Safety of Monkeypox Vaccine Using Active Surveillance, Two-Center Observational Study in Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Outcomes
2.3. Procedure and Questionnaire
2.4. Data Collection and Analysis
3. Results
3.1. Population Characteristics
3.2. Administration Context and AEFIs
3.3. AEFI Grading
3.4. Single-Dose Recipients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO–World Health Organization. Monkeypox. Available online: https://www.who.int/news-room/fact-sheets/detail/monkeypox (accessed on 5 May 2023).
- Tiecco, G.; Degli Antoni, M.; Storti, S.; Tomasoni, L.R.; Castelli, F.; Quiros-Roldan, E. Monkeypox, a Literature Review: What Is New and Where Does This concerning Virus Come From? Viruses 2022, 14, 1894. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; White, A. Monkeypox virus emerges from the shadow of its more infamous cousin: Family biology matters. Emerg. Microbes Infect. 2022, 11, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Riopelle, J.C.; Munster, V.J.; Port, J.R. Atypical and Unique Transmission of Monkeypox Virus during the 2022 Outbreak: An Overview of the Current State of Knowledge. Viruses 2022, 14, 2012. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Director-General’s Statement at the Press Conference following the IHR Emergency Committee Regarding the Multi-country Outbreak of Monkeypox—23 July 2022. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022 (accessed on 5 May 2023).
- Epidemia di Vaiolo delle Scimmie in Paesi Non Endemici, Maggio 2022. Istituto Superiore di Sanità, Epicentro–L’epidemiologia per la Sanità Pubblica. Available online: https://www.epicentro.iss.it/monkeypox/epidemia-2022 (accessed on 5 May 2023).
- Communicable Disease Threats Report, 24–30 July, Week 30. ECDC. Available online: https://www.ecdc.europa.eu/en/publications-data/communicable-disease-threats-report-24-30-july-week-30 (accessed on 5 May 2023).
- Gomez-Lucia, E. Monkeypox: Some Keys to Understand This Emerging Disease. Animals 2022, 12, 2190. [Google Scholar] [CrossRef] [PubMed]
- Beer, E.M.; Rao, V.B. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl. Trop. Dis. 2019, 13, e0007791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO–World Health Organization. 2022 Mpox (Monkeypox) Outbreak: Global Trends. Available online: https://worldhealthorg.shinyapps.io/mpx_global/ (accessed on 5 May 2023).
- Ministero della Salute. Vaiolo delle Scimmie–Mpox. Bollettino Nazionale. Available online: https://www.salute.gov.it/portale/vaioloScimmie/dettaglioContenutiVaioloScimmie.jsp?lingua=italiano&id=5943&area=vaioloScimmie&menu=vuoto (accessed on 5 May 2023).
- Ministero della Salute. Circolare 2 Agosto 2022. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2022&codLeg=88439&parte=1%20&serie=null (accessed on 5 May 2023).
- Poland, G.A.; Kennedy, R.B.; Tosh, P.K. Prevention of monkeypox with vaccines: A rapid review. Lancet Infect. Dis. 2022, 22, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Ministero della Salute. Circolare 5 Agosto 2022–Indicazioni ad Interim sulla Strategia Vaccinale Contro il Vaiolo delle Scimmie (MPX). Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2022&codLeg=88498&parte=1%20&serie=null (accessed on 5 May 2023).
- FDA–Food and Drug Administration. Package Insert–JYNNEOS. Available online: https://www.fda.gov/media/131078/download (accessed on 5 May 2023).
