boucke, K.; Kucera, T.; Zbinden, A.; et al. MxB is an interferon-induced restriction factor of human herpesviruses. Nat. Commun. 2018, 9, 1980. [Google Scholar] [CrossRef]
- Liu, S.Y.; Sanchez, D.J.; Aliyari, R.; Lu, S.; Cheng, G. Systematic identification of type I and type II interferon-induced antiviral factors. Proc. Natl. Acad. Sci. USA 2012, 109, 4239–4244. [Google Scholar] [CrossRef]
- Schilling, M.; Bulli, L.; Weigang, S.; Graf, L.; Naumann, S.; Patzina, C.; Wagner, V.; Bauersfeld, L.; Goujon, C.; Hengel, H.; et al. Human MxB Protein Is a Pan-herpesvirus Restriction Factor. J. Virol. 2018, 92, e01056-18. [Google Scholar] [CrossRef]
- Betancor, G.; Bangham, M.; Jeon, J.K.; Shah, K.; Lynham, S.; Jimenez-Guardeno, J.M.; Malim, M.H. MX2 Viral Substrate Breadth and Inhibitory Activity Are Regulated by Protein Phosphorylation. mBio 2022, 13, e0171422. [Google Scholar] [CrossRef]
- Busnadiego, I.; Kane, M.; Rihn, S.J.; Preugschas, H.F.; Hughes, J.; Blanco-Melo, D.; Strouvelle, V.P.; Zang, T.M.; Willett, B.J.; Boutell, C.; et al. Host and viral determinants of Mx2 antiretroviral activity. J. Virol. 2014, 88, 7738–7752. [Google Scholar] [CrossRef]
- Meier, K.; Vasudevan, A.A.J.; Zhang, Z.; Bahr, A.; Kochs, G.; Haussinger, D.; Munk, C. Equine MX2 is a restriction factor of equine infectious anemia virus (EIAV). Virology 2018, 523, 52–63. [Google Scholar] [CrossRef]
- Ji, S.; Na, L.; Ren, H.; Wang, Y.; Wang, X. Equine Myxovirus Resistance Protein 2 Restricts Lentiviral Replication by Blocking Nuclear Uptake of Capsid Protein. J. Virol. 2018, 92, e00499-18. [Google Scholar] [CrossRef]
- Wang, H.; Bai, J.; Fan, B.; Li, Y.; Zhang, Q.; Jiang, P. The Interferon-Induced Mx2 Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication. J. Interferon. Cytokine. Res. 2016, 36, 129–139. [Google Scholar] [CrossRef]
- Morozumi, T.; Naito, T.; Lan, P.D.; Nakajima, E.; Mitsuhashi, T.; Mikawa, S.; Hayashi, T.; Awata, T.; Uenishi, H.; Nagata, K.; et al. Molecular cloning and characterization of porcine Mx2 gene. Mol. Immunol. 2009, 46, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Tungtrakoolsub, P.; Morozumi, T.; Uenishi, H.; Kawahara, M.; Watanabe, T. A single nucleotide polymorphism of porcine MX2 gene provides antiviral activity against vesicular stomatitis virus. Immunogenetics 2014, 66, 25–32. [Google Scholar] [CrossRef]
