NS1 Protein N-Linked Glycosylation Site Affects the Virulence and Pathogenesis of Dengue Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses, Plasmids, Cells, and Animals
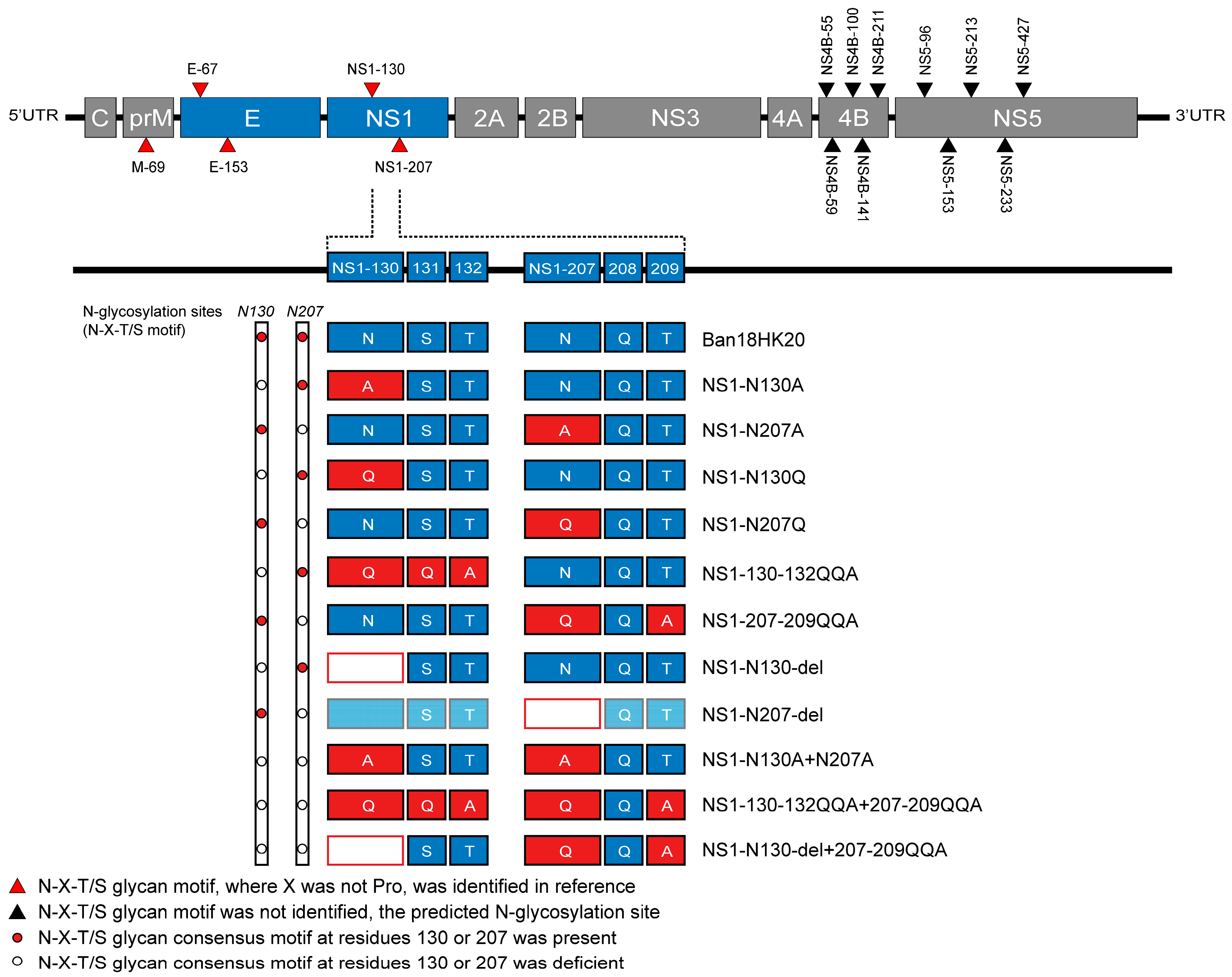
2.2. Design of Dengue Virus NS1 Protein N-Glycosylation Site Mutations
2.3. Construction Strategy for Molecular Cloning
2.4. In Vitro Transcription, Transfection, and Virus Rescue
2.5. Virus Titer Assay
2.6. Western Blotting
2.7. Indirect Immunofluorescence Assays
2.8. Virus Genome Sequencing and Genetic Stability Assay
2.9. Mouse Experiments
2.10. Virus Plaque Purification Assay
2.11. Construction of Dengue Chimeric Virus
2.12. Statistical Analysis
3. Results
3.1. Identification of Rescue Viruses
3.2. Dengue Virus Possesses an Attenuated Phenotype after Deletion of the N-Glycosylation Site in the NS1 Protein
3.3. Plaque Purification of the Mutant N130del+207-209QQA Strain and Screening of Attenuated Strains
3.4. Identification of Virulence Loci for Attenuated Mutants
3.5. Genetic Stability of Plaque-Purified Attenuated Dengue Virus Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitehead, S.S.; Blaney, J.E.; Durbin, A.P.; Murphy, B.R. Prospects for a dengue virus vaccine. Nat. Rev. Microbiol. 2007, 5, 518–528. [Google Scholar] [CrossRef]
- WHO. Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 12 December 2022).
