Development and Appraisal of a Web-Based Decision Aid for HPV Vaccination for Young Adults and Parents of Children in Israel—A Quasi-Experimental Study
Abstract
1. Introduction
2. Methods
2.1. Setting
2.2. Participants
2.3. Ethical Considerations
2.4. Study Design
2.5. Procedure
2.6. Measures
2.7. Data Analysis
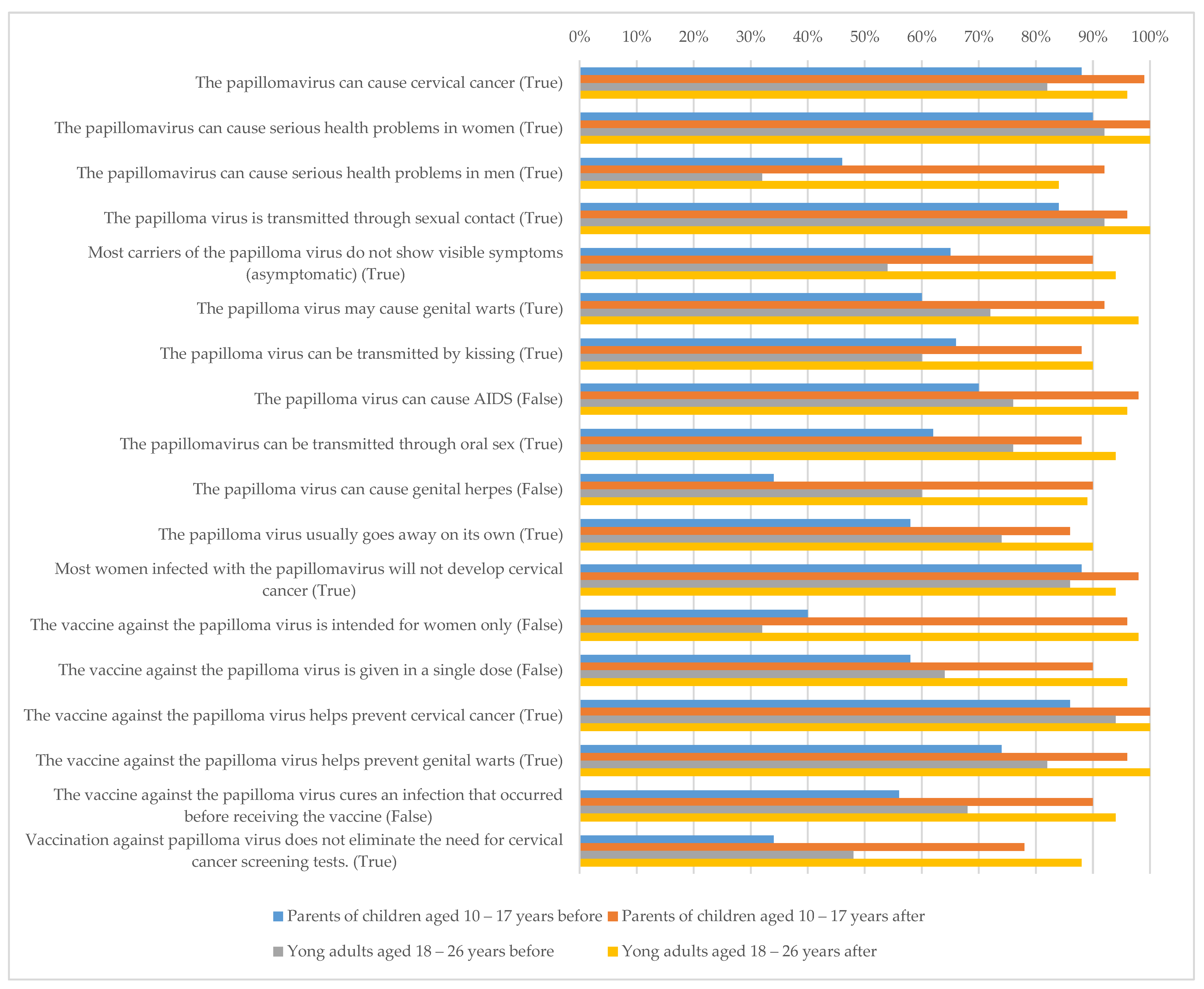
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| HPV | Human papillomavirus |
| IPDAS | International Patient Decision Aid Standards |
| WHO | World Health Organization |
| ILS | Israeli Shekel |
| DCS | Decisional Conflict Scale |
| DSE | Decision Self Efficacy |
References
- Harper, D.M.; DeMars, L.R. HPV vaccines—A review of the first decade. Gynecol. Oncol. 2017, 146, 196–204. [Google Scholar] [CrossRef]
- Daniels, V.; Saxena, K.; Roberts, C.; Kothari, S.; Corman, S.; Yao, L.; Niccolai, L. Impact of reduced human papillomavirus vaccination coverage rates due to COVID-19 in the United States: A model based analysis. Vaccine 2021, 39, 2731–2735. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, P.; Faivre, P.; Lopalco, P.L.; Joura, E.A.; Bergroth, T.; Varga, S.; Gemayel, N.; Drury, R. The status of human papillomavirus vaccination recommendation, funding, and coverage in WHO Europe countries (2018–2019). Expert Rev. Vaccines 2020, 19, 1073–1083. [Google Scholar] [CrossRef]
- Bogani, G.; Leone Roberti Maggiore, U.; Signorelli, M.; Martinelli, F.; Ditto, A.; Sabatucci, I.; Mosca, L.; Lorusso, D.; Raspagliesi, F. The role of human papillomavirus vaccines in cervical cancer: Prevention and treatment. Crit. Rev. Oncol. Hematol. 2018, 122, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Strategic Advisory Group of Experts (SAGE) on Immunization. Strategic Advisory Group of Experts (SAGE) Working Group on Potential Contribution of HPV Vaccines and Immunization towards Cervical Cancer Elimination. 2022. Available online: https://cdn.who.int/media/docs/default-source/immunization/position_paper_documents/human-papillomavirus-(hpv)/hpv-background-document--report-march-2022.pdf?sfvrsn=b600e252_1 (accessed on 25 March 2023).
- Bruni, L.; Diaz, M.; Barrionuevo-Rosas, L.; Herrero, R.; Bray, F.; Bosch, F.X.; de Sanjosé, S.; Castellsagué, X. Global estimates of human papillomavirus vaccination coverage by region and income level: A pooled analysis. Lancet Glob. Health 2016, 4, e453–e463. [Google Scholar] [CrossRef]