- Babiker, H.A.; Nakatsu, Y.; Yamada, K.; Yoneda, A.; Takada, A.; Ueda, J.; Hata, H.; Watanabe, T. Bovine and water buffalo Mx2 genes: Polymorphism and antiviral activity. Immunogenetics 2007, 59, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mao, L.; Xiao, F.; Liao, Z.; Yin, J.; Li, W.; Sun, M.; Liu, M.; Ji, X.; Liu, C.; et al. Interferon-stimulated genes inhibit caprine parainfluenza virus type 3 replication in Madin-Darby bovine kidney cells. Vet. Microbiol. 2020, 241, 108573. [Google Scholar] [CrossRef]
- Goujon, C.; Moncorge, O.; Bauby, H.; Doyle, T.; Barclay, W.S.; Malim, M.H. Transfer of the amino-terminal nuclear envelope targeting domain of human MX2 converts MX1 into an HIV-1 resistance factor. J. Virol. 2014, 88, 9017–9026. [Google Scholar] [CrossRef] [PubMed]
- Goujon, C.; Greenbury, R.A.; Papaioannou, S.; Doyle, T.; Malim, M.H. A triple-arginine motif in the amino-terminal domain and oligomerization are required for HIV-1 inhibition by human MX2. J. Virol. 2015, 89, 4676–4680. [Google Scholar] [CrossRef]
- Gao, S.; von der Malsburg, A.; Paeschke, S.; Behlke, J.; Haller, O.; Kochs, G.; Daumke, O. Structural basis of oligomerization in the stalk region of dynamin-like MxA. Nature 2010, 465, 502–506. [Google Scholar] [CrossRef]
- Gao, S.; von der Malsburg, A.; Dick, A.; Faelber, K.; Schroder, G.F.; Haller, O.; Kochs, G.; Daumke, O. Structure of myxovirus resistance protein a reveals intra- and intermolecular domain interactions required for the antiviral function. Immunity 2011, 35, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Haller, O.; Gao, S.; von der Malsburg, A.; Daumke, O.; Kochs, G. Dynamin-like MxA GTPase: Structural insights into oligomerization and implications for antiviral activity. J. Biol. Chem. 2010, 285, 28419–28424. [Google Scholar] [CrossRef]
- Alvarez, F.J.D.; He, S.; Perilla, J.R.; Jang, S.; Schulten, K.; Engelman, A.N.; Scheres, S.H.W.; Zhang, P. CryoEM structure of MxB reveals a novel oligomerization interface critical for HIV restriction. Sci. Adv. 2017, 3, e1701264. [Google Scholar] [CrossRef] [PubMed]