- Mustafa, M.S.; Rasotgi, V.; Jain, S.; Gupta, V. Discovery of fifth serotype of dengue virus (DENV-5): A new public health dilemma in dengue control. Med. J. Armed Forces India 2015, 71, 67–70. [Google Scholar] [CrossRef]
- Muller, D.A.; Young, P.R. The flavivirus NS1 protein: Molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antiviral Res. 2013, 98, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Akey, D.L.; Brown, W.C.; Dutta, S.; Konwerski, J.; Jose, J.; Jurkiw, T.J.; DelProposto, J.; Ogata, C.M.; Skiniotis, G.; Kuhn, R.J.; et al. Flavivirus NS1 structures reveal surfaces for associations with membranes and the immune system. Science 2014, 343, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Libraty, D.H.; Young, P.R.; Pickering, D.; Endy, T.P.; Kalayanarooj, S.; Green, S.; Vaughn, D.W.; Nisalak, A.; Ennis, F.A.; Rothman, A.L. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J. Infect. Dis. 2002, 186, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Beatty, P.R.; Puerta-Guardo, H.; Killingbeck, S.S.; Glasner, D.R.; Hopkins, K.; Harris, E. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci. Transl. Med. 2015, 7, 304ra141. [Google Scholar] [CrossRef]
- Guy, B.; Briand, O.; Lang, J.; Saville, M.; Jackson, N. Development of the Sanofi Pasteur tetravalent dengue vaccine: One more step forward. Vaccine 2015, 33, 7100–7111. [Google Scholar] [CrossRef]
- Halstead, S.B.; Katzelnick, L.C.; Russell, P.K.; Markoff, L.; Aguiar, M.; Dans, L.R.; Dans, A.L. Ethics of a partially effective dengue vaccine: Lessons from the Philippines. Vaccine 2020, 38, 5572–5576. [Google Scholar] [CrossRef]
- Dayan, G.H.; Langevin, E.; Gilbert, P.B.; Wu, Y.; Moodie, Z.; Forrat, R.; Price, B.; Frago, C.; Bouckenooghe, A.; Cortes, M.; et al. Assessment of the long-term efficacy of a dengue vaccine against symptomatic, virologically-confirmed dengue disease by baseline dengue serostatus. Vaccine 2020, 38, 3531–3536. [Google Scholar] [CrossRef]
- Kinney, R.M.; Butrapet, S.; Chang, G.J.; Tsuchiya, K.R.; Roehrig, J.T.; Bhamarapravati, N.; Gubler, D.J. Construction of infectious cDNA clones for dengue 2 virus: Strain 16681 and its attenuated vaccine derivative, strain PDK-53. Virology 1997, 230, 300–308. [Google Scholar] [CrossRef]
- Osorio, J.E.; Huang, C.Y.; Kinney, R.M.; Stinchcomb, D.T. Development of DENVax: A chimeric dengue-2 PDK-53-based tetravalent vaccine for protection against dengue fever. Vaccine 2011, 29, 7251–7260. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.E.; Partidos, C.D.; Wallace, D.; Stinchcomb, D.T. Development of a recombinant, chimeric tetravalent dengue vaccine candidate. Vaccine 2015, 33, 7112–7120. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Kinney, R.M.; Livengood, J.A.; Bolling, B.; Arguello, J.J.; Luy, B.E.; Silengo, S.J.; Boroughs, K.L.; Stovall, J.L.; Kalanidhi, A.P.; et al. Genetic and phenotypic characterization of manufacturing seeds for a tetravalent dengue vaccine (DENVax). PLoS Negl. Trop. Dis. 2013, 7, e2243. [Google Scholar] [CrossRef] [PubMed]
