Intranasal Single-Replication Influenza Vector Induces Cross-Reactive Serum and Mucosal Antibodies against SARS-CoV-2 Variants
Abstract
1. Introduction
2. Materials and Methods
2.1. M2SR Expressing Membrane-Anchored SARS-CoV-2 RBD Antigen
2.2. SARS-CoV-2 M2SR and Sing2016 M2SR Vaccine Viruses
2.3. Production of Vaccines
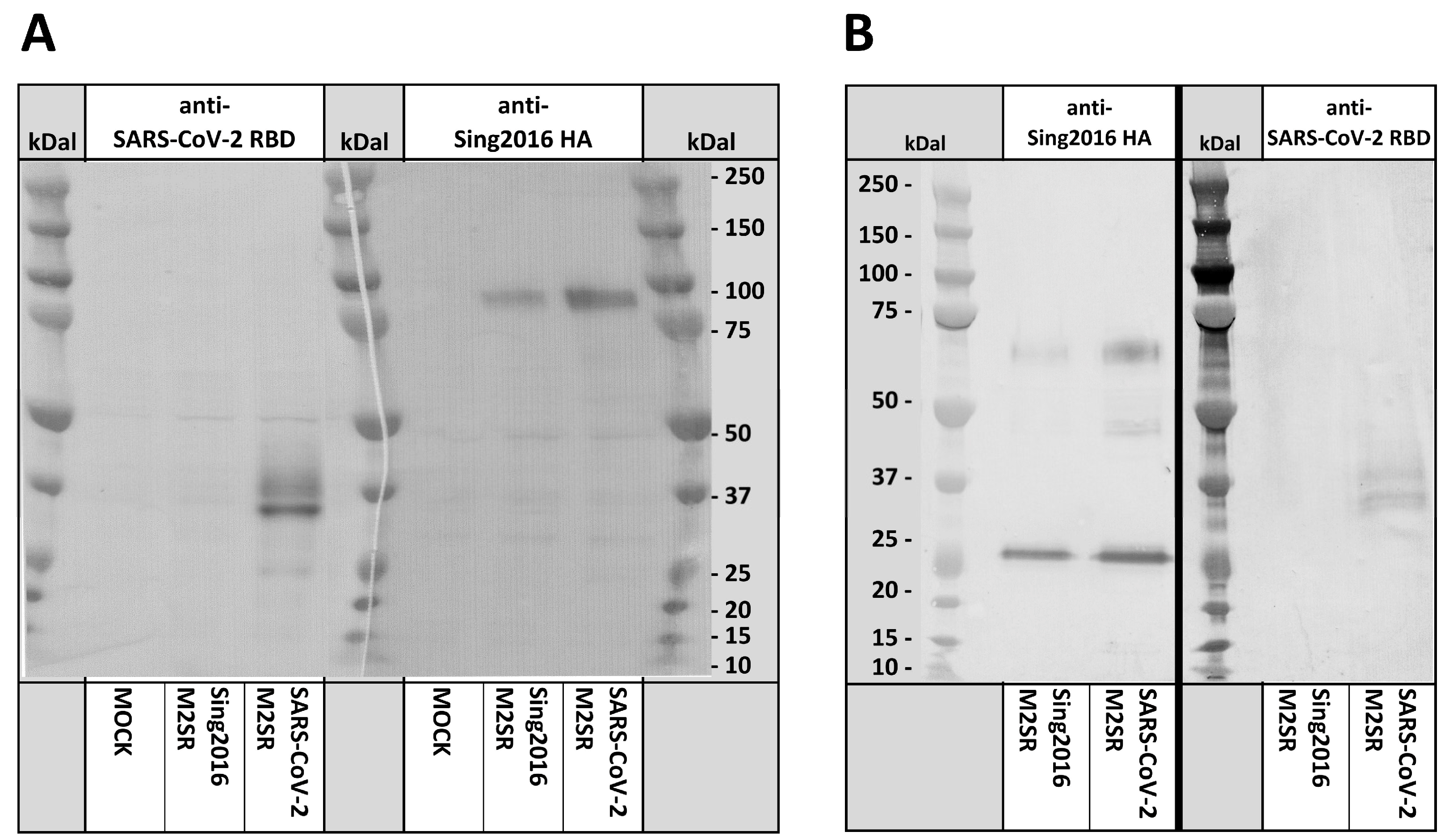
2.4. Immunoblot Analysis
2.5. Immunofluorescence Microscopy of SARS-CoV-2 RBD and Sing2016 H3N2 HA
2.6. Flow Cytometric Analysis of SARS-CoV-2 RBD and Sing2016 H3N2 HA Cell Surface Expression
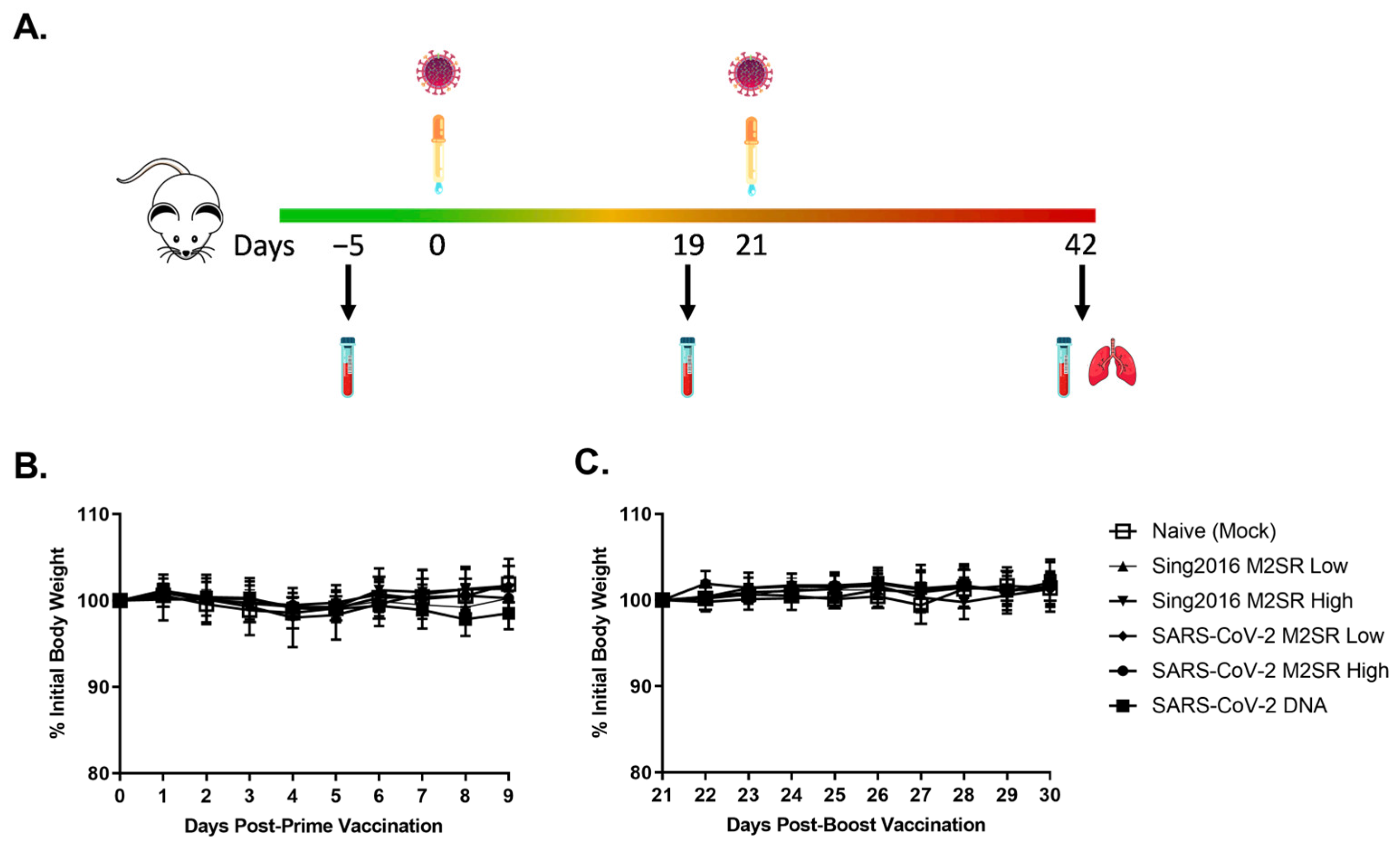
2.7. Mouse Studies
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Hemagglutination Inhibition Assay (HAI)