- Buffone, C.; Schulte, B.; Opp, S.; Diaz-Griffero, F. Contribution of MxB oligomerization to HIV-1 capsid binding and restriction. J. Virol. 2015, 89, 3285–3294. [Google Scholar] [CrossRef] [PubMed]
- Dicks, M.D.; Goujon, C.; Pollpeter, D.; Betancor, G.; Apolonia, L.; Bergeron, J.R.; Malim, M.H. Oligomerization Requirements for MX2-Mediated Suppression of HIV-1 Infection. J. Virol. 2016, 90, 22–32. [Google Scholar] [CrossRef]
- Betancor, G.; Jimenez-Guardeno, J.M.; Lynham, S.; Antrobus, R.; Khan, H.; Sobala, A.; Dicks MD, J.; Malim, M.H. MX2-mediated innate immunity against HIV-1 is regulated by serine phosphorylation. Nat. Microbiol. 2021, 6, 1031–1042. [Google Scholar] [CrossRef]
- Schulte, B.; Buffone, C.; Opp, S.; Di Nunzio, F.; De Souza Aranha Vieira, D.A.; Brandariz-Nunez, A.; Diaz-Griffero, F. Restriction of HIV-1 Requires the N-Terminal Region of MxB as a Capsid-Binding Motif but Not as a Nuclear Localization Signal. J. Virol. 2015, 89, 8599–8610. [Google Scholar] [CrossRef] [PubMed]
- Melen, K.; Keskinen, P.; Ronni, T.; Sareneva, T.; Lounatmaa, K.; Julkunen, I. Human MxB protein, an interferon-alpha-inducible GTPase, contains a nuclear targeting signal and is localized in the heterochromatin region beneath the nuclear envelope. J. Biol. Chem. 1996, 271, 23478–23486. [Google Scholar] [CrossRef]
- Melen, K.; Julkunen, I. Nuclear cotransport mechanism of cytoplasmic human MxB protein. J. Biol. Chem. 1997, 272, 32353–32359. [Google Scholar] [CrossRef]
- Ganser-Pornillos, B.K.; Yeager, M.; Pornillos, O. Assembly and architecture of HIV. Adv. Exp. Med. Biol. 2012, 726, 441–465. [Google Scholar]
- Bulli, L.; Apolonia, L.; Kutzner, J.; Pollpeter, D.; Goujon, C.; Herold, N.; Schwarz, S.M.; Giernat, Y.; Keppler, O.T.; Malim, M.H.; et al. Complex Interplay between HIV-1 Capsid and MX2-Independent Alpha Interferon-Induced Antiviral Factors. J. Virol. 2016, 90, 7469–7480. [Google Scholar] [CrossRef]
- Opp, S.; Vieira, D.A.; Schulte, B.; Chanda, S.K.; Diaz-Griffero, F. MxB Is Not Responsible for the Blocking of HIV-1 Infection Observed in Alpha Interferon-Treated Cells. J. Virol. 2015, 90, 3056–3064. [Google Scholar] [CrossRef] [PubMed]