- Tricou, V.; Sáez-Llorens, X.; Yu, D.; Rivera, L.; Jimeno, J.; Villarreal, A.C.; Dato, E.; Saldaña de Suman, O.; Montenegro, N.; DeAntonio, R.; et al. Safety and immunogenicity of a tetravalent dengue vaccine in children aged 2-17 years: A randomised, placebo-controlled, phase 2 trial. Lancet 2020, 395, 1434–1443. [Google Scholar] [CrossRef]
- Men, R.; Bray, M.; Clark, D.; Chanock, R.M.; Lai, C.J. Dengue type 4 virus mutants containing deletions in the 3’ noncoding region of the RNA genome: Analysis of growth restriction in cell culture and altered viremia pattern and immunogenicity in rhesus monkeys. J. Virol. 1996, 70, 3930–3937. [Google Scholar] [CrossRef]
- Whitehead, S.S.; Hanley, K.A.; Blaney, J.E., Jr.; Gilmore, L.E.; Elkins, W.R.; Murphy, B.R. Substitution of the structural genes of dengue virus type 4 with those of type 2 results in chimeric vaccine candidates which are attenuated for mosquitoes, mice, and rhesus monkeys. Vaccine 2003, 21, 4307–4316. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, S.S.; Falgout, B.; Hanley, K.A.; Blaney, J.E., Jr.; Markoff, L.; Murphy, B.R. A live, attenuated dengue virus type 1 vaccine candidate with a 30-nucleotide deletion in the 3’ untranslated region is highly attenuated and immunogenic in monkeys. J. Virol. 2003, 77, 1653–1657. [Google Scholar] [CrossRef]
- Blaney, J.E., Jr.; Hanson, C.T.; Hanley, K.A.; Murphy, B.R.; Whitehead, S.S. Vaccine candidates derived from a novel infectious cDNA clone of an American genotype dengue virus type 2. BMC Infect. Dis. 2004, 4, 39. [Google Scholar] [CrossRef]
- Blaney, J.E., Jr.; Hanson, C.T.; Firestone, C.Y.; Hanley, K.A.; Murphy, B.R.; Whitehead, S.S. Genetically modified, live attenuated dengue virus type 3 vaccine candidates. Am. J. Trop. Med. Hyg. 2004, 71, 811–821. [Google Scholar] [CrossRef]
- Blaney, J.E., Jr.; Sathe, N.S.; Goddard, L.; Hanson, C.T.; Romero, T.A.; Hanley, K.A.; Murphy, B.R.; Whitehead, S.S. Dengue virus type 3 vaccine candidates generated by introduction of deletions in the 3’ untranslated region (3’-UTR) or by exchange of the DENV-3 3’-UTR with that of DENV-4. Vaccine 2008, 26, 817–828. [Google Scholar] [CrossRef]
- Whitehead, S.S. Development of TV003/TV005, a single dose, highly immunogenic live attenuated dengue vaccine; what makes this vaccine different from the Sanofi-Pasteur CYD™ vaccine? Expert Rev. Vaccines 2016, 15, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Nivarthi, U.K.; Swanstrom, J.; Delacruz, M.J.; Patel, B.; Durbin, A.P.; Whitehead, S.S.; Kirkpatrick, B.D.; Pierce, K.K.; Diehl, S.A.; Katzelnick, L.; et al. A tetravalent live attenuated dengue virus vaccine stimulates balanced immunity to multiple serotypes in humans. Nat. Commun. 2021, 12, 1102. [Google Scholar] [CrossRef]
- Chandler, K.B.; Pompach, P.; Goldman, R.; Edwards, N. Exploring site-specific N-glycosylation microheterogeneity of haptoglobin using glycopeptide CID tandem mass spectra and glycan database search. J. Proteome Res. 2013, 12, 3652–3666. [Google Scholar] [CrossRef]