- IOMS/WHO Working Group on Vaccine Pharmacovigilance. Definition and Application of Terms for Vaccine Pharmacovigilance: Report of Cioms/Who Working Group on Vaccine Pharmacovigilance; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Cowling, B.J.; Perera, R.A.P.M.; Valkenburg, S.A.; Leung, N.H.L.; Iuliano, A.D.; Tam, Y.H.; Wong, J.H.F.; Fang, V.J.; Li, A.P.Y.; So, H.C. Comparative immunogenicity of several enhanced influenza vaccine options for older adults: A randomized, controlled trial. Clin. Infect. Dis. 2020, 71, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C. Safety and efficacy of the BNT162b2 MRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B. Efficacy and safety of the MRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Quach, H.Q.; Kennedy, R.B. Enhancing Immunogenicity of Influenza Vaccine in the Elderly through Intradermal Vaccination: A Literature Analysis. Viruses 2022, 14, 2438. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Emerging adjuvants for intradermal vaccination. Int. J. Pharm. 2023, 632, 122559. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.; Marquez, P.; Moro, P.; Weintraub, E.; Yu, Y.; Boersma, P.; Donahue, J.G.; Glanz, J.M.; Goddard, K.; Hambidge, S.J.; et al. Safety Monitoring of JYNNEOS Vaccine During the 2022 Mpox Outbreak–United States, 22 May–21 October 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Turner Overton, E.; Schmidt, D.; Vidojkovic, S.; Menius, E.; Nopora, K.; Maclennan, J.; Weidenthaler, H. A randomized phase 3 trial to assess the immunogenicity and safety of 3 consecutively produced lots of freeze-dried MVA-BN® vaccine in healthy adults. Vaccine 2023, 41, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Lopez, L.K.; Glover, C.; Cashman, P.; Reynolds, R.; Macartney, K.; Wood, N. Short-term Adverse Events Following Immunization with Modified Vaccinia Ankara-Bavarian Nordic (MVA-BN) Vaccine for Mpox. JAMA 2023, 329, 2091–2094. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | N (%) | ||
|---|---|---|---|
| Age, years (mean (SD)) | 36.4 (8.7) | ||
| BMI > 30 | No | 136 (96.5) | |
| Yes | 5 (3.6) | ||
| Preexisting health conditions | No | 112 (79.4) | |
| Yes | 29 (20.6) | ||
| Immunodepression | 9 (6.4) | ||
| Psychiatric conditions | 6 (4.3) | ||
| Heart disease | 3 (2.1) | ||
| Bone and joint disease | 2 (1.4) | ||
| Diabetes | 1 (0.7) | ||
| Hypertension | 1 (0.7) | ||
| Liver disease | 1 (0.7) | ||
| Kidney disease | 1 (0.7) | ||
| Cancer | 1 (0.7) | ||
| Other | 7 (5) | ||
| Drugs | No | 81 (57.5) | |
| Yes | 60 (42.6) | ||
| Antiviral | 39 (27.7) | ||
