Cholangiocarcinoma in the Era of Immunotherapy
Abstract
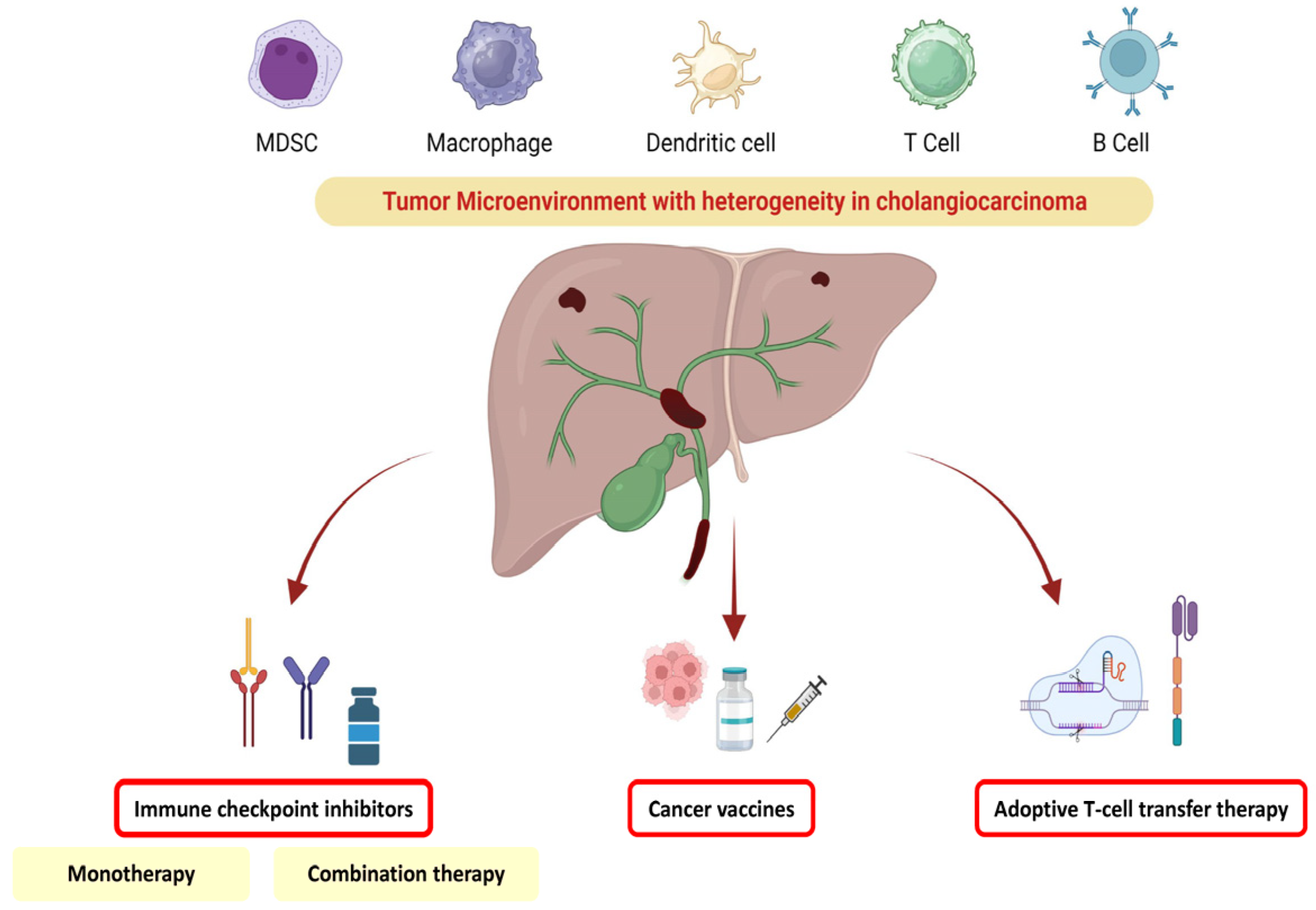
:1. Introduction
2. Literature Search
3. Tumoral Heterogeneity in Cholangiocarcinoma and Impact on Therapeutic Management
4. ICI Immunotherapy
4.1. Keynote 28
4.2. Keynote 158
4.3. Nivolumab
4.4. Durvalumab
4.5. Bintrafusp Alfa
5. Combination Therapies
5.1. ICI Combination Therapy
5.2. ICIs and Chemotherapy
5.3. ICIs and Antiangiogenic Agents
6. Adoptive Cell Therapy
6.1. Chimeric Antigen Receptor T-Cell (CAR-T Cell)
6.2. Tumor-Infiltrating Lymphocytes (TILs)
7. Vaccine Therapy
8. Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef] [Green Version]
- Greten, T.F.; Schwabe, R.; Bardeesy, N.; Ma, L.; Goyal, L.; Kelley, R.K.; Wang, X.W. Immunology and immunotherapy of cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2023, 22, 349–365. [Google Scholar] [CrossRef]
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Hepatol. 2020, 72, 95–103. [Google Scholar] [CrossRef]
- Meserve, J.; Facciorusso, A.; Holmer, A.K.; Annese, V.; Sandborn, W.J.; Singh, S. Systematic review with meta-analysis: Safety and tolerability of immune checkpoint inhibitors in patients with pre-existing inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2021, 53, 374–382. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okusaka, T.; Nakachi, K.; Fukutomi, A.; Mizuno, N.; Ohkawa, S.; Funakoshi, A.; Nagino, M.; Kondo, S.; Nagaoka, S.; Funai, J.; et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: A comparative multicentre study in Japan. Br. J. Cancer 2010, 103, 469–474. [Google Scholar] [CrossRef] [Green Version]
- Facciorusso, A.; Del Prete, V.; Crucinio, N.; Muscatiello, N.; Carr, B.I.; Di Leo, A.; Barone, M. Angiotensin receptor blockers improve survival outcomes after radiofrequency ablation in hepatocarcinoma patients. J. Gastroenterol. Hepatol. 2015, 30, 1643–1650. [Google Scholar] [CrossRef]
- Ito, T.; Sakurai-Yageta, M.; Goto, A.; Pairojkul, C.; Yongvanit, P.; Murakami, Y. Genomic and transcriptional alterations of cholangiocarcinoma. J. Hepatobiliary Pancreat Sci. 2014, 21, 380–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.M.; Esmail, A.; Raza, A.; Dacha, S.; Abdelrahim, M. Timeline of FDA-Approved Targeted Therapy for Cholangiocarcinoma. Cancers 2022, 14, 2641. [Google Scholar] [CrossRef] [PubMed]