- Orenstein, W.A.; Gellin, B.G.; Beigi, R.H.; Despres, S.; Lynfield, R.; Maldonado, Y.; Mouton, C.; Rawlins, W.; Rothholz, M.C.; Smith, N.; et al. Overcoming barriers to low hpv vaccine uptake in the United States: Recommendations from the national vaccine advisory committee. Public Health Rep. 2016, 131, 17–25. [Google Scholar]
- Khoo, S.P.; Adriana, N.; Ridzuan, M.; Rajasuriar, R.; Nasir, H.; Gravitt, P.; Ng, C.W.; Ling, Y.; Id, W. Changes in genital Human Papillomavirus (HPV) prevalence among urban females a decade after the Malaysian HPV vaccination program. PLoS ONE 2022, 17, e0278477. [Google Scholar] [CrossRef]
- Khamisy-Farah, R.; Adawi, M.; Jeries-Ghantous, H.; Bornstein, J.; Farah, R.; Bragazzi, N.L.; Odeh, M. Knowledge of human papillomavirus (HPV), attitudes and practices towards anti-HPV vaccination among Israeli pediatricians, gynecologists, and internal medicine doctors: Development and validation of an ad hoc questionnaire. Vaccines 2019, 7, 157. [Google Scholar] [CrossRef]
- Shavit, O.; Roura, E.; Barchana, M.; Diaz, M.; Bornstein, J. Burden of Human Papillomavirus Infection and Related Diseases in Israel. Vaccine 2013, 31 (Suppl. S8), 132–141. [Google Scholar] [CrossRef]
- Lavie, M.; Lavie, I.; Laskov, I.; Cohen, A.; Grisaru, D.; Grisaru-Soen, G.; Michaan, N. Impact of COVID-19 Pandemic on Human Papillomavirus Vaccine Uptake in Israel. J. Low. Genit. Tract Dis. 2023, 27, 168–172. [Google Scholar] [CrossRef]
- Shahbari, N.A.E.; Gesser-Edelsburg, A.; Davidovitch, N.; Brammli-Greenberg, S.; Grifat, R.; Mesch, G.S. Factors associated with seasonal influenza and HPV vaccination uptake among different ethnic groups in Arab and Jewish society in Israel. Int. J. Equity Health 2021, 20, 201. [Google Scholar] [CrossRef]
- Shibli, R.; Rishpon, S. The factors associated with maternal consent to human papillomavirus vaccination among adolescents in Israel. Hum. Vaccines Immunother. 2019, 15, 3009–3015. [Google Scholar] [CrossRef]
- Highet, M.; Jessiman-Perreault, G.; Hilton, E.; Law, G.; Allen-Scott, L. Understanding the decision to immunize: Insights into the information needs and priorities of people who have utilized an online human papillomavirus (HPV) vaccine decision aid tool. Can. J. Public Health 2021, 112, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Shahbari, N.A.E.; Gesser-Edelsburg, A.; Mesch, G.S. Case of paradoxical cultural sensitivity: Mixed method study of web-based health informational materials about the human papillomavirus vaccine in Israel. J. Med. Internet Res. 2019, 21, e13373. [Google Scholar] [CrossRef]