- Summers, B.J.; Digianantonio, K.M.; Smaga, S.S.; Huang, P.T.; Zhou, K.; Gerber, E.E.; Wang, W.; Xiong, Y. Modular HIV-1 Capsid Assemblies Reveal Diverse Host-Capsid Recognition Mechanisms. Cell Host Microbe 2019, 26, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Smaga, S.S.; Xu, C.; Summers, B.J.; Digianantonio, K.M.; Perilla, J.R.; Xiong, Y. MxB Restricts HIV-1 by Targeting the Tri-hexamer Interface of the Viral Capsid. Structure 2019, 27, 1234–1245.e5. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhao, F.; Zhao, X.; Zhang, D.; Liu, X.; Hu, S.; Mei, S.; Fan, Z.; Huang, Y.; Sun, H.; et al. Pro-515 of the dynamin-like GTPase MxB contributes to HIV-1 inhibition by regulating MxB oligomerization and binding to HIV-1 capsid. J. Biol. Chem. 2020, 295, 6447–6456. [Google Scholar] [CrossRef]
- Pitossi, F.; Blank, A.; Schroder, A.; Schwarz, A.; Hussi, P.; Schwemmle, M.; Pavlovic, J.; Staeheli, P. A functional GTP-binding motif is necessary for antiviral activity of Mx proteins. J. Virol. 1993, 67, 6726–6732. [Google Scholar] [CrossRef]
- Ponten, A.; Sick, C.; Weeber, M.; Haller, O.; Kochs, G. Dominant-negative mutants of human MxA protein: Domains in the carboxy-terminal moiety are important for oligomerization and antiviral activity. J. Virol. 1997, 71, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Ju, Z.; Zhong, C.; Wu, Y.; Zan, Y.; Hou, W.; Feng, Y. GTPase Activity of MxB Contributes to Its Nuclear Location, Interaction with Nucleoporins and Anti-HIV-1 Activity. Virol. Sin. 2021, 36, 85–94. [Google Scholar] [CrossRef]
- Merindol, N.; El-Far, M.; Sylla, M.; Masroori, N.; Dufour, C.; Li, J.X.; Cherry, P.; Plourde, M.B.; Tremblay, C.; Berthoux, L. HIV-1 capsids from B27/B57+ elite controllers escape Mx2 but are targeted by TRIM5alpha, leading to the induction of an antiviral state. PLoS Pathog. 2018, 14, e1007398. [Google Scholar] [CrossRef]
- Miles, R.J.; Kerridge, C.; Hilditch, L.; Monit, C.; Jacques, D.A.; Towers, G.J. MxB sensitivity of HIV-1 is determined by a highly variable and dynamic capsid surface. Elife 2020, 9, e56910. [Google Scholar] [CrossRef]
- King, M.C.; Raposo, G.; Lemmon, M.A. Inhibition of nuclear import and cell-cycle progression by mutated forms of the dynamin-like GTPase MxB. Proc. Natl. Acad. Sci. USA 2004, 101, 8957–8962. [Google Scholar] [CrossRef]