- Elvevi, A.; Laffusa, A.; Scaravaglio, M.; Rossi, R.E.; Longarini, R.; Stagno, A.M.; Cristoferi, L.; Ciaccio, A.; Cortinovis, D.L.; Invernizzi, P.; et al. Clinical treatment of cholangiocarcinoma: An updated comprehensive review. Ann. Hepatol. 2022, 27, 100737. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Larrañaga, M.; González-López, E.; Roa-Bautista, A.; Rodrigues, P.M.; Díaz-González, Á.; Banales, J.M.; López-Hoyos, M.; Santos-Laso, A.; Crespo, J. Immune Checkpoint Inhibitors: The Emerging Cornerstone in Cholangiocarcinoma Therapy? Liver Cancer 2021, 10, 545–560. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Gaggar, S.; Gögenur, I. Cancer-Associated Fibroblasts and Tumor-Associated Macrophages in Cancer and Cancer Immunotherapy. Front. Oncol. 2021, 11, 668731. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, C.; Lu, S.; Xu, Y.; Li, Z.; Jiang, H.; Ma, Y. Tumor-associated macrophages in cholangiocarcinoma: Complex interplay and potential therapeutic target. EBioMedicine 2021, 67, 103375. [Google Scholar] [CrossRef]
- Martin-Serrano, M.A.; Kepecs, B.; Torres-Martin, M.; Bramel, E.R.; Haber, P.K.; Merritt, E.; Rialdi, A.; Param, N.J.; Maeda, M.; Lindblad, K.E.; et al. Novel microenvironment-based classification of intrahepatic cholangiocarcinoma with therapeutic implications. Gut 2023, 72, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Job, S.; Rapoud, D.; Dos Santos, A.; Gonzalez, P.; Desterke, C.; Pascal, G.; Elarouci, N.; Ayadi, M.; Adam, R.; Azoulay, D.; et al. Identification of Four Immune Subtypes Characterized by Distinct Composition and Functions of Tumor Microenvironment in Intrahepatic Cholangiocarcinoma. Hepatology 2020, 72, 965–981. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients With Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888–894. [Google Scholar] [CrossRef]
- Monge, C.; Pehrsson, E.C.; Xie, C.; Duffy, A.G.; Mabry, D.; Wood, B.J.; Kleiner, D.E.; Steinberg, S.M.; Figg, W.D.; Redd, B.; et al. A Phase II Study of Pembrolizumab in Combination with Capecitabine and Oxaliplatin with Molecular Profiling in Patients with Advanced Biliary Tract Carcinoma. Oncologist 2022, 27, e273–e285. [Google Scholar] [CrossRef]
- Piha-Paul, S.A.; Oh, D.Y.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int. J. Cancer 2020, 147, 2190–2198. [Google Scholar] [CrossRef]
- Ueno, M.; Ikeda, M.; Morizane, C.; Kobayashi, S.; Ohno, I.; Kondo, S.; Okano, N.; Kimura, K.; Asada, S.; Namba, Y.; et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: A non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol. Hepatol. 2019, 4, 611–621. [Google Scholar] [CrossRef]
- Facciorusso, A.; Abd El Aziz, M.A.; Tartaglia, N.; Ramai, D.; Mohan, B.P.; Cotsoglou, C.; Pusceddu, S.; Giacomelli, L.; Ambrosi, A.; Sacco, R. Microwave Ablation Versus Radiofrequency Ablation for Treatment of Hepatocellular Carcinoma: A Meta-Analysis of Randomized Controlled Trials. Cancers 2020, 12, 3796. [Google Scholar] [CrossRef] [PubMed]