2.10. Plaque Reduction Neutralization Titer (PRNT)
2.11. Statistical Analyses
3. Results
3.1. Generation of SARS-CoV-2 H3N2 M2SR Vaccine
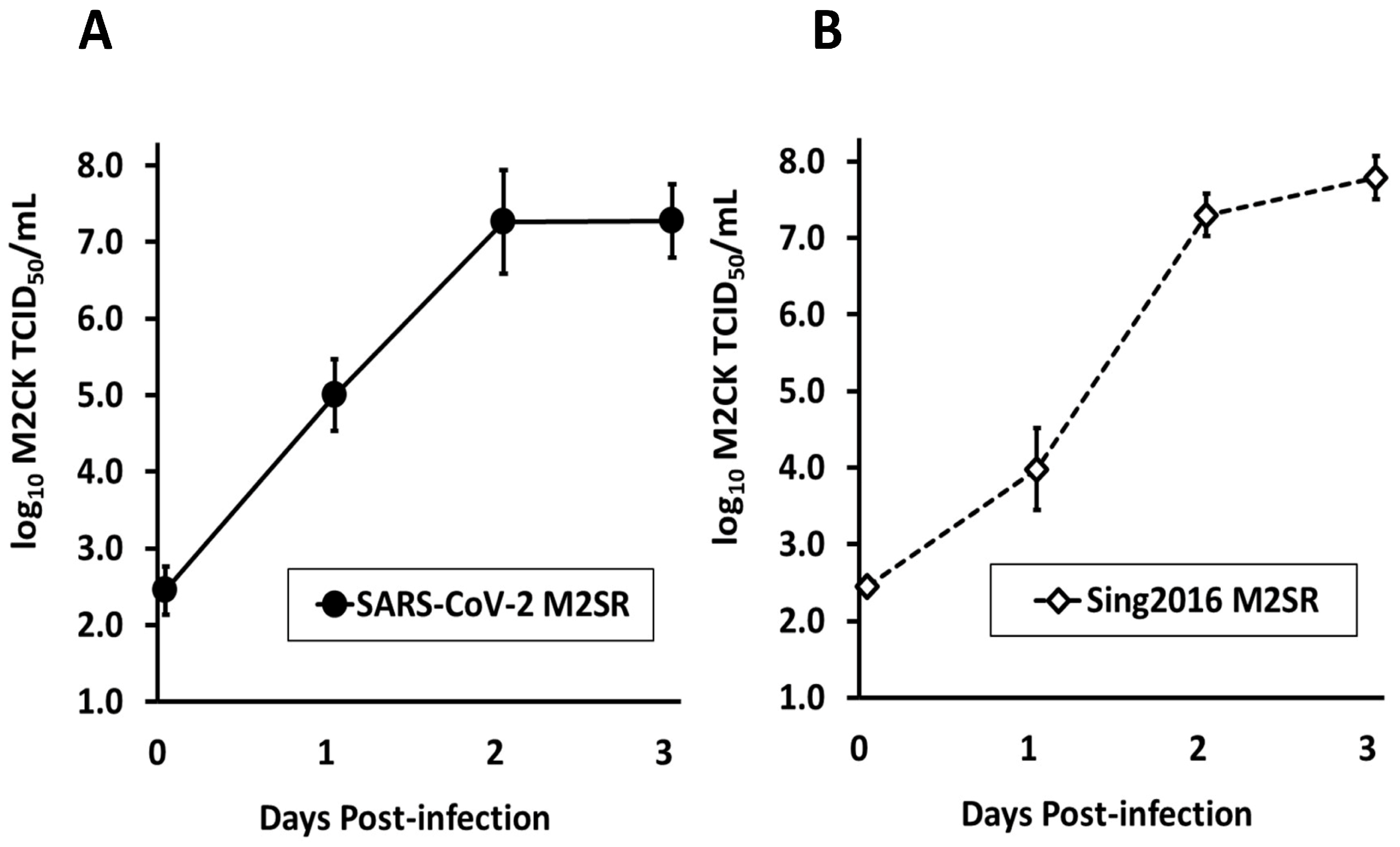
3.2. Growth Kinetics of SARS-CoV-2 M2SR in Tissue Culture
3.3. RBD Expression in SARS-CoV-2 M2SR-Infected Cells and Virions
3.4. Sub-Cellular Detection of RBD Expression by Immunofluorescence
3.5. RBD Surface Expression Detection by Flow Cytometry
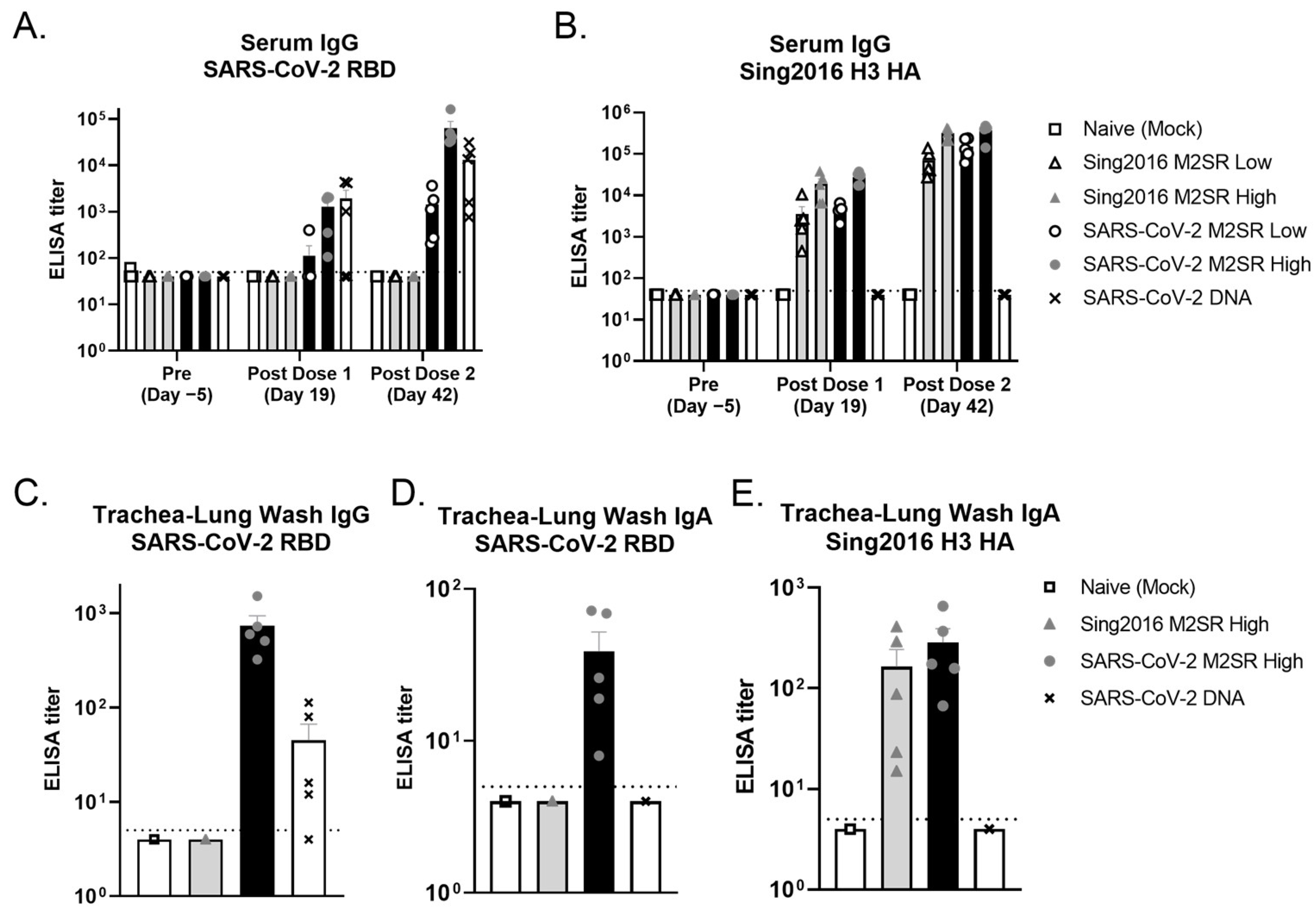
3.6. Evaluation of In Vivo Immune Responses Induced by SARS-CoV-2 M2SR Vaccine
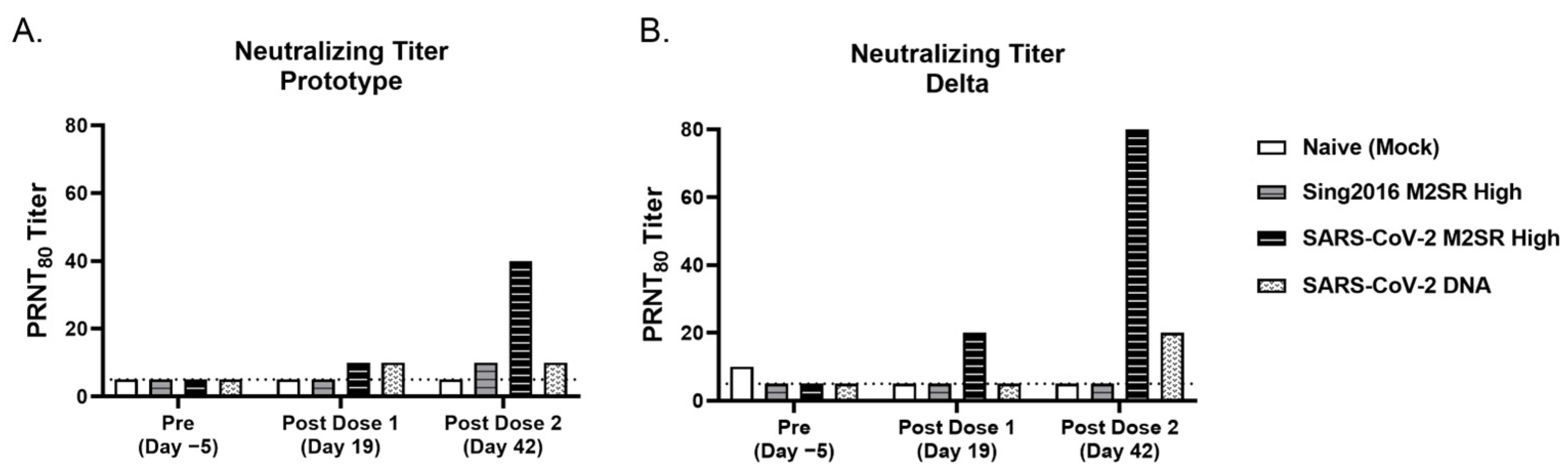
3.7. SARS-CoV-2 M2SR Vaccine Induces Functional Antibodies against Influenza and SARS-CoV-2 Variants