- Kane, M.; Rebensburg, S.V.; Takata, M.A.; Zang, T.M.; Yamashita, M.; Kvaratskhelia, M.; Bieniasz, P.D. Nuclear pore heterogeneity influences HIV-1 infection and the antiviral activity of MX2. Elife 2018, 7, e35738. [Google Scholar] [CrossRef]
- Dicks, M.D.J.; Betancor, G.; Jimenez-Guardeno, J.M.; Pessel-Vivares, L.; Apolonia, L.; Goujon, C.; Malim, M.H. Multiple components of the nuclear pore complex interact with the amino-terminus of MX2 to facilitate HIV-1 restriction. PLoS Pathog. 2018, 14, e1007408. [Google Scholar] [CrossRef] [PubMed]
- Luban, J.; Bossolt, K.L.; Franke, E.K.; Kalpana, G.V.; Goff, S.P. Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B. Cell 1993, 73, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Braaten, D.; Luban, J. Cyclophilin A regulates HIV-1 infectivity, as demonstrated by gene targeting in human T cells. EMBO J. 2001, 20, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Pan, Q.; Liang, Z.; Qiao, W.; Cen, S.; Liang, C. The highly polymorphic cyclophilin A-binding loop in HIV-1 capsid modulates viral resistance to MxB. Retrovirology 2015, 12, 1–6. [Google Scholar] [CrossRef]
- Xie, L.; Chen, L.; Zhong, C.; Yu, T.; Ju, Z.; Wang, M.; Xiong, H.; Zeng, Y.; Wang, J.; Hu, H.; et al. MxB impedes the NUP358-mediated HIV-1 pre-integration complex nuclear import and viral replication cooperatively with CPSF6. Retrovirology 2020, 17, 16. [Google Scholar] [CrossRef]
- Laguette, N.; Sobhian, B.; Casartelli, N.; Ringeard, M.; Chable-Bessia, C.; Segeral, E.; Yatim, A.; Emiliani, S.; Schwartz, O.; Benkirane, M. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 2011, 474, 654–657. [Google Scholar] [CrossRef]
- Goldstone, D.C.; Ennis-Adeniran, V.; Hedden, J.J.; Groom, H.C.; Rice, G.I.; Christodoulou, E.; Walker, P.A.; Kelly, G.; Haire, L.F.; Yap, M.W.; et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 2011, 480, 379–382. [Google Scholar] [CrossRef]
- Buffone, C.; Kutzner, J.; Opp, S.; Martinez-Lopez, A.; Selyutina, A.; Coggings, S.A.; Studdard, L.R.; Ding, L.; Kim, B.; Spearman, P.; et al. The ability of SAMHD1 to block HIV-1 but not SIV requires expression of MxB. Virology 2019, 531, 260–268. [Google Scholar] [CrossRef]