- Doki, Y.; Ueno, M.; Hsu, C.H.; Oh, D.Y.; Park, K.; Yamamoto, N.; Ioka, T.; Hara, H.; Hayama, M.; Nii, M.; et al. Tolerability and efficacy of durvalumab, either as monotherapy or in combination with tremelimumab, in patients from Asia with advanced biliary tract, esophageal, or head-and-neck cancer. Cancer Med. 2022, 11, 2550–2560. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Oh, D.Y.; Choi, H.J.; Kudo, M.; Ueno, M.; Kondo, S.; Chen, L.T.; Osada, M.; Helwig, C.; Dussault, I.; et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. J. Immunother. Cancer 2020, 8, e000564. [Google Scholar] [CrossRef] [PubMed]
- Trifylli, E.M.; Koustas, E.; Papadopoulos, N.; Sarantis, P.; Aloizos, G.; Damaskos, C.; Garmpis, N.; Garmpi, A.; Karamouzis, M.V. An Insight into the Novel Immunotherapy and Targeted Therapeutic Strategies for Hepatocellular Carcinoma and Cholangiocarcinoma. Life 2022, 12, 665. [Google Scholar] [CrossRef]
- Kang, S.; El-Rayes, B.F.; Akce, M. Evolving Role of Immunotherapy in Advanced Biliary Tract Cancers. Cancers 2022, 14, 1748. [Google Scholar] [CrossRef]
- Song, X.; Hu, Y.; Li, Y.; Shao, R.; Liu, F.; Liu, Y. Overview of current targeted therapy in gallbladder cancer. Signal Transduct. Target. Ther. 2020, 5, 230. [Google Scholar] [CrossRef]
- Lee, H.T.; Lee, S.H.; Heo, Y.S. Molecular Interactions of Antibody Drugs Targeting PD-1, PD-L1, and CTLA-4 in Immuno-Oncology. Molecules 2019, 24, 1190. [Google Scholar] [CrossRef] [Green Version]
- Klein, O.; Kee, D.; Nagrial, A.; Markman, B.; Underhill, C.; Michael, M.; Jackett, L.; Lum, C.; Behren, A.; Palmer, J.; et al. Evaluation of Combination Nivolumab and Ipilimumab Immunotherapy in Patients With Advanced Biliary Tract Cancers: Subgroup Analysis of a Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2020, 6, 1405–1409. [Google Scholar] [CrossRef]
- Sahai, V.; Griffith, K.A.; Beg, M.S.; Shaib, W.L.; Mahalingam, D.; Zhen, D.B.; Deming, D.A.; Zalupski, M.M. A randomized phase 2 trial of nivolumab, gemcitabine, and cisplatin or nivolumab and ipilimumab in previously untreated advanced biliary cancer: BilT-01. Cancer 2022, 128, 3523–3530. [Google Scholar] [CrossRef]
- Ramai, D.; Singh, J.; Lester, J.; Khan, S.R.; Chandan, S.; Tartaglia, N.; Ambrosi, A.; Serviddio, G.; Facciorusso, A. Systematic review with meta-analysis: Bariatric surgery reduces the incidence of hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2021, 53, 977–984. [Google Scholar] [CrossRef]
- Ciardiello, D.; Maiorano, B.A.; Parente, P.; Rodriquenz, M.G.; Latiano, T.P.; Chiarazzo, C.; Pazienza, V.; Guerrera, L.P.; Amoruso, B.; Normanno, N.; et al. Immunotherapy for Biliary Tract Cancer in the Era of Precision Medicine: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 820. [Google Scholar] [CrossRef]