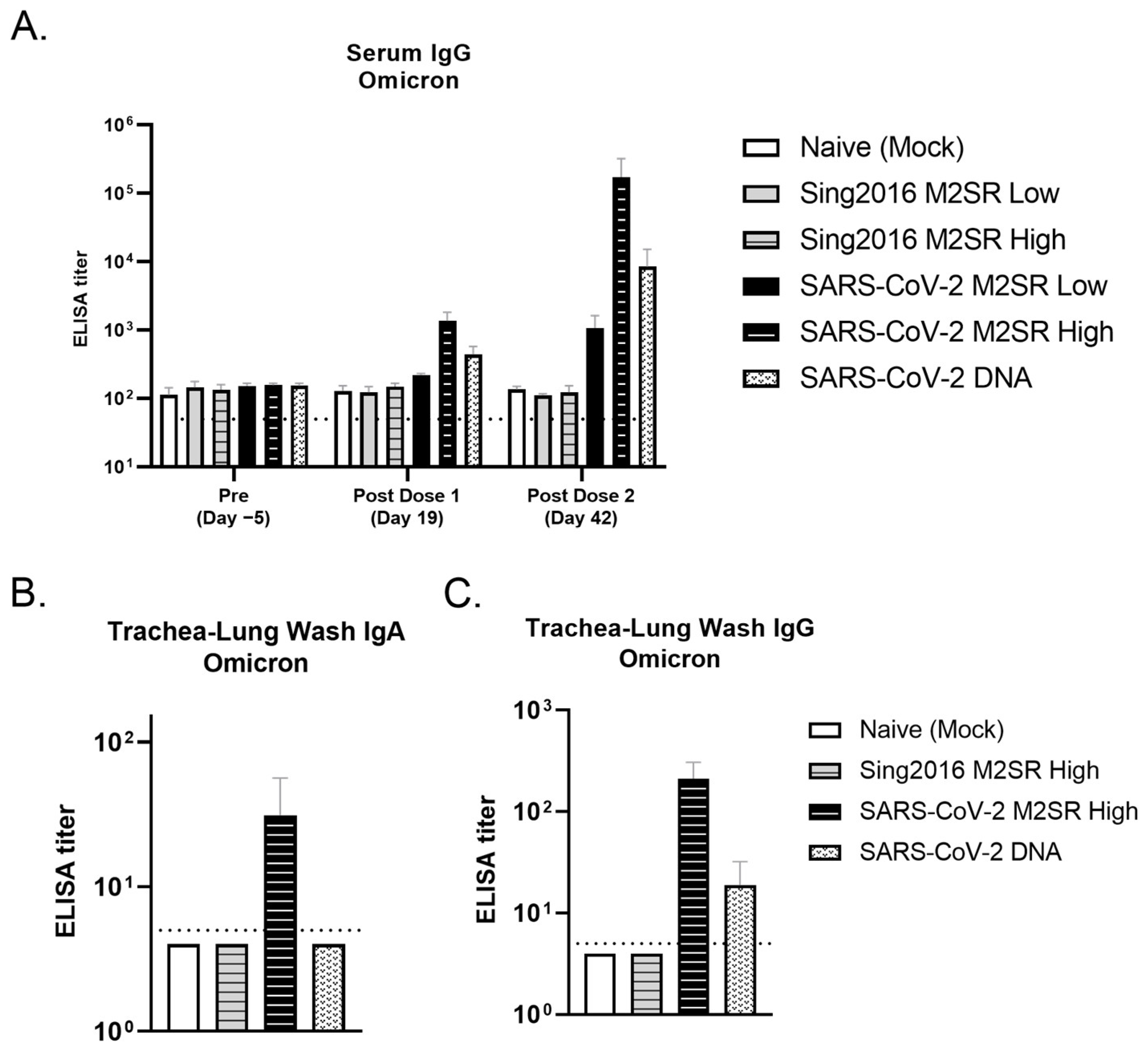
3.8. Evaluation of SARS-CoV-2 M2SR-Induced Antibody Response to SARS-CoV-2 Omicron Variant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 31 March 2023).
- Rasul, G.; Nepal, A.K.; Hussain, A.; Maharjan, A.; Joshi, S.; Lama, A.; Gurung, P.; Ahmad, F.; Mishra, A.; Sharma, E. Socio-Economic Implications of COVID-19 Pandemic in South Asia: Emerging Risks and Growing Challenges. Front. Sociol. 2021, 6, 629693. [Google Scholar] [CrossRef]
- Perugini, C.; Vladisavljevic, M. Social stability challenged by Covid-19: Pandemics, inequality and policy responses. J. Policy Model. 