- Yap, S.S.L.; Nguyen-Khuong, T.; Rudd, P.M.; Alonso, S. Dengue Virus Glycosylation: What Do We Know? Front. Microbiol. 2017, 8, 1415. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.E.; Calvert, A.E.; Mesesan, K.; Crabtree, M.B.; Volpe, K.E.; Silengo, S.; Kinney, R.M.; Huang, C.Y.; Miller, B.R.; Roehrig, J.T. Glycosylation of the dengue 2 virus E protein at N67 is critical for virus growth in vitro but not for growth in intrathoracically inoculated Aedes aegypti mosquitoes. Virology 2007, 366, 415–423. [Google Scholar] [CrossRef]
- Krumm, S.A.; Doores, K.J. Targeting Glycans on Human Pathogens for Vaccine Design. Curr. Top. Microbiol. Immunol. 2020, 428, 129–163. [Google Scholar] [CrossRef]
- Whiteman, M.C.; Wicker, J.A.; Kinney, R.M.; Huang, C.Y.; Solomon, T.; Barrett, A.D. Multiple amino acid changes at the first glycosylation motif in NS1 protein of West Nile virus are necessary for complete attenuation for mouse neuroinvasiveness. Vaccine 2011, 29, 9702–9710. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.; Wang, L.; Zhao, D.; Li, M.; Liu, M.; Li, Y. Construction, characterization and stability analysis of infectious clone of live attenuated dengue virus type 4 Ban18HK20 strain. Chin. J. Microbiol. Immunol. 2019, 12, 827–834. [Google Scholar]
- Fang, E.; Liu, X.; Li, M.; Liu, J.; Zhang, Z.; Liu, X.; Li, X.; Li, W.; Peng, Q.; Yu, Y.; et al. Construction of a Dengue NanoLuc Reporter Virus for In Vivo Live Imaging in Mice. Viruses 2022, 14, 1253. [Google Scholar] [CrossRef] [PubMed]
- Eckels, K.H.; Harrison, V.R.; Summers, P.L.; Russell, P.K. Dengue-2 vaccine: Preparation from a small-plaque virus clone. Infect. Immun. 1980, 27, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-F.; Deng, Y.-Q.; Yang, H.-Q.; Zhao, H.; Jiang, T.; Yu, X.-D.; Li, S.-H.; Ye, Q.; Zhu, S.-Y.; Wang, H.-J. A chimeric dengue virus vaccine using japanese encephalitis virus vaccine strain sa14-14-2 as backbone is immunogenic and protective against either parental virus in mice and nonhuman primates. J. Virol. 2013, 87, 13694–13705. [Google Scholar] [CrossRef]
- Somnuke, P.; Hauhart, R.E.; Atkinson, J.P.; Diamond, M.S.; Avirutnan, P. N-linked glycosylation of dengue virus NS1 protein modulates secretion, cell-surface expression, hexamer stability, and interactions with human complement. Virology 2011, 413, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Basset, J.; Burlaud-Gaillard, J.; Feher, M.; Roingeard, P.; Rey, F.A.; Pardigon, N. A Molecular Determinant of West Nile Virus Secretion and Morphology as a Target for Viral Attenuation. J. Virol. 2020, 94, e00086-20. [Google Scholar] [CrossRef] [PubMed]
- Tajima, S.; Takasaki, T.; Kurane, I. Characterization of Asn130-to-Ala mutant of dengue type 1 virus NS1 protein. Virus Genes 2008, 36, 323–329. [Google Scholar] [CrossRef]
- Wang, C.; Puerta-Guardo, H.; Biering, S.B.; Glasner, D.R.; Tran, E.B.; Patana, M.; Gomberg, T.A.; Malvar, C.; Lo, N.T.N.; Espinosa, D.A.; et al. Endocytosis of flavivirus NS1 is required for NS1-mediated endothelial hyperpermeability and is abolished by a single N-glycosylation site mutation. PLoS Pathog. 2019, 15, e1007938. [Google Scholar] [CrossRef]
- Crabtree, M.B.; Kinney, R.M.; Miller, B.R. Deglycosylation of the NS1 protein of dengue 2 virus, strain 16681: Construction and characterization of mutant viruses. Arch. Virol. 2005, 150, 771–786. [Google Scholar] [CrossRef]