- Feng, K.; Liu, Y.; Zhao, Y.; Yang, Q.; Dong, L.; Liu, J.; Li, X.; Zhao, Z.; Mei, Q.; Han, W. Efficacy and biomarker analysis of nivolumab plus gemcitabine and cisplatin in patients with unresectable or metastatic biliary tract cancers: Results from a phase II study. J. Immunother. Cancer 2020, 8, e000367. [Google Scholar] [CrossRef]
- Chen, X.; Wu, X.; Wu, H.; Gu, Y.; Shao, Y.; Shao, Q.; Zhu, F.; Li, X.; Qian, X.; Hu, J.; et al. Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: A single-arm, open-label, phase II trial. J. Immunother. Cancer 2020, 8, e001240. [Google Scholar] [CrossRef]
- Chen, X.; Qin, S.; Gu, S.; Ren, Z.; Chen, Z.; Xiong, J.; Liu, Y.; Meng, Z.; Zhang, X.; Wang, L.; et al. Camrelizumab plus oxaliplatin-based chemotherapy as first-line therapy for advanced biliary tract cancer: A multicenter, phase 2 trial. Int. J. Cancer 2021, 149, 1944–1954. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.; Zhang, Y.; Liu, T.; Si, H.; Wang, Z.; Yan, H.; Qian, N.; Dai, G. PD-1 Inhibitors Could Improve the Efficacy of Chemotherapy as First-Line Treatment in Biliary Tract Cancers: A Propensity Score Matching Based Analysis. Front. Oncol. 2021, 11, 648068. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; He, A.R.; Qin, S.; Chen, L.T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Lee, T.; Lee, M.A.; Kitano, M.; et al. 78P Updated overall survival (OS) from the phase III TOPAZ-1 study of durvalumab (D) or placebo (PBO) plus gemcitabine and cisplatin (+ GC) in patients (pts) with advanced biliary tract cancer (BTC). Ann. Oncol. 2022, 33, S1462–S1463. [Google Scholar] [CrossRef]
- Peng, L.; Qin, B.D.; Xiao, K.; Xu, S.; Yang, J.S.; Zang, Y.S.; Stebbing, J.; Xie, L.P. A meta-analysis comparing responses of Asian versus non-Asian cancer patients to PD-1 and PD-L1 inhibitor-based therapy. Oncoimmunology 2020, 9, 1781333. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Welsh, E.A.; Fulp, W.J.; Chen, L.; Teer, J.K.; Thompson, Z.J.; Engel, B.E.; Xie, M.; Berglund, A.E.; Creelan, B.C.; et al. Differential association of STK11 and TP53 with KRAS mutation-associated gene expression, proliferation and immune surveillance in lung adenocarcinoma. Oncogene 2016, 35, 3209–3216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Wang, X.; Bajaj, G.; Agrawal, S.; Bello, A.; Lestini, B.; Finckenstein, F.G.; Park, J.S.; Roy, A. Nivolumab Exposure-Response Analyses of Efficacy and Safety in Previously Treated Squamous or Nonsquamous Non-Small Cell Lung Cancer. Clin. Cancer Res. 2017, 23, 5394–5405. [Google Scholar] [CrossRef] [Green Version]