2021, 43, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Whitacre, R.; Oni-Orisan, A.; Gaber, N.; Martinez, C.; Buchbinder, L.; Herd, D.; Holmes, S.M. COVID-19 and the political geography of racialisation: Ethnographic cases in San Francisco, Los Angeles and Detroit. Glob. Public Health 2021, 16, 1396–1410. [Google Scholar] [CrossRef] [PubMed]
- Surie, D.; DeCuir, J.; Zhu, Y.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; Zepeski, A.; et al. Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19-Associated Hospitalization Among Immunocompetent Adults Aged >/=65 Years-IVY Network, 18 States, September 8-November 30, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1625–1630. [Google Scholar] [CrossRef]
- Lytras, T.; Kontopidou, F.; Lambrou, A.; Tsiodras, S. Comparative effectiveness and durability of COVID-19 vaccination against death and severe disease in an ongoing nationwide mass vaccination campaign. J. Med. Virol. 2022, 94, 5044–5050. [Google Scholar] [CrossRef] [PubMed]
- Chuenkitmongkol, S.; Solante, R.; Burhan, E.; Chariyalertsak, S.; Chiu, N.C.; Do-Van, D.; Husin, M.; Hwang, K.P.; Kiertiburanakul, S.; Kulkarni, P.S.; et al. Expert review on global real-world vaccine effectiveness against SARS-CoV-2. Expert Rev. Vaccines 2022, 21, 1255–1268. [Google Scholar] [CrossRef]
- CDC. COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker/#datatracker-home (accessed on 31 March 2023).