- O’Connor, A. Using patient decision aids to promote evidence-based decision making. ACP J. Club. 2001, 135, 100–102. [Google Scholar] [CrossRef]
- Stacey, D.; Légaré, F.; Lewis, K.; Barry, M.J.; Bennett, C.L.; Eden, K.B.; Holmes-Rovner, M.; Llewellyn-Thomas, H.; Lyddiatt, A.; Thomson, R.; et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst. Rev. 2017, 2017, CD001431. [Google Scholar] [CrossRef] [PubMed]
- Stacey, D.; Légaré, F.; Boland, L.; Lewis, K.B.; Loiselle, M.C.; Hoefel, L.; Garvelink, M.; O’Connor, A. 20th Anniversary Ottawa Decision Support Framework: Part 3 Overview of Systematic Reviews and Updated Framework. Med. Decis. Mak. 2020, 40, 379–398. [Google Scholar] [CrossRef]
- Martin, R.W.; Brogård Andersen, S.; O’Brien, M.A.; Bravo, P.; Hoffmann, T.; Olling, K.; Shepherd, H.L.; Dankl, K.; Stacey, D.; Dahl Steffensen, K. Providing Balanced Information about Options in Patient Decision Aids: An Update from the International Patient Decision Aid Standards. Med. Decis. Mak. 2021, 41, 780–800. [Google Scholar] [CrossRef]
- Wang, L.D.L.; Lam, W.W.T.; Fielding, R. Development and pre/post testing of a decision aid supporting Chinese parental and young women’s HPV vaccination decision-making. Women Health 2020, 60, 330–340. [Google Scholar] [CrossRef]
- Elwyn, G.; O’Connor, A.; Stacey, D.; Volk, R.; Edwards, A.; Coulter, A.; Thomson, R.; Barratt, A.; Butow, P.; Barry, M.; et al. Developing a quality criteria framework for patient decision aids: Online international Delphi consensus process. Br. Med. J. 2006, 333, 417–419. [Google Scholar] [CrossRef]
- Sepucha, K.R.; Abhyankar, P.; Hoffman, A.S.; Bekker, H.L.; LeBlanc, A.; Levin, C.A.; Ropka, M.; Shaffer, V.A.; Sheridan, S.L.; Stacey, D.; et al. Standards for UNiversal reporting of patient Decision Aid Evaluation studies: The development of SUNDAE Checklist. BMJ Qual. Saf. 2018, 27, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Garvelink, M.M.; Boland, L.; Klein, K.; Nguyen, D.V.; Menear, M.; Bekker, H.L.; Eden, K.B.; LeBlanc, A.; O’Connor, A.M.; Stacey, D.; et al. Decisional Conflict Scale Findings among Patients and Surrogates Making Health Decisions: Part II of an Anniversary Review. Med. Decis. Mak. 2019, 39, 315–326. [Google Scholar] [CrossRef]
- O’Connor, A.M. User Manual—Decisional Conflict Scale; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 1993; Available online: Https://DecisionaidOhriCa/Eval_DcsHtml (accessed on 20 March 2023).