- Arru, C.; De Miglio, M.R.; Cossu, A.; Muroni, M.R.; Carru, C.; Zinellu, A.; Paliogiannis, P. Durvalumab Plus Tremelimumab in Solid Tumors: A Systematic Review. Adv. Ther. 2021, 38, 3674–3693. [Google Scholar] [CrossRef]
- Villanueva, L.; Lwin, Z.; Chung, H.C.; Gomez-Roca, C.; Longo, F.; Yanez, E.; Senellart, H.; Doherty, M.; García-Corbacho, J.; Hendifar, A.E.; et al. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase II LEAP-005 study. J. Clin. Oncol. 2021, 39 (Suppl. 3), 321. [Google Scholar] [CrossRef]
- Shi, G.M.; Huang, X.Y.; Wu, D.; Sun, H.C.; Liang, F.; Ji, Y.; Chen, Y.; Yang, G.H.; Lu, J.C.; Meng, X.L.; et al. Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: A single-center, single-arm, phase 2 study. Signal Transduct. Target. Ther. 2023, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Ruff, S.M.; Shannon, A.H.; Pawlik, T.M. Advances in Targeted Immunotherapy for Hepatobiliary Cancers. Int. J. Mol. Sci. 2022, 23, 13961. [Google Scholar] [CrossRef]
- Supimon, K.; Sangsuwannukul, T.; Sujjitjoon, J.; Phanthaphol, N.; Chieochansin, T.; Poungvarin, N.; Wongkham, S.; Junking, M.; Yenchitsomanus, P.T. Anti-mucin 1 chimeric antigen receptor T cells for adoptive T cell therapy of cholangiocarcinoma. Sci. Rep. 2021, 11, 6276. [Google Scholar] [CrossRef] [PubMed]
- Phanthaphol, N.; Somboonpatarakun, C.; Suwanchiwasiri, K.; Chieochansin, T.; Sujjitjoon, J.; Wongkham, S.; Maher, J.; Junking, M.; Yenchitsomanus, P.T. Chimeric Antigen Receptor T Cells Targeting Integrin αvβ6 Expressed on Cholangiocarcinoma Cells. Front. Oncol. 2021, 11, 657868. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Liu, Y.; Guo, Y.; Qiu, J.; Wu, Z.; Dai, H.; Yang, Q.; Wang, Y.; Han, W. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell 2018, 9, 838–847. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, K.; Liu, Y.; Wu, Z.; Dai, H.; Yang, Q.; Wang, Y.; Jia, H.; Han, W. Phase I Study of Chimeric Antigen Receptor-Modified T Cells in Patients with EGFR-Positive Advanced Biliary Tract Cancers. Clin. Cancer Res. 2018, 24, 1277–1286. [Google Scholar] [CrossRef] [Green Version]
- Anwar, M.Y.; Williams, G.R.; Paluri, R.K. CAR T Cell Therapy in Pancreaticobiliary Cancers: A Focused Review of Clinical Data. J. Gastrointest. Cancer 2021, 52, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Ranganathan, R.; Jiang, S.; Fang, C.; Sun, J.; Kim, S.; Newick, K.; Lo, A.; June, C.H.; Zhao, Y.; et al. A Chimeric Switch-Receptor Targeting PD1 Augments the Efficacy of Second-Generation CAR T Cells in Advanced Solid Tumors. Cancer Res. 2016, 76, 1578–1590. [Google Scholar] [CrossRef] [Green Version]