- Ioannou, G.N.; Bohnert, A.S.B.; O’Hare, A.M.; Boyko, E.J.; Maciejewski, M.L.; Smith, V.A.; Bowling, C.B.; Viglianti, E.; Iwashyna, T.J.; Hynes, D.M.; et al. Effectiveness of mRNA COVID-19 Vaccine Boosters Against Infection, Hospitalization, and Death: A Target Trial Emulation in the Omicron (B.1.1.529) Variant Era. Ann. Intern. Med. 2022, 175, 1693–1706. [Google Scholar] [CrossRef]
- Pormohammad, A.; Zarei, M.; Ghorbani, S.; Mohammadi, M.; Aghayari Sheikh Neshin, S.; Khatami, A.; Turner, D.L.; Djalalinia, S.; Mousavi, S.A.; Mardani-Fard, H.A.; et al. Effectiveness of COVID-19 Vaccines against Delta (B.1.617.2) Variant: A Systematic Review and Meta-Analysis of Clinical Studies. Vaccines 2021, 10, 23. [Google Scholar] [CrossRef]
- Ferdinands, J.M.; Rao, S.; Dixon, B.E.; Mitchell, P.K.; DeSilva, M.B.; Irving, S.A.; Lewis, N.; Natarajan, K.; Stenehjem, E.; Grannis, S.J.; et al. Waning of vaccine effectiveness against moderate and severe covid-19 among adults in the US from the VISION network: Test negative, case-control study. BMJ 2022, 379, e072141. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Fritz, D.K.; Afkhami, S.; Aguirre, E.; Howie, K.J.; Zganiacz, A.; Dvorkin-Gheva, A.; Thompson, M.R.; Silver, R.F.; Cusack, R.P.; et al. Aerosol delivery, but not intramuscular injection, of adenovirus-vectored tuberculosis vaccine induces respiratory-mucosal immunity in humans. JCI Insight 2022, 7, e155655. [Google Scholar] [CrossRef]
- CDC. FluView Interactive. Available online: https://www.cdc.gov/flu/weekly/fluviewinteractive.htm (accessed on 31 March 2023).
- Adams, K.; Tastad, K.J.; Huang, S.; Ujamaa, D.; Kniss, K.; Cummings, C.; Reingold, A.; Roland, J.; Austin, E.; Kawasaki, B.; et al. Prevalence of SARS-CoV-2 and Influenza Coinfection and Clinical Characteristics Among Children and Adolescents Aged <18 Years Who Were Hospitalized or Died with Influenza—United States, 2021–2022 Influenza Season. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1589–1596. [Google Scholar] [CrossRef]
- Garg, I.; Gangu, K.; Shuja, H.; Agahi, A.; Sharma, H.; Bobba, A.; Nasrullah, A.; Chourasia, P.; Pal, S.; Sheikh, A.B.; et al. COVID-19 and Influenza Coinfection Outcomes among Hospitalized Patients in the United States: A Propensity Matched Analysis of National Inpatient Sample. Vaccines 2022, 10, 2159. [Google Scholar] [CrossRef] [PubMed]
- CDC. Influenza, CDC Seasonal Flu Vaccine Effectiveness Studies. Available online: https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm (accessed on 30 May 2023).
- Hatta, Y.; Boltz, D.; Sarawar, S.; Kawaoka, Y.; Neumann, G.; Bilsel, P. Novel influenza vaccine M2SR protects against drifted H1N1 and H3N2 influenza virus challenge in ferrets with pre-existing immunity. Vaccine 2018, 36, 5097–5103. [Google Scholar] [CrossRef] [PubMed]
- Hatta, Y.; Boltz, D.; Sarawar, S.; Kawaoka, Y.; Neumann, G.; Bilsel, P. M2SR, a novel live influenza vaccine, protects mice and ferrets against highly pathogenic avian influenza. Vaccine 2017, 35, 4177–4183. [Google Scholar] [CrossRef]
- Hill-Batorski, L.H.; Hatta, Y.; Moser, M.J.; Sarawar, S.; Neumann, G.; Kawaoka, Y.; Bilsel, P. Quadrivalent Formulation of Intranasal Influenza Vaccine M2SR (M2-Deficient Single Replication) Protects against Drifted Influenza A and B Virus Challenge. Vaccines 2023, 11, 798. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Watanabe, T.; Kawaoka, Y. Influenza A virus lacking M2 protein as a live attenuated vaccine. J. Virol. 2009, 83, 5947–5950. [Google Scholar] [CrossRef]
- Moser, M.J.; Hatta, Y.; Gabaglia, C.; Sanchez, A.; Dias, P.; Sarawar, S.; Kawaoka, Y.; Hatta, M.; Neumann, G.; Bilsel, P. Single-replication BM2SR vaccine provides sterilizing immunity and cross-lineage influenza B virus protection in mice. Vaccine 2019, 37, 4533–4542. [Google Scholar] [CrossRef]
- Eiden, J.; Volckaert, B.; Rudenko, O.; Aitchison, R.; Herber, R.; Belshe, R.; Greenberg, H.; Coelingh, K.; Marshall, D.; Kawaoka, Y.; et al. M2-Deficient Single-Replication Influenza Vaccine-Induced Immune Responses Associated with Protection Against Human Challenge with Highly Drifted H3N2 Influenza Strain. J. Infect. Dis. 2022, 226, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Eiden, J.; Fierro, C.; Schwartz, H.; Adams, M.; Ellis, K.J.; Aitchison, R.; Herber, R.; Hatta, Y.; Marshall, D.; Moser, M.J.; et al. Intranasal M2SR (M2-Deficient Single Replication) H3N2 Influenza Vaccine Provides Enhanced Mucosal and Serum Antibodies in Adults. J. Infect. Dis. 2022, 227, 103–112. [Google Scholar] [CrossRef]