- Pryor, M.J.; Wright, P.J. Glycosylation mutants of dengue virus NS1 protein. J. Gen. Virol. 1994, 75 Pt 5, 1183–1187. [Google Scholar] [CrossRef]
- Muylaert, I.R.; Chambers, T.J.; Galler, R.; Rice, C.M. Mutagenesis of the N-linked glycosylation sites of the yellow fever virus NS1 protein: Effects on virus replication and mouse neurovirulence. Virology 1996, 222, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.C.; Li, L.; Wicker, J.A.; Kinney, R.M.; Huang, C.; Beasley, D.W.; Chung, K.M.; Diamond, M.S.; Solomon, T.; Barrett, A.D. Development and characterization of non-glycosylated E and NS1 mutant viruses as a potential candidate vaccine for West Nile virus. Vaccine 2010, 28, 1075–1083. [Google Scholar] [CrossRef]
- Annamalai, A.S.; Pattnaik, A.; Sahoo, B.R.; Guinn, Z.P.; Bullard, B.L.; Weaver, E.A.; Steffen, D.; Natarajan, S.K.; Petro, T.M.; Pattnaik, A.K. An Attenuated Zika Virus Encoding Non-Glycosylated Envelope (E) and Non-Structural Protein 1 (NS1) Confers Complete Protection against Lethal Challenge in a Mouse Model. Vaccines 2019, 7, 112. [Google Scholar] [CrossRef]
- Whiteman, M.C.; Popov, V.; Sherman, M.B.; Wen, J.; Barrett, A.D. Attenuated West Nile virus mutant NS1130-132QQA/175A/207A exhibits virus-induced ultrastructural changes and accumulation of protein in the endoplasmic reticulum. J. Virol. 2015, 89, 1474–1478. [Google Scholar] [CrossRef]
- Pryor, M.J.; Gualano, R.C.; Lin, B.; Davidson, A.D.; Wright, P.J. Growth restriction of dengue virus type 2 by site-specific mutagenesis of virus-encoded glycoproteins. J. Gen. Virol. 1998, 79 Pt 11, 2631–2639. [Google Scholar] [CrossRef]
- Butrapet, S.; Huang, C.Y.; Pierro, D.J.; Bhamarapravati, N.; Gubler, D.J.; Kinney, R.M. Attenuation markers of a candidate dengue type 2 vaccine virus, strain 16681 (PDK-53), are defined by mutations in the 5’ noncoding region and nonstructural proteins 1 and 3. J. Virol. 2000, 74, 3011–3019. [Google Scholar] [CrossRef]
- Messer, W.B.; Yount, B.; Hacker, K.E.; Donaldson, E.F.; Huynh, J.P.; de Silva, A.M.; Baric, R.S. Development and characterization of a reverse genetic system for studying dengue virus serotype 3 strain variation and neutralization. PLoS Negl. Trop. Dis. 2012, 6, e1486. [Google Scholar] [CrossRef]
- Deng, C.L.; Zhang, Q.Y.; Chen, D.D.; Liu, S.Q.; Qin, C.F.; Zhang, B.; Ye, H.Q. Recovery of the Zika virus through an in vitro ligation approach. J. Gen. Virol. 2017, 98, 1739–1743. [Google Scholar] [CrossRef]
- Rice, C.M.; Grakoui, A.; Galler, R.; Chambers, T.J. Transcription of infectious yellow fever RNA from full-length cDNA templates produced by in vitro ligation. New Biol. 1989, 1, 285–296. [Google Scholar] [PubMed]
- Sumiyoshi, H.; Hoke, C.H.; Trent, D.W. Infectious Japanese encephalitis virus RNA can be synthesized from in vitro-ligated cDNA templates. J. Virol. 1992, 66, 5425–5431. [Google Scholar] [CrossRef]