- O’Connor, A.M. User Manual—Decision Self-Efficacy Scale [Document on the Internet]; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 1995; (Modified 2020, Assessed on 1 April 2023). [Google Scholar]
- Barry, M.J.; Härter, M.; Househ, M.; Steffensen, K.D.; Stacey, D. What can we learn from rapidly developed patient decision aids produced during the COVID-19 pandemic? BMJ 2022, 378, e071530. [Google Scholar] [CrossRef]
- Kryworuchko, J.; Stacey, D.; Bennett, C.; Graham, I.D. Appraisal of primary outcome measures used in trials of patient decision support. Patient Educ. Couns. 2008, 73, 497–503. [Google Scholar] [CrossRef]
- Serrano, B.; Brotons, M.; Bosch, F.X.; Bruni, L. Epidemiology and burden of HPV-related disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, J.M.L.; Bloem, P.N. Population-based HPV vaccination programmes are safe and effective: 2017 update and the impetus for achieving better global coverage. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 42–58. [Google Scholar] [CrossRef]
- Fiks, A.G.; Hughes, C.C.; Gafen, A.; Guevara, J.P.; Barg, F.K. Contrasting parents’ and pediatricians’ perspectives on shared decision-making in ADHD. Pediatrics 2011, 127, e188–e196. [Google Scholar] [CrossRef] [PubMed]
- Grandahl, M.; Nevéus, T. Barriers towards HPV vaccinations for boys and young men: A narrative review. Viruses 2021, 13, 1644. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.; Shapiro, G.K.; Brown, C.A.; Dube, E.; Ogilvie, G.; Rosberger, Z. “I didn’t even know boys could get the vaccine”: Parents’ reasons for human papillomavirus (HPV) vaccination decision making for their sons. Psychooncology 2015, 24, 1316–1323. [Google Scholar] [CrossRef]
- Dempsey, A.F.; Zimet, G.D.; Davis, R.L.; Koutsky, L. Factors that are associated with parental acceptance of human papillomavirus vaccines: A randomized intervention study of written information about HPV. Pediatrics 2006, 117, 1486–1493. [Google Scholar] [CrossRef]
- Bruel, S.; Leclercq, T.; Ginzarly, M.; Botelho-Nevers, E.; Frappé, P.; Gagneux-Brunon, A. Patient decision aid in vaccination: A systematic review of the literature. Expert Rev. Vaccines 2020, 19, 305–311. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.M. Validation of a Decisional Conflict Scale. Med. Decis. Mak. 1995, 15, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Vujovich-Dunn, C.; Kaufman, J.; King, C.; Skinner, S.R.; Wand, H.; Guy, R.; Leask, J. A systematic review and meta-analysis of effectiveness of decision aids for vaccination decision-making. Vaccine 2021, 39, 3655–3665. [Google Scholar] [CrossRef] [PubMed]
- Wroe, A.L.; Turner, N.; Owens, R.G. Evaluation of a decision-making aid for parents regarding childhood immunizations. Health Psychol. 2005, 24, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Shourie, S.; Jackson, C.; Cheater, F.M.; Bekker, H.L.; Edlin, R.; Tubeuf, S.; Harrison, W.; McAleese, E.; Schweiger, M.; Bleasby, B.; et al. A cluster randomised controlled trial of a web based decision aid to support parents’ decisions about their child’s Measles Mumps and Rubella (MMR) vaccination. Vaccine 2013, 31, 6003–6010. [Google Scholar] [CrossRef] [PubMed]
- Witteman, H.O.; Chipenda Dansokho, S.; Exe, N.; Dupuis, A.; Provencher, T.; Zikmund-Fisher, B.J. Risk Communication, Values Clarification, and Vaccination Decisions. Risk Anal. 2015, 35, 1801–1819. [Google Scholar] [CrossRef]
- Wegwarth, O.; Kurzenhäuser-Carstens, S.; Gigerenzer, G. Overcoming the knowledge-behavior gap: The effect of evidence-based HPV vaccination leaflets on understanding, intention, and actual vaccination decision. Vaccine 2014, 32, 1388–1393. [Google Scholar] [CrossRef]
- Gendler, Y.; Ofri, L. Investigating the influence of vaccine literacy, vaccine perception and vaccine hesitancy on Israeli parents’ acceptance of the COVID-19 vaccine for their children: A cross-sectional study. Vaccines 2021, 9, 1391. [Google Scholar] [CrossRef]
| Variable | n (%) or Mean (SD) |
|---|---|
| Gender | |
| Male | 48 (40%) |
| Female | 72 (60%) |
| Age (years) | 43.6 (4.3) |
| Population group | |
| Jews | 84 (70%) |