- Eiden, J.; Gordon, G.; Fierro, C.; Herber, R.; Aitchison, R.; Belshe, R.; Greenberg, H.; Hoft, D.; Hatta, Y.; Moser, M.J.; et al. Safety and Immunogenicity of M2-Deficient, Single Replication, Live Influenza Vaccine (M2SR) in Adults. Vaccines 2021, 9, 1388. [Google Scholar] [CrossRef]
- Luytjes, W.; Krystal, M.; Enami, M.; Parvin, J.D.; Palese, P. Amplification, expression, and packaging of foreign gene by influenza virus. Cell 1989, 59, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Enami, M.; Luytjes, W.; Krystal, M.; Palese, P. Introduction of site-specific mutations into the genome of influenza virus. Proc. Natl. Acad. Sci. USA 1990, 87, 3802–3805. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Watanabe, T.; Ito, H.; Watanabe, S.; Goto, H.; Gao, P.; Hughes, M.; Perez, D.R.; Donis, R.; Hoffmann, E.; et al. Generation of influenza A viruses entirely from cloned cDNAs. Proc. Natl. Acad. Sci. USA 1999, 96, 9345–9350. [Google Scholar] [CrossRef] [PubMed]
- Fodor, E.; Devenish, L.; Engelhardt, O.G.; Palese, P.; Brownlee, G.G.; Garcia-Sastre, A. Rescue of influenza A virus from recombinant DNA. J. Virol. 1999, 73, 9679–9682. [Google Scholar] [CrossRef] [PubMed]
- Langley, W.A.; Bradley, K.C.; Li, Z.N.; Smith, M.E.; Schnell, M.J.; Steinhauer, D.A. Induction of neutralizing antibody responses to anthrax protective antigen by using influenza virus vectors: Implications for disparate immune system priming pathways. J. Virol. 2010, 84, 8300–8307. [Google Scholar] [CrossRef]
- Muster, T.; Ferko, B.; Klima, A.; Purtscher, M.; Trkola, A.; Schulz, P.; Grassauer, A.; Engelhardt, O.G.; Garcia-Sastre, A.; Palese, P.; et al. Mucosal model of immunization against human immunodeficiency virus type 1 with a chimeric influenza virus. J. Virol. 1995, 69, 6678–6686. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- de Felipe, P.; Luke, G.A.; Hughes, L.E.; Gani, D.; Halpin, C.; Ryan, M.D. E unum pluribus: Multiple proteins from a self-processing polyprotein. Trends Biotechnol. 2006, 24, 68–75. [Google Scholar] [CrossRef]
- Loes, A.N.; Gentles, L.E.; Greaney, A.J.; Crawford, K.H.D.; Bloom, J.D. Attenuated Influenza Virions Expressing the SARS-CoV-2 Receptor-Binding Domain Induce Neutralizing Antibodies in Mice. Viruses 2020, 12, 987. [Google Scholar] [CrossRef]
- Eiden, J.R.E.C.F.H.S.K.E.R.A.R.H.P.B. Intranasal M2SR (M2-deficient Single Replication) Live H3N2 Influenza Investigational Vaccine Induces Serum HAI & Broad Immune Responses in High Proportion of Adults. Open Forum. Infect. Dis. 2020, 7, S40–S41. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Arifin, W.N.; Zahiruddin, W.M. Sample Size Calculation in Animal Studies Using Resource Equation Approach. Malays. J. Med. Sci. 2017, 24, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.T. Resource equation method for sample size calculation in animal studies. Am. J. Emerg. Med. 2023, 63, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Honda-Okubo, Y.; Baldwin, J.; Bowen, R.; Bielefeldt-Ohmann, H.; Petrovsky, N. Covax-19/Spikogen(R) vaccine based on recombinant spike protein extracellular domain with Advax-CpG55.2 adjuvant provides single dose protection against SARS-CoV-2 infection in hamsters. Vaccine 2022, 40, 3182–3192. [Google Scholar] [CrossRef]
- Sarawar, S.; Hatta, Y.; Watanabe, S.; Dias, P.; Neumann, G.; Kawaoka, Y.; Bilsel, P. M2SR, a novel live single replication influenza virus vaccine, provides effective heterosubtypic protection in mice. Vaccine 2016, 34, 5090–5098. [Google Scholar] [CrossRef]
- Topol, E.J.; Iwasaki, A. Operation Nasal Vaccine-Lightning speed to counter COVID-19. Sci. Immunol. 2022, 7, eadd9947. [Google Scholar] [CrossRef]
- Lund, F.E.; Randall, T.D. Scent of a vaccine. Science 2021, 373, 397–399. [Google Scholar] [CrossRef]