- Lee, K.; Ambrose, Z.; Martin, T.D.; Oztop, I.; Mulky, A.; Julias, J.G.; Vandegraaff, N.; Baumann, J.G.; Wang, R.; Yuen, W.; et al. Flexible use of nuclear import pathways by HIV-1. Cell Host Microbe 2010, 7, 221–233. [Google Scholar] [CrossRef]
- Cao, H.; Krueger, E.W.; Chen, J.; Drizyte-Miller, K.; Schulz, M.E.; McNiven, M.A. The anti-viral dynamin family member MxB participates in mitochondrial integrity. Nat. Commun. 2020, 11, 1048. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chai, K.; Liu, Q.; Yi, D.R.; Pan, Q.; Huang, Y.; Tan, J.; Qiao, W.; Guo, F.; Cen, S.; et al. HIV-1 resists MxB inhibition of viral Rev protein. Emerg. Microbes. Infect. 2020, 9, 2030–2045. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Young, J.M.; Emerman, M.; Malik, H.S. Evolutionary Analyses Suggest a Function of MxB Immunity Proteins Beyond Lentivirus Restriction. PLoS Pathog. 2015, 11, e1005304. [Google Scholar] [CrossRef] [PubMed]
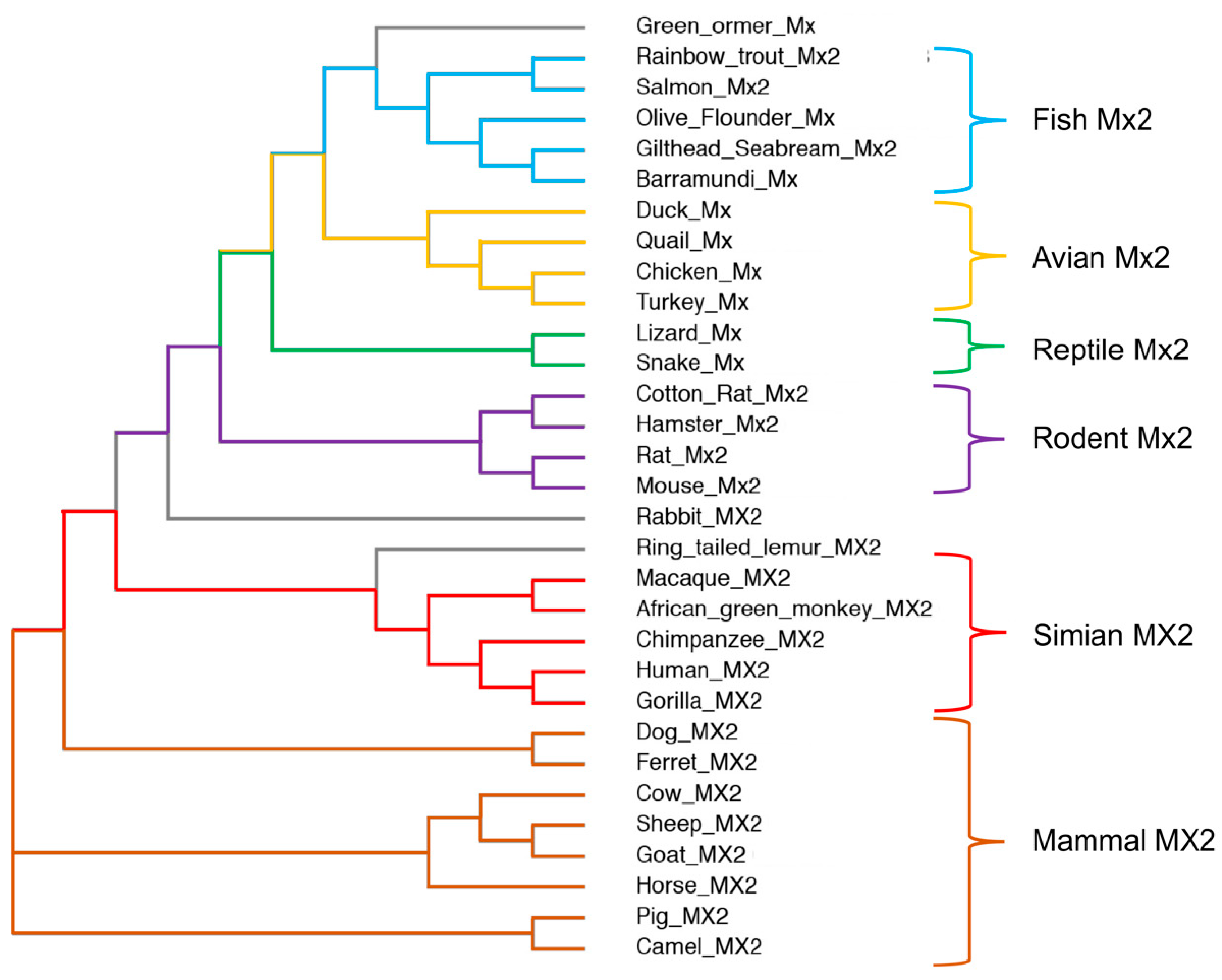
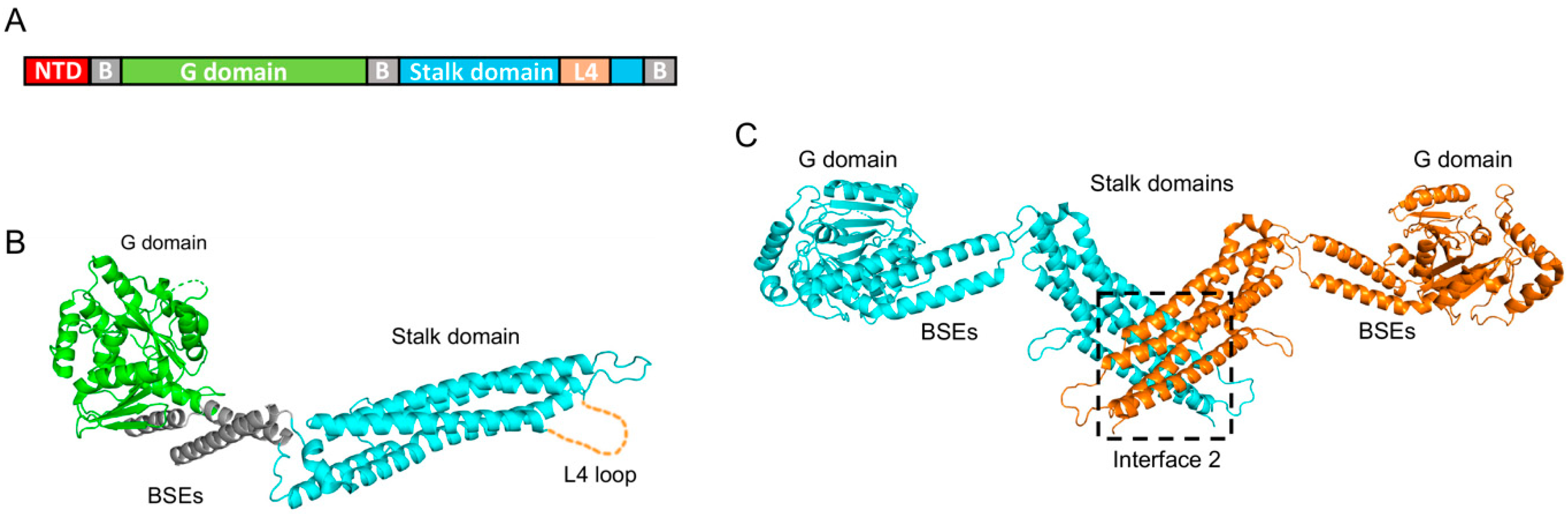
| MX2 | Virus Family/Subfamily | Virus | Technique | Ref |
|---|---|---|---|---|
| Human | Hantaviridae | Hantavirus | PO | [44] |
| Rhabdoviridae | Vesicular stomatitis virus (VSV) | PO | [45,46] | |
| Hepadnaviridae | Hepatitis B virus (HBV) | PD/PO | [41] | |
| Flaviviridae | Hepatitis C virus (HCV) | PD/PO | [38] | |
| Japanese encephalitis virus (JEV) | PO | [38] | ||
| Dengue virus | PO | [38] | ||
| Alphaherpesvirinae | Herpes simplex virus 1 (HSV-1) | PD/PO | [39,40,45,47] | |
| Herpes simplex virus 2 (HSV-2) | PO | [40,45] | ||
| Varicella zoster virus (VZV) | PO | [40] | ||
| Betaherpesvirinae | Murine cytomegalovirus (MCMV) | PO | [42,47] | |
| Gammaherpesvirinae | Kaposi-sarcoma-associated virus (KSHV) | PO | [45] | |
| Murine gamma herpesvirus 68 (MHV68) | PO | [47] | ||
| Orthoretrovirinae | Human immunodeficiency virus 1 (HIV-1) | PD/PO | [31,32,33] | |
| Human immunodeficiency virus 2 (HIV-2) | PO | [32] | ||
| Simian immunodeficiency virus (SIV) | PO | [31,32,43] | ||
| Equine infectious anemia virus (EIAV) | PO * | [48] | ||
| Murine leukemia virus (MLV) | PO * | [48] | ||
| Mason–Pfizer monkey virus (MPMV) | PO | [49] | ||