- Facciorusso, A.; Abd El Aziz, M.A.; Singh, S.; Pusceddu, S.; Milione, M.; Giacomelli, L.; Sacco, R. Statin Use Decreases the Incidence of Hepatocellular Carcinoma: An Updated Meta-Analysis. Cancers 2020, 12, 874. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Heij, L.R.; Czigany, Z.; Dahl, E.; Lang, S.A.; Ulmer, T.F.; Luedde, T.; Neumann, U.P.; Bednarsch, J. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 127. [Google Scholar] [CrossRef]
- Nishida, N.; Aoki, T.; Morita, M.; Chishina, H.; Takita, M.; Ida, H.; Hagiwara, S.; Minami, Y.; Ueshima, K.; Kudo, M. Non-Inflamed Tumor Microenvironment and Methylation/Downregulation of Antigen-Presenting Machineries in Cholangiocarcinoma. Cancers 2023, 15, 2379. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.R.; Corrales, L.; Gajewski, T.F. The STING pathway and the T cell-inflamed tumor microenvironment. Trends Immunol. 2015, 36, 250–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morisaki, T.; Umebayashi, M.; Kiyota, A.; Koya, N.; Tanaka, H.; Onishi, H.; Katano, M. Combining cetuximab with killer lymphocytes synergistically inhibits human cholangiocarcinoma cells in vitro. Anticancer. Res. 2012, 32, 2249–2256. [Google Scholar]
- Sawasdee, N.; Thepmalee, C.; Sujjitjoon, J.; Yongpitakwattana, P.; Junking, M.; Poungvarin, N.; Yenchitsomanus, P.T.; Panya, A. Gemcitabine enhances cytotoxic activity of effector T-lymphocytes against chemo-resistant cholangiocarcinoma cells. Int. Immunopharmacol. 2020, 78, 106006. [Google Scholar] [CrossRef]
- Diggs, L.P.; Ruf, B.; Ma, C.; Heinrich, B.; Cui, L.; Zhang, Q.; McVey, J.C.; Wabitsch, S.; Heinrich, S.; Rosato, U.; et al. CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma. J. Hepatol. 2021, 74, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.R.; Wu, C.E.; Chen, M.H.; Huang, W.K.; Shih, H.J.; Lan, K.L.; Yeh, C.N. Comprehensive Evaluation of Immune-Checkpoint DNA Cancer Vaccines in a Rat Cholangiocarcinoma Model. Vaccines 2020, 8, 703. [Google Scholar] [CrossRef]
- Huang, X.; Tang, T.; Zhang, G.; Liang, T. Identification of tumor antigens and immune subtypes of cholangiocarcinoma for mRNA vaccine development. Mol. Cancer 2021, 20, 50. [Google Scholar] [CrossRef]
- Oh, D.Y.; He, A.R.; Qin, S.; Chen, L.T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. J. Clin. Oncol. 2022, 40, 378. [Google Scholar] [CrossRef]
- Kaida, M.; Morita-Hoshi, Y.; Soeda, A.; Wakeda, T.; Yamaki, Y.; Kojima, Y.; Ueno, H.; Kondo, S.; Morizane, C.; Ikeda, M.; et al. Phase 1 trial of Wilms tumor 1 (WT1) peptide vaccine and gemcitabine combination therapy in patients with advanced pancreatic or biliary tract cancer. J. Immunother. 2011, 34, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Aruga, A.; Takeshita, N.; Kotera, Y.; Okuyama, R.; Matsushita, N.; Ohta, T.; Takeda, K.; Yamamoto, M. Phase I clinical trial of multiple-peptide vaccination for patients with advanced biliary tract cancer. J. Transl. Med. 2014, 12, 61. [Google Scholar] [CrossRef] [Green Version]