- Hassan, A.O.; Case, J.B.; Winkler, E.S.; Thackray, L.B.; Kafai, N.M.; Bailey, A.L.; McCune, B.T.; Fox, J.M.; Chen, R.E.; Alsoussi, W.B.; et al. A SARS-CoV-2 Infection Model in Mice Demonstrates Protection by Neutralizing Antibodies. Cell 2020, 182, 744–753.e744. [Google Scholar] [CrossRef]
- Hassan, A.O.; Kafai, N.M.; Dmitriev, I.P.; Fox, J.M.; Smith, B.K.; Harvey, I.B.; Chen, R.E.; Winkler, E.S.; Wessel, A.W.; Case, J.B.; et al. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell 2020, 183, 169–184.e113. [Google Scholar] [CrossRef]
- Afkhami, S.; D’Agostino, M.R.; Zhang, A.; Stacey, H.D.; Marzok, A.; Kang, A.; Singh, R.; Bavananthasivam, J.; Ye, G.; Luo, X.; et al. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell 2022, 185, 896–915.e819. [Google Scholar] [CrossRef]
- Alu, A.; Chen, L.; Lei, H.; Wei, Y.; Tian, X.; Wei, X. Intranasal COVID-19 vaccines: From bench to bed. EBioMedicine 2022, 76, 103841. [Google Scholar] [CrossRef] [PubMed]
- Akst, J. Nasal Vaccines are commercially high risk, perhaps high reward. Scientist 2022, 2, 23–26. [Google Scholar]
- Chen, J.; Wang, P.; Yuan, L.; Zhang, L.; Zhang, L.; Zhao, H.; Chen, C.; Wang, X.; Han, J.; Chen, Y.; et al. A live attenuated virus-based intranasal COVID-19 vaccine provides rapid, prolonged, and broad protection against SARS-CoV-2. Sci. Bull. 2022, 67, 1372–1387. [Google Scholar] [CrossRef]
- Koonpaew, S.; Kaewborisuth, C.; Srisutthisamphan, K.; Wanitchang, A.; Thaweerattanasinp, T.; Saenboonrueng, J.; Poonsuk, S.; Jengarn, J.; Viriyakitkosol, R.; Kramyu, J.; et al. A Single-Cycle Influenza A Virus-Based SARS-CoV-2 Vaccine Elicits Potent Immune Responses in a Mouse Model. Vaccines 2021, 9, 850. [Google Scholar] [CrossRef] [PubMed]
- Krug, R.M. Functions of the influenza A virus NS1 protein in antiviral defense. Curr. Opin. Virol. 2015, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ayllon, J.; Garcia-Sastre, A. The NS1 protein: A multitasking virulence factor. Curr. Top. Microbiol. Immunol. 2015, 386, 73–107. [Google Scholar] [CrossRef]
- Egorov, A.; Brandt, S.; Sereinig, S.; Romanova, J.; Ferko, B.; Katinger, D.; Grassauer, A.; Alexandrova, G.; Katinger, H.; Muster, T. Transfectant influenza A viruses with long deletions in the NS1 protein grow efficiently in Vero cells. J. Virol. 1998, 72, 6437–6441. [Google Scholar] [CrossRef]
- Garcia-Sastre, A.; Egorov, A.; Matassov, D.; Brandt, S.; Levy, D.E.; Durbin, J.E.; Palese, P.; Muster, T. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 1998, 252, 324–330. [Google Scholar] [CrossRef]
- Zhu, F.; Zhuang, C.; Chu, K.; Zhang, L.; Zhao, H.; Huang, S.; Su, Y.; Lin, H.; Yang, C.; Jiang, H.; et al. Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: Randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Respir. Med. 2022, 10, 749–760. [Google Scholar] [CrossRef]
- Mossler, C.; Groiss, F.; Wolzt, M.; Wolschek, M.; Seipelt, J.; Muster, T. Phase I/II trial of a replication-deficient trivalent influenza virus vaccine lacking NS1. Vaccine 2013, 31, 6194–6200. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moser, M.J.; Hill-Batorski, L.; Bowen, R.A.; Matejka, S.M.; Marshall, D.; Kawaoka, Y.; Neumann, G.; Bilsel, P. Intranasal Single-Replication Influenza Vector Induces Cross-Reactive Serum and Mucosal Antibodies against SARS-CoV-2 Variants. Vaccines 2023, 11, 1063. https://doi.org/10.3390/vaccines11061063
Moser MJ, Hill-Batorski L, Bowen RA, Matejka SM, Marshall D, Kawaoka Y, Neumann G, Bilsel P. Intranasal Single-Replication Influenza Vector Induces Cross-Reactive Serum and Mucosal Antibodies against SARS-CoV-2 Variants. Vaccines. 2023; 11(6):1063. https://doi.org/10.3390/vaccines11061063
Chicago/Turabian StyleMoser, Michael J., Lindsay Hill-Batorski, Richard A. Bowen, Sarah M. Matejka, David Marshall, Yoshihiro Kawaoka, Gabriele Neumann, and Pamuk Bilsel. 2023. "Intranasal Single-Replication Influenza Vector Induces Cross-Reactive Serum and Mucosal Antibodies against SARS-CoV-2 Variants" Vaccines 11, no. 6: 1063. https://doi.org/10.3390/vaccines11061063
APA StyleMoser, M. J., Hill-Batorski, L., Bowen, R. A., Matejka, S. M., Marshall, D., Kawaoka, Y., Neumann, G., & Bilsel, P. (2023). Intranasal Single-Replication Influenza Vector Induces Cross-Reactive Serum and Mucosal Antibodies against SARS-CoV-2 Variants. Vaccines, 11(6), 1063. https://doi.org/10.3390/vaccines11061063