| Muslim-Arabs | 36 (30%) |
| Religious affiliation | |
| Secular | 58 (48.3%) |
| Traditional | 12 (10%) |
| Religious | 32 (26.7%) |
| Orthodox | 18 (15%) |
| Highest education level achieved | |
| Primary education | 14 (11.7%) |
| Secondary education | 25 (20.8%) |
| Diploma | 38 (31.7%) |
| Academic degree | 43 (35.8%) |
| Marital status | |
| Married | 116 (96.7%) |
| Divorced/separated | 4 (3.3%) |
| No. of children | |
| 1 | 18 (15%) |
| 2 | 46 (38.3%) |
| 3 | 34 (28.3%) |
| 4 and above | 22 (18.4%) |
| Heard of HPV vaccine before | 102 (85%) |
| Variable | n (%) or Mean (SD) |
|---|---|
| Gender | |
| Male | 52 (32.5%) |
| Female | 108 (67.5%) |
| Age (years) | 22.6 (2.8) |
| Population group | |
| Jews | 118 (73.8%) |
| Muslim-Arabs | 42 (26.2%) |
| Religious affiliation | |
| Secular | 87 (54.4%) |
| Traditional | 18 (11.3%) |
| Religious | 44 (27.5%) |
| Orthodox | 11 (6.8%) |
| Highest education level achieved | |
| Primary education | 20 (12.5%) |
| Secondary education | 88 (55%) |
| Diploma | 35 (21.9%) |
| Academic degree | 17 (10.6%) |
| Marital status | |
| Single | 122 (76.2%) |
| Married | 38 (23.8%) |
| Monogamous relationship | |
| Yes | 74 (46.3%) |
| No | 86 (53.7%) |
| Heard of HPV vaccine before | 145 (90.6%) |
| Parents of Children Aged 10–17 Years | Young Adults Aged 18–26 Years | |
|---|---|---|
| n = 120 (%) | n = 160 (%) | |
| The decision aid is easy to understand | ||
| Agree/strongly agree | 112 (93.3%) | 148 (92.5%) |
| Neutral | 5 (4.2%) | 12 (7.5%) |
| Disagree/strongly disagree | 3 (2.5%) | 0 |
| Length of the decision aid | ||
| Too long | 8 (6.7%) | 38 (23.8%) |
| Too short | 8 (6.7%) | 6 (3.7%) |
| Just right | 104 (86.6%) | 116 (72.5%) |
| Amount of information | ||
| Too much information | 10 (8.3%) | 21 (13.1%) |
| Too little information | 12 (10%) | 17 (10.6%) |
| Just right | 98 (81.7%) | 122 (76.3%) |
| Information presented | ||
| Encourage to receive the HPV vaccine | 10 (8.3%) | 18 (11.2%) |
| Discourage to receive the HPV vaccine | 0 | 0 |
| Balanced | 110 (91.7) | 142 (88.8%) |
| Others who are making decisions about HPV vaccination may find this decision aid helpful | ||
| Agree/strongly agree | 114 (95%) | 150 (93.8%) |
| Neutral | 2 (1.7%) | 8 (5%) |
| Disagree/strongly disagree | 4 (3.3%) | 2 (1.2%) |
| Parents of Children Aged 10–17 Years (n = 120) | Young Adults Aged 18–26 Years (n = 160) | |||||||
|---|---|---|---|---|---|---|---|---|
| Before | After | p | d # | Before | After | p | d # | |
| Vaccine hesitancy | 6.1 (3.4) | 4.5 (3.1) | <0.001 | 0.49 | 4.9 (3.2) | 2.6 (1.9) | <0.001 | 0.87 |
| Vaccine safety | 6.9 (3.1) | 8.4 (2.2) | <0.001 | 0.56 | 7.1 (2.5) | 8.6 (1.8) | 0.002 | 0.69 |
| Vaccine effectiveness | 6.7 (2.5) | 7.6 (2.4) | 0.02 | 0.36 | 6.4 (3.1) | 8.2 (2.8) | <0.001 | 0.61 |
| Decisional conflict | 40.6 (18.8) | 16.9 (11.2) | <0.001 | 1.52 | 39.2(13.6) | 14.4 (6.9) | <0.001 | 2.29 |
| Decision self-efficacy | 60.1 (14.7) | 81.9 (16.5) | <0.001 | 1.41 | 55.2 (18.4) | 78.8 (16.1) | <0.001 | 1.37 |
| Vaccination intentions | 46% | 75% | <0.001 | 64% | 92% | <0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gendler, Y. Development and Appraisal of a Web-Based Decision Aid for HPV Vaccination for Young Adults and Parents of Children in Israel—A Quasi-Experimental Study. Vaccines 2023, 11, 1038. https://doi.org/10.3390/vaccines11061038
Gendler Y. Development and Appraisal of a Web-Based Decision Aid for HPV Vaccination for Young Adults and Parents of Children in Israel—A Quasi-Experimental Study. Vaccines. 2023; 11(6):1038. https://doi.org/10.3390/vaccines11061038
Chicago/Turabian StyleGendler, Yulia. 2023. "Development and Appraisal of a Web-Based Decision Aid for HPV Vaccination for Young Adults and Parents of Children in Israel—A Quasi-Experimental Study" Vaccines 11, no. 6: 1038. https://doi.org/10.3390/vaccines11061038
APA StyleGendler, Y. (2023). Development and Appraisal of a Web-Based Decision Aid for HPV Vaccination for Young Adults and Parents of Children in Israel—A Quasi-Experimental Study. Vaccines, 11(6), 1038. https://doi.org/10.3390/vaccines11061038