- van Willigen, W.W.; Bloemendal, M.; Gerritsen, W.R.; Schreibelt, G.; de Vries, I.J.M.; Bol, K.F. Dendritic Cell Cancer Therapy: Vaccinating the Right Patient at the Right Time. Front. Immunol. 2018, 9, 2265. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Sakabe, T.; Abe, H.; Tanii, M.; Takahashi, H.; Chiba, A.; Yanagida, E.; Shibamoto, Y.; Ogasawara, M.; Tsujitani, S.; et al. DC-vaccine study group at the Japan Society of Innovative Cell Therapy (J-SICT). Dendritic cell-based immunotherapy targeting synthesized peptides for advanced biliary tract cancer. J. Gastrointest. Surg. 2013, 17, 1609–1617. [Google Scholar] [CrossRef]
- Jiraviriyakul, A.; Songjang, W.; Kaewthet, P.; Tanawatkitichai, P.; Bayan, P.; Pongcharoen, S. Honokiol-enhanced cytotoxic T lymphocyte activity against cholangiocarcinoma cells mediated by dendritic cells pulsed with damage-associated molecular patterns. World J. Gastroenterol. 2019, 25, 3941–3955. [Google Scholar] [CrossRef]
- Ciombor, K.K.; Goldberg, R.M. Hypermutated Tumors and Immune Checkpoint Inhibition. Drugs 2018, 78, 155–162. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Duffy, A.G.; Mabry-Hrones, D.; Wood, B.; Levy, E.; Krishnasamy, V.; Khan, J.; Wei, J.S.; Agdashian, D.; Tyagi, M.; et al. Tremelimumab in Combination With Microwave Ablation in Patients With Refractory Biliary Tract Cancer. Hepatology 2019, 69, 2048–2060. [Google Scholar] [CrossRef] [PubMed]
- Robbrecht, D.; Jungels, C.; Sorensen, M.M.; Spanggaard, I.; Eskens, F.; Fretland, S.Ø.; Guren, T.K.; Aftimos, P.; Liberg, D.; Svedman, C.; et al. First-in-human phase 1 dose-escalation study of CAN04, a first-in-class interleukin-1 receptor accessory protein (IL1RAP) antibody in patients with solid tumours. Br. J. Cancer 2022, 126, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.E.; Fisher, J.L.; Flick, H.L.; Wang, C.; Sun, L.; Ernstoff, M.S.; Alvarez, J.C.; Losey, H.C. ALKS 4230: A novel engineered IL-2 fusion protein with an improved cellular selectivity profile for cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000673. [Google Scholar] [CrossRef]
| Treatment | Trial Name | Target | Phase | Line of Therapy | ORR (%) | OS (m) | PFS (m) |
|---|---|---|---|---|---|---|---|
| Pembrolizumab | KEYNOTE-028 | PD-1 | I | second | 13 | 5.7 | 1.8 |
| Pembrolizumab | KEYNOTE-158 | PD-1 | II | second | 5.8 | 7.4 | 2 |