- Kapoor, M.; Zhang, L.; Mohan, P.M.; Padmanabhan, R. Synthesis and characterization of an infectious dengue virus type-2 RNA genome (New Guinea C strain). Gene 1995, 162, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Muruato, A.; Lokugamage, K.G.; Narayanan, K.; Zhang, X.; Zou, J.; Liu, J.; Schindewolf, C.; Bopp, N.E.; Aguilar, P.V.; et al. An Infectious cDNA Clone of SARS-CoV-2. Cell Host Microbe 2020, 27, 841–848.e3. [Google Scholar] [CrossRef] [PubMed]



| Virus (Strain) | Dose (log10 PFU) | No. of Dead/Total (% Mortality) | LD50 (PFU) | AST ± SEM a |
|---|---|---|---|---|
| Ban18HK20 | 2.2 | 6/6 (100) | 1.20 | 9.2 ± 0.3 |
| 1.2 | 3/4 (75) | 12.0 ± 1.0 | ||
| 0.2 | 4/6 (67) | 14.5 ± 1.3 | ||
| −0.8 | 0/6 (0) | NA | ||
| #1 N130A | 3.4 | 4/4 (100) | 1.23 | 9.0 ± 0.4 |
| 2.4 | 6/6 (100) | 10.3 ± 0.9 | ||
| 1.4 | 6/6 (100) | 11.3 ± 1.0 | ||
| 0.4 | 3/6 (50) | 12.0 ± 0.6 | ||
| #2 N207A | 3.6 | 7/7 (100) | 6.92 | 9.6 ± 0.4 |
| 2.6 | 3/3 (100) | 12.3 ± 0.3 | ||
| 1.6 | 5/6 (83) | 15.2 ± 1.6 | ||
| 0.6 | 2/6 (33) | 11.0 ± 2.0 | ||
| #3 N130Q | 3.2 | 5/5 (100) | 2.09 | 9.6 ± 0.7 |
| 2.2 | 6/6 (100) | 10.3 ± 0.6 | ||
| 1.2 | 6/6 (100) | 11.3 ± 0.7 | ||
| 0.2 | 2/6 (33) | 15.5 ± 0.5 | ||
| #4 N207Q | 3.5 | 6/6 (100) | 1.55 | 9.2 ± 0.4 |
| 2.5 | 5/5 (100) | 11.4 ± 0.8 | ||
| 1.5 | 6/6 (100) | 10.8 ± 0.5 | ||
| 0.5 | 3/5 (60) | 16.0 ± 0.6 | ||
| −0.5 | 1/6 (17) | 16.0 ± 0.0 | ||
| #5 130-132QQA | 3.4 | 6/6 (100) | 2.51 | 10.3 ± 0.5 |
| 2.4 | 7/7 (100) | 10.6 ± 0.6 | ||
| 1.4 | 5/5 (100) | 13.4 ± 0.7 | ||
| 0.4 | 3/7 (43) | 17.3 ± 1.5 | ||
| #6 207-209QQA | 3.6 | 6/6 (100) | 12.59 | 11.2 ± 0.9 |
| 2.6 | 4/4 (100) | 9.3 ± 0.8 | ||
| 1.6 | 6/6 (100) | 11.7 ± 0.4 | ||
| 0.6 | 0/4 (0) | NA | ||
| #7 N130-del | 3.9 | 6/6 (100) | 13.8 | 10.7 ± 0.8 |
| 2.9 | 6/6 (100) | 11.7 ± 0.6 | ||
| 1.9 | 5/6 (83) | 13.2 ± 0.6 | ||
| 0.9 | 3/7 (43) | 13.0 ± 0.0 | ||
| #9 N130A+N207A | 3.1 | 5/5 (100) | 1.26 | 10.0 ± 0.3 |
| 2.1 | 6/6 (100) | 10.0 ± 0.3 | ||
| 1.1 | 6/6 (100) | 11.8 ± 0.3 | ||
| 0.1 | 3/6 (50) | 15.0 ± 2.1 | ||
| #10 130-132QQA+207-209QQA | 3.7 | 6/6 (100) | 0.5 | 11.3 ± 0.3 |
| 2.7 | 5/5 (100) | 12.8 ± 0.2 | ||
| 1.7 | 6/6 (100) | 13.0 ± 0.4 | ||
| 0.7 | 6/6 (100) | 16.0 ± 1.0 | ||
| −0.3 | 3/6 (50) | 14.7 ± 1.5 | ||
| #11 N130del+207-209QQA | 5.1 | 6/6 (100) | 5754.40 | 11.3 ± 0.6 |
| 4.1 | 2/6 (33) | 16.5 ± 1.5 | ||
| 3.1 | 1/6 (17) | 12.0 ± 0.0 | ||
| 2.1 | 3/6 (50) | 16.5 ± 1.5 | ||
| 1.1 | 0/6(0) | NA | ||
| PBS | NA | 0/6(0) | NA | NA |
| Virus (Strain) | Dose (PFU) | No. of Dead/Total (% Mortality) | LD50 (PFU) | AST ± SEM a |
|---|---|---|---|---|
| Ban18HK20 | 100 | 10/11 (91) | 4.90 | 11.5 ± 0.3 |
| 10 | 8/11 (73) | 11.8 ± 0.6 | ||
| 1 | 1/10 (10) | 15.0 ± 0.0 | ||
| #1 N130A | 100 | 12/12 (100) | 3.16 | 12.8 ± 0.5 |
| 10 | 10/11 (91) | 14.2 ± 0.6 | ||
| 1 | 1/10 (10) | 14.0 ± 0.0 | ||
| #2 N207A | 100 | 10/10 (100) | 6.17 | 12.0 ± 0.6 |
| 10 | 7/11 (64) | 15.0 ± 0.8 | ||
| 1 | 0/9 (0) | NA | ||
| #3 N130Q | 100 | 10/11 (91) | 3.80 | 12.9 ± 0.5 |
| 10 | 6/10 (60) | 14.7 ± 0.4 | ||
| 1 | 4/10 (40) | 15.0 ± 0.6 | ||
| #4 N207Q | 100 | 9/12 (75) | 13.80 | 13.0 ± 0.8 |
| 10 | 4/11 (36) | 14.5 ± 1.2 | ||
| 1 | 4/11 (36) | 18.5 ± 0.3 | ||
| #5 130-132QQA | 100 | 8/10 (80) | 11.48 | 12.1 ± 0.6 |
| 10 | 2/10 (20) | 14.0 ± 0.0 | ||
| 1 | 7/11 (64) | 17.0 ± 0.5 | ||
