Coronavirus Disease 2019 (COVID-19) Reinfection Rates in Malawi: A Possible Tool to Guide Vaccine Prioritisation and Immunisation Policies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design, Population, Setting and Study Period
2.2. Definition and Determination of Reinfection
2.3. Data Collection Procedures
2.4. Data Analysis
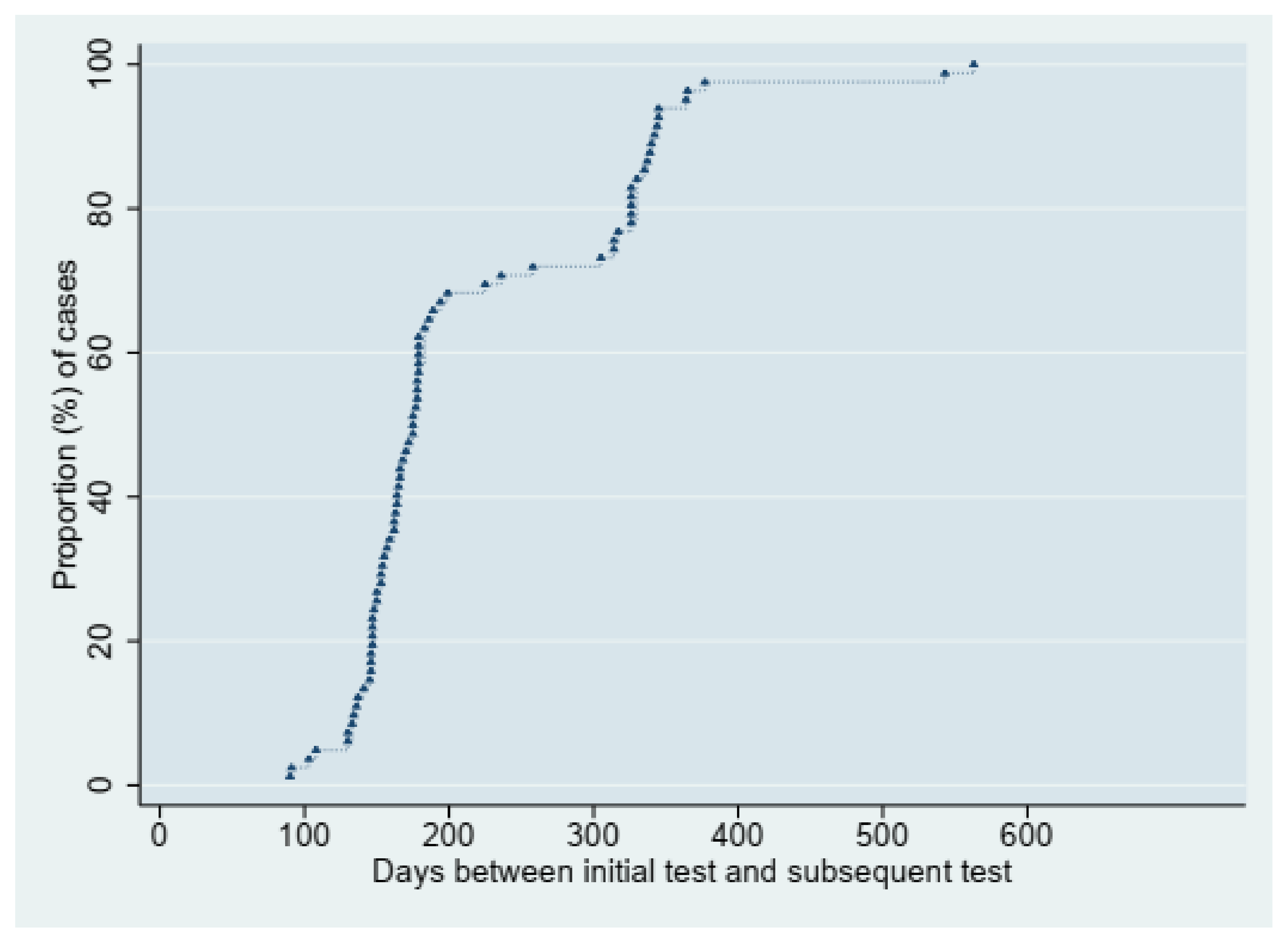
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Worldometer. Worldometer COVID-19 for Malawi. Online. 2022. Available online: https://www.worldometers.info/coronavirus/country/malawi/ (accessed on 31 March 2022).
- Griffin, S. COVID-19: Antibodies protect against reinfection for at least six months, study finds. BMJ 2020, 371, m4961. Available online: https://www.bmj.com/content/371/bmj.m4961 (accessed on 18 June 2023). [CrossRef]
- Conti, M.G.; Terreri, S.; Piano Mortari, E.; Albano, C.; Natale, F.; Boscarino, G.; Zacco, G.; Palobma, P.; Cascioli, S.; Corrente, F.; et al. Immune Response of Neonates Born to Mothers Infected with SARS-CoV-2. JAMA Netw. Open. 2021, 11, e2132563. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhong, J.; Zhang, D. Transplacental Transfer of Maternal Antibody against SARS-CoV-2 and Its Influencing Factors: A Review. Vaccines 2022, 10, 1083. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.A.; Keith, M.; Pace, R.M.; Williams, J.E.; Ley, S.H.; Barbosa-Leiker, C.; Caffe, B.; Smith, C.B.; Kunkle, A.; Lackey, K.A.; et al. SARS-CoV-2 specific antibody trajectories in mothers and infants over two months following maternal infection. Front. Immunol. 2022, 13, 1015002. [Google Scholar] [CrossRef] [PubMed]
- The New York Times. Tracking Coronavirus Vaccinations around the World. 2023. Available online: https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html (accessed on 2 January 2023).
- Financial Times. COVID-19 Vaccine Tracking. Available online: https://ig.ft.com/coronavirus-vaccine-tracker/?areas=gbr&areas=isr&areas=usa&areas=eue&areas=are&areas=chn&areas=chl&cumulative=1&doses=total&populationAdjusted=1 (accessed on 2 January 2023).
- Reuters. COVID-19 Vaccine Tracker. 2023. Available online: https://www.reuters.com/graphics/world-coronavirus-tracker-and-maps/vaccination-rollout-and-access/ (accessed on 2 January 2023).
- World Health Organization. COVID-19 Vaccinations. 2023. Available online: https://covid19.who.int/?mapFilter=vaccinations (accessed on 2 January 2023).
- Chhikara, B.S.; Rathi, B.; Singh, J. Corona virus SARS-CoV-2 disease COVID-19: Infection, prevention and clinical advances of the prospective chemical drug therapeutics. Chem. Biol. 2020, 7, 63–72. [Google Scholar]
- Dickson, M.; Mathews, R.A.; Menon, G.G.; Aid, N.C. Analysis of COVID-19 Reinfection Rates and Its Underlying Causes: A Systematic Review. 2020. Available online: https://www.researchgate.net/publication/345990834_ANALYSIS_OF_COVID-19_REINFECTION_RATES_AND_ITS_UNDERLYING_CAUSES_A_SYSTEMATIC_REVIEW (accessed on 2 January 2023).
- Bobrovitz, N.; Arora, R.K.; Cao, C.; Boucher, E.; Liu, M.; Donnici, C.; Yanes-Lane, M.; Whelan, M.; Perlman-Arrow, S.; Chen, J.; et al. Global seroprevalence of SARS-CoV-2 antibodies: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0252617. Available online: https://dx.plos.org/10.1371/journal.pone.0252617 (accessed on 29 May 2022). [