| Nivolumab | NCT02829918 | PD-1 | II | second | 22 | 14.2 | 3.68 |
| Nivolumab | JapicCTI-153098 | PD-1 | I | second | 3 | 5.2 | 1.4 |
| Durvalumab | NCT01938612 | PDL1 | I | second | 4.8 | 8.1 | 2 |
| Bintrafusp alfa | NCT02699514 | PD-L1 TGFβ-RII | I | second | 20 | 12.5 | 2.5 |
| Treatment | Trial Name | Target | Phase | Line of Therapy | ORR (%) | OS (m) | PFS (m) |
|---|---|---|---|---|---|---|---|
| Nivolumab Ipilimumab | CA209-538 | PD-1 CTLA4 | II | second | 23 | 5.7 | 2.9 |
| Durvalumab + Tremelimumab | NCT01938612 | PDL1, CTLA4 | II | second | 10.8 | 10.1 | - |
| Treatment | Trial Name | Target | Phase | Line of Therapy | ORR (%) | OS (m) | PFS (m) |
|---|---|---|---|---|---|---|---|
| Nivolumab cisplatin/gemcitabine | JapicCTI-153098 | PD-1 | II | first | 37 | 15.4 | 4.2 |
| Nivolumab cisplatin/gemcitabine | NCT03311789 | PD-1 | II | first | 55.6 | 8.5 | 6.1 |
| Camrelizumab Gemcitabine/oxaliplatin | NCT03486678 | PD-1 | II | first | 54 | 11.8 | 6.1 |
| Camrelizumab Gemcitabine/oxaliplatin or FOLFOX | NCT03092895 | PD-1 | II | first | 16.3 | 12.4 | 5.3 |
| Durvalumab Cisplatin/gemcitabine | NCT03046862 | PD-L1 | II | first | 73.3 | 18.1 | 11 |
| Durvalumab + GEMCIS | TOPAZ-1 | PD-L1 | III | first | 26.7 vs. 18.7 | 12.8 vs. 11.5 | 7.2 vs. 5.7 |
| Treatment | Trial Name | Target | Phase | Line of Therapy | ORR (%) | OS (m) | PFS (m) |
|---|---|---|---|---|---|---|---|
| Lenvatinib + Pembrolizumab | LEAP-005 | PD-1 | II | second | 10 | 8.6 | 6.1 |
| Toripalimab and lenvatinib + GEMOX | NCT03951597 | PD-1 | II | first | 80 | NA | 10 |
| Treatment | Trial Name | Phase | Primary Outcome |
|---|---|---|---|
| GEMCIS ± Pembrolizumab | NCT04003636 | III | OS |
| GEMCIS ± Bintrafusp alfa | NCT04066491 | II | OS |
| Pembrolizumab + GEMCIS | NCT03260712 | II | PFS |
| Nivolumab + GEMCIS vs. Nivolumab + Ipilimumab | NCT03101566 | II | PFS |
| Nivolumab + Gemcitabine + TS-1 | NCT04172402 | II | ORR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manthopoulou, E.; Ramai, D.; Dhar, J.; Samanta, J.; Ioannou, A.; Lusina, E.; Sacco, R.; Facciorusso, A. Cholangiocarcinoma in the Era of Immunotherapy. Vaccines 2023, 11, 1062. https://doi.org/10.3390/vaccines11061062
Manthopoulou E, Ramai D, Dhar J, Samanta J, Ioannou A, Lusina E, Sacco R, Facciorusso A. Cholangiocarcinoma in the Era of Immunotherapy. Vaccines. 2023; 11(6):1062. https://doi.org/10.3390/vaccines11061062
Chicago/Turabian StyleManthopoulou, Eleni, Daryl Ramai, Jahnvi Dhar, Jayanta Samanta, Alexandros Ioannou, Ekaterina Lusina, Rodolfo Sacco, and Antonio Facciorusso. 2023. "Cholangiocarcinoma in the Era of Immunotherapy" Vaccines 11, no. 6: 1062. https://doi.org/10.3390/vaccines11061062