| #6 207-209QQA | 100 | 9/10 (90) | 5.37 | 13.2 ± 0.8 |
| 10 | 5/10 (50) | 15.4 ± 0.9 | ||
| 1 | 4/11 (36) | 18.3 ± 0.3 | ||
| #7 N130-del | 100 | 2/12 (17) | >100 | 14.0 ± 0.0 |
| 10 | 5/12 (42) | 15.2 ± 0.5 | ||
| 1 | 1/11 (9) | 16.0 ± 0.0 | ||
| #9 N130A+N207A | 100 | 1/11 (9) | 67.61 | 14.0 ± 0.0 |
| 10 | 5/11 (45) | 14.0 ± 0.6 | ||
| 1 | 5/13 (38) | 16.0 ± 0.6 | ||
| #10 130-132QQA+207-209QQA | 100 | 7/12 (58) | 20.89 | 14.3 ± 1.0 |
| 10 | 5/11 (45) | 16.6 ± 0.9 | ||
| 1 | 2/11 (18) | 17.0 ± 1.0 | ||
| #11 N130del+207-209QQA | 100 | 3/11 (27) | 100 | 17.3 ± 1.3 |
| 10 | 3/12 (25) | 19.3 ± 0.3 | ||
| 1 | 2/11 (18) | 18.0 ± 1.0 | ||
| PBS | NA | 0/12 (0) | NA | NA |
| Locus/Protein | Virus Nucleotide Changes (Amino Acid Changes) | ||||
|---|---|---|---|---|---|
| Ban18HK20 | #11-P0 | #11-P1 | #11-P3 | #11-P5 | |
| NS1-112 | AAA(K) | AAA(K) | AAA(K) | AAC(N) | AAC(N) |
| NS1-129 | AAA(K) | AAA(K) | AAA(K) | ACA(T) | ACA(T) |
| NS1-130 | AAT(N) | del | del | AAA(K) | AAA(K) |
| NS1-207 | AAC(N) | CAG(Q) | CAG(Q) | CAG(Q) | CAG(Q) |
| NS1-209 | ACC(T) | GCG(A) | GCG(A) | GCG(A) | GCG(A) |
| Virus (Strain) | Dose (PFU) | No. of Dead/Total (% Mortality) | LD50 (PFU) | AST ± SEM a |
|---|---|---|---|---|
| #11-puri1 | 100 | 1/6 (17) | >100 | 13.0 ± 0.0 |
| 10 | 1/6 (17) | 19.0 ± 0.0 | ||
| 1 | 0/6 (0) | NA | ||
| #11-puri2 | 100 | 4/7 (57) | 75.86 | 15.0 ± 1.1 |
| 10 | 0/5 (0) | NA | ||
| 1 | 0/5 (0) | NA | ||
| #11-puri3 | 100 | 2/6 (33) | >100 | 17.5 ± 2.5 |
| 10 | 1/7 (14) | 19.0 ± 0.0 | ||
| 1 | 0/6 (0) | NA | ||
| #11-puri4 | 100 | 0/5 (0) | >100 | NA |
| 10 | 1/5 (20) | 18.0 ± 0.0 | ||
| 1 | 0/5 (0) | NA | ||
| #11-puri5 | 100 | 1/5 (20) | >100 | 19.0 ± 0.0 |
| 10 | 1/5 (20) | 17.0 ± 0.0 | ||
| 1 | 0/5 (0) | NA | ||
| #11-puri6 | 100 | 1/6 (17) | >100 | 20.0 ± 0.0 |
| 10 | 1/6 (17) | 20.0 ± 0.0 | ||
| 1 | 0/7 (0) | NA | ||
| #11-puri7 | 100 | 0/6 (0) | >100 | NA |
| 10 | 0/5 (0) | NA | ||
| 1 | 0/6 (0) | NA | ||
| #11-puri8 | 100 | 4/6 (67) | 56.23 | 17.8 ± 0.8 |
| 10 | 0/6 (0) | NA | ||
| 1 | 0/6 (0) | NA | ||
| #11-puri9 | 100 | 0/5 (0) | >100 | NA |
| 10 | 0/6 (0) | NA | ||
| 1 | 0/5 (0) | NA | ||
| #11-puri10 | 100 | 0/13(0) | >100 | NA |
| 10 | 2/12(17) | 19.5 ± 0.5 | ||
| 1 | 1/11(9) | 19.0 ± 0.0 | ||
| PBS | NA | 0/6 (0) | NA | NA |
| Locus/Protein | Virus Nucleotide Changes (Amino Acid Changes) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ban18HK20 | #11 | #11-Puri1 | #11-Puri2 | #11-Puri3 | #11-Puri4 | #11-Puri5 | #11-Puri6 | #11-Puri7 | #11-Puri8 | #11-Puri9 | #11-Puri10 | |
| NS1-112 | AAA(K) | ··· | AAC(N) | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· | AAC(N) |
| NS1-129 | AAA(K) | ··· | ··· | ACA(T) | ACA(T) | ACA(T) | ACA(T) | ACA(T) | ACA(T) | ACA(T) | ACA(T) | ··· |
| NS1-130 | AAT(N) | del | del | AAA(K) | AAA(K) | AAA(K) | AAA(K) | AAA(K) | AAA(K) | AAA(K) | AAA(K) | del |
| NS1-207 | AAC(N) | CAG(Q) | CAG(Q) | CAG(Q) | CAG(Q) | CAG(Q) | CAG(Q) | CAG(Q) | CAG(Q) | CAG(Q) | CAG(Q) | CAG(Q) |
| NS1-209 | ACC(T) | GCG(A) | GCG(A) | GCG(A) | GCG(A) | GCG(A) | GCG(A) | GCG(A) | GCG(A) | GCG(A) | GCG(A) | GCG(A) |
| NS2A-99 | GAG(E) | ··· | GAT(D) | ··· | ··· | ··· | ··· | ··· | GAT(D) | ··· | GAT(D) | ··· |