| Psychiatric drugs | 7 (5) | ||
| Pain medication | 5 (3.6) | ||
| Anti-hypertensive | 2 (1.4) | ||
| Antihistamines | 2 (1.4) | ||
| Corticosteroids | 2 (1.4) | ||
| Other | 6 (4.3) | ||
| Allergies | No | 92 (65.3) | |
| Yes | 49 (34.8) | ||
| Rhinitis | 31 (22) | ||
| Drug allergy | 13 (9.2) | ||
| Asthma | 6 (4.3) | ||
| Contact dermatitis/Hives | 2 (1.4) | ||
| Food allergy | 1 (0.7) | ||
| Insects | 1 (0.7) | ||
| Other | 8 (5.7) | ||
| Healthcare worker | No | 118 (83.7) | |
| Yes | 23 (16.3) | ||
| Medical Doctor | 9 (6.4) | ||
| Other healthcare worker | 9 (6.4) | ||
| Pharmacist | 2 (1.4) | ||
| Volunteer | 2 (1.4) | ||
| Laboratory technician | 1 (0.7) | ||
| First Dose (n = 135) | Second Dose (n = 50) | |||
|---|---|---|---|---|
| Did you take one or more drugs before the vaccine? | No | 124 (91.9) | 44 (88.0) | |
| Yes | 11 (8.1) | 6 (12.0) | ||
| Antiinflammatory drugs | 6 (4.4) | 5 (10.0) | ||
| Antihistamines | 5 (3.7) | 1 (2.0) | ||
| Acetaminophen | 0 (0.0) | 0 (0.0) | ||
| Were other vaccines administered at the same time as the vaccine? c | No | 108 (80.0) | 33 (66.0) | |
| Yes | 27 (20.0) | 17 (34.0) | ||
| Did you experience any AEFI within the first week after the vaccine? | No | 1 (0.7) | 0 (0.0) | |
| Yes | 134 (99.3) | 50 (100.0) | ||
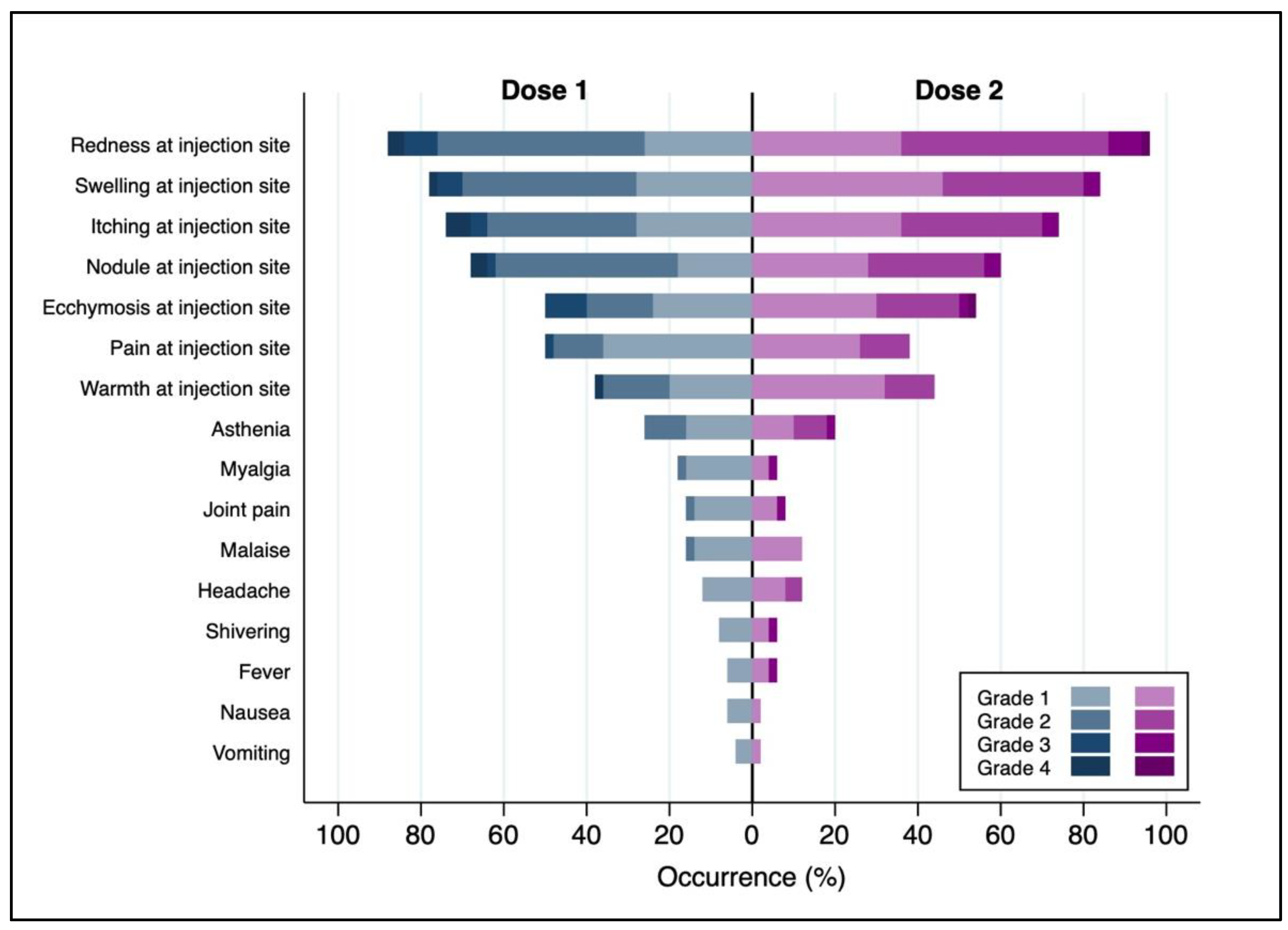
| Local AEFIs | 131 (97.0) | 50 (100.0) | ||
| Redness at injection site | 127 (94.1) | 48 (96.0) | ||
| Swelling at injection site | 114 (84.4) | 42 (84.0) | ||
| Itching at injection site | 107 (79.3) | 37 (74.0) | ||
| Nodule at injection site | 97 (71.9) | 30 (60.0) | ||
| Ecchymosis at injection site | 69 (51.1) | 19 (38.0) | ||
| Pain at injection site | 62 (45.9) | 27 (54.0) | ||
| Warmth at injection site | 61 (45.2) | 22 (44.0) | ||
| Systemic AEFIs | 53 (39.3) | 13 (26.0) | ||
| Asthenia | 40 (29.6) | 10 (20.0) | ||
| Headache | 22 (16.3) | 3 (6.0) | ||
| Malaise | 20 (14.8) | 4 (8.0) | ||
| Myalgia | 19 (14.1) | 6 (12.0) | ||
| Joint pain | 16 (11.9) | 6 (12.0) | ||
| Shivering | 9 (6.7) | 3 (6.0) | ||
| Nausea | 7 (5.2) | 3 (6.0) | ||
| Fever | 6 (4.4) | 1 (2.0) | ||
| Vomiting | 4 (3.0) | 1 (2.0) | ||
| Other | 6 (4.4) | 2 (4.0) | ||
| Within how long after the vaccination did symptoms appear? | No AEFIs | 1 (0.7) | 4 (8.0) | |
| Seconds/minutes | 17 (12.6) | 13 (26.0) | ||
| Two days | 61 (45.2) | 26 (52.0) | ||
| One week | 14 (10.4) | 6 (12.0) | ||
| Does not recall | 6 (4.4) | 1 (2.0) | ||
| No answer | 36 (26.7) | 0 (0.0) | ||
| Did any adverse effects appear later than one week after the vaccination? | No | 99 (73.3) | 42 (84.0) | |