| Equine | Orthoretrovirinae | HIV-1 | PO | [50,51] |
| HIV-2 | PO | [50] | ||
| SIV | PO | [50,51] | ||
| EIAV | PD/PO | [50,51] | ||
| SIV | PO | [50] | ||
| MLV | PO | [50] | ||
| Porcine | Arteriviridae | Porcine reproductive and respiratory syndrome virus (PRRSV) | PO | [52] |
| Orthomyxoviridae | Influenza A virus (IAV) | PO | [53] | |
| Rhabdoviridae | VSV | PO | [54] | |
| Bovine | Rhabdoviridae | VSV | PO | [55] |
| Paramyxoviridae | Caprine parainfluenza virus 3 (CPIV3) | PD/PO | [56] | |
| African green monkey | Arteriviridae | PRRSV | PD/PO | [52] |
| Orthoretrovirinae | HIV-1 | PO | [49] | |
| MPMV | PO | [49] | ||
| Rhesus macaque | Orthoretrovirinae | HIV-1 | PO | [49] |
| MPMV | PO | [32,49] | ||
| Canine | Orthoretrovirinae | HIV-1 | PO | [57,58] |
| Mutation | MX2 Domain | Antiviral Activity | Ref |
|---|---|---|---|
| ∆1-25 | NTD | Canceled | [32,43,57] |
| R11-13A | NTD | Canceled | [58] |
| S14, 17-18D | NTD | Canceled | [65] |
| K20A | NTD | Unaffected | [66] |
| Y21A | NTD | ND | [66] |
| S28D | NTD | Increased | [48,65] |
| K131A | G domain | Reduced | [31,32,43] |
| T151A | G domain | Unaffected | [32,43] |
| T151D | G domain | Increased | [48] |
| E285K | G domain | Unaffected | [62] |
| T334D | G domain | Decreased | [48] |
| T343A | G domain | Decreased | [48] |
| T343D | G domain | Increased | [48] |
| F420D | Stalk domain | Unaffected | [62] |
| E484K | Stalk domain | Decreased | [62] |
| E491K | Stalk domain | Unaffected | [62] |
| M574D | Stalk domain | Canceled | [35,63,64] |
| Y651D | Stalk domain | Canceled | [35,63,64] |
| W677D | Stalk domain | Unaffected | [62] |
| K693D | Stalk domain | Unaffected | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betancor, G. You Shall Not Pass: MX2 Proteins Are Versatile Viral Inhibitors. Vaccines 2023, 11, 930. https://doi.org/10.3390/vaccines11050930
Betancor G. You Shall Not Pass: MX2 Proteins Are Versatile Viral Inhibitors. Vaccines. 2023; 11(5):930. https://doi.org/10.3390/vaccines11050930
Chicago/Turabian StyleBetancor, Gilberto. 2023. "You Shall Not Pass: MX2 Proteins Are Versatile Viral Inhibitors" Vaccines 11, no. 5: 930. https://doi.org/10.3390/vaccines11050930