| NS3-4 | CTG(L) | ··· | CTA(L) | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· |
| NS4A-20 | AGG(R) | ··· | AGA(R) | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· | AGA(R) |
| NS4B-197 | CCA(P) | ··· | CCT(P) | CCT(P) | CCT(P) | CCT(P) | CCT(P) | CCT(P) | CCT(P) | CCT(P) | CCT(P) | ··· |
| NS5-362 | AGA(R) | ··· | ··· | CGA(R) | ··· | CGA(R) | CGA(R) | CGA(R) | CGA(R) | CGA(R) | CGA(R) | ··· |
| NS5-699 | AAG(K) | ··· | ··· | ··· | AAT(N) | ··· | ··· | ··· | ··· | ··· | ··· | ··· |
| NS5-827 | GAC(D) | ··· | ··· | ··· | GAT(D) | ··· | ··· | ··· | ··· | ··· | ··· | ··· |
| Virus (Strain) | Dose (PFU) | No. of Dead/Total (% Mortality) | LD50 (PFU) | AST ± SEM a |
|---|---|---|---|---|
| Ban18HK20 (Mut-NS1-K129T, N130K, N207Q, T209A, NS2A-E99D) | 100 | 0/12 (0) | >100 | NA |
| 10 | 0/12 (0) | NA | ||
| 1 | 0/13 (0) | NA | ||
| Ban18HK20 | 100 | 12/12 (100) | 19.05 | 10.5 ± 0.3 |
| 10 | 3/12 (25) | 15.3 ± 0.9 | ||
| 1 | 1/13 (8) | 14.0 ± 0.0 | ||
| PBS | NA | 0/12 (0) | NA | NA |
| Virus (Strain) | Dose (PFU) | No. of Dead/Total (% Mortality) | LD50 (PFU) | AST ± SEM a |
|---|---|---|---|---|
| rDENV4/1(N207Q+T209A+E99D) | 100 | 14/14 (100) | 2.24 | 9.4 ± 0.2 |
| 10 | 8/11 (73) | 11.3 ± 0.4 | ||
| 1 | 5/12 (42) | 11.8 ± 0.4 | ||
| rDENV4/1(K129T+N130K+N207Q+T209A+E99D) | 100 | 0/19 (0) | >100 | NA |
| 10 | 0/15 (0) | NA | ||
| 1 | 0/20 (0) | NA | ||
| DENV4/1 | 100 | 15/15 (100) | 2.51 | 8.7 ± 0.2 |
| 10 | 15/16 (94) | 10.1 ± 0.2 | ||
| 1 | 3/14 (21) | 10.7 ± 0.3 | ||
| PBS | NA | 0/12 (0) | NA | NA |
| Locus/Protein | Virus Nucleotide Changes (Amino Acid Changes) | ||||||
|---|---|---|---|---|---|---|---|
| Ban18HK20 | #11 | #11-Puri9 | #11-Puri9-P3 | #11-Puri9-P5 | #11-Puri9 (Rescued)-P3 | #11-Puri9 (Rescued)-P5 | |
| NS1-129 | AAA(K) | ··· | ACA(T) | ACA(T) | ACA(T) | ACA(T) | ACA(T) |
| NS1-130 | AAT(N) | del | AAA(K) | AAA(K) | AAA(K) | AAA(K) | AAA(K) |
| NS1-207 | AAC(N) | CAG(Q) | CAG(Q) | CAG(Q) | CAG(Q) | CAG(Q) | CAG(Q) |
| NS1-209 | ACC(T) | GCG(A) | GCG(A) | GCG(A) | GCG(A) | GCG(A) | GCG(A) |
| NS2A-99 | GAG(E) | ··· | GAT(D) | GAT(D) | GAT(D) | GAT(D) | GAT(D) |
| NS4B-197 | CCA(P) | ··· | CCT(P) | CCT(P) | CCT(P) | CCA(P) | CCA(P) |
| NS5-362 | AGA(R) | ··· | CGA(R) | CGA(R) | CGA(R) | AGA(R) | AGA(R) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, E.; Li, M.; Liu, X.; Hu, K.; Liu, L.; Zhang, Z.; Li, X.; Peng, Q.; Li, Y. NS1 Protein N-Linked Glycosylation Site Affects the Virulence and Pathogenesis of Dengue Virus. Vaccines 2023, 11, 959. https://doi.org/10.3390/vaccines11050959
Fang E, Li M, Liu X, Hu K, Liu L, Zhang Z, Li X, Peng Q, Li Y. NS1 Protein N-Linked Glycosylation Site Affects the Virulence and Pathogenesis of Dengue Virus. Vaccines. 2023; 11(5):959. https://doi.org/10.3390/vaccines11050959
Chicago/Turabian StyleFang, Enyue, Miao Li, Xiaohui Liu, Kongxin Hu, Lijuan Liu, Zelun Zhang, Xingxing Li, Qinhua Peng, and Yuhua Li. 2023. "NS1 Protein N-Linked Glycosylation Site Affects the Virulence and Pathogenesis of Dengue Virus" Vaccines 11, no. 5: 959. https://doi.org/10.3390/vaccines11050959