| Yes | 36 (26.7) | 8 (16.0) | ||
| After how long did the AEFI disappear? a | No AEFIs | 1 (0.7) | n.a. | |
| Within one week | 22 (16.3) | n.a. | ||
| Later than one week | 63 (46.7) | n.a. | ||
| Still present | 29 (21.5) | n.a. | ||
| No answer | 20 (14.8) | n.a. | ||
| Reported maximum severity of AEFIs b | Local AEFIs | Grade 1 | 26 (19.8) | 10 (20.0) |
| Grade 2 | 83 (63.4) | 33 (66.0) | ||
| Grade 3 | 9 (6.9) | 5 (10.0) | ||
| Grade 4 | 13 (9.9) | 2 (4.0) | ||
| Systemic AEFIs | Grade 1 | 36 (70.6) | 7 (53.8) | |
| Grade 2 | 14 (27.5) | 5 (38.5) | ||
| Grade 3 | 0 (0) | 1 (7.7) | ||
| Grade 4 | 1 (2) | 0 (0) |
| Local second-dose AEFIs | ||||
| No | Yes | Total | ||
| Local first-dose AEFIs | No | 0 (0.0) | 3 (6.0) | 3 (6.0) |
| Yes | 1 (2.0) | 46 (92.0) | 47 (94.0) | |
| Total | 1 (2.0) | 49 (98.0) | 50 (100.0) | |
| Systemic second-dose AEFIs | ||||
| Systemic first-dose AEFIs | No | 19 (38.0) | 10 (20.0) | 29 (58.0) |
| Yes | 11 (22.0) | 10 (20.0) | 21 (42.0) | |
| Total | 30 (60.0) | 20 (40.0) | 50 (100.0) | |
| Any second-dose AEFIs | ||||
| Any first-dose AEFI | No | 0 (0.0) | 1 (2.0) | 1 (2.0) |
| Yes | 1 (2.0) | 48 (96.0) | 49 (98.0) | |
| Total | 1 (2.0) | 49 (98.0) | 50 (100.0) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montalti, M.; Di Valerio, Z.; Angelini, R.; Bovolenta, E.; Castellazzi, F.; Cleva, M.; Pandolfi, P.; Reali, C.; Resi, D.; Todeschini, R.; et al. Safety of Monkeypox Vaccine Using Active Surveillance, Two-Center Observational Study in Italy. Vaccines 2023, 11, 1163. https://doi.org/10.3390/vaccines11071163
Montalti M, Di Valerio Z, Angelini R, Bovolenta E, Castellazzi F, Cleva M, Pandolfi P, Reali C, Resi D, Todeschini R, et al. Safety of Monkeypox Vaccine Using Active Surveillance, Two-Center Observational Study in Italy. Vaccines. 2023; 11(7):1163. https://doi.org/10.3390/vaccines11071163
Chicago/Turabian StyleMontalti, Marco, Zeno Di Valerio, Raffaella Angelini, Elena Bovolenta, Federica Castellazzi, Marta Cleva, Paolo Pandolfi, Chiara Reali, Davide Resi, Renato Todeschini, and et al. 2023. "Safety of Monkeypox Vaccine Using Active Surveillance, Two-Center Observational Study in Italy" Vaccines 11, no. 7: 1163. https://doi.org/10.3390/vaccines11071163
APA StyleMontalti, M., Di Valerio, Z., Angelini, R., Bovolenta, E., Castellazzi, F., Cleva, M., Pandolfi, P., Reali, C., Resi, D., Todeschini, R., & Gori, D. (2023). Safety of Monkeypox Vaccine Using Active Surveillance, Two-Center Observational Study in Italy. Vaccines, 11(7), 1163. https://doi.org/10.3390/vaccines11071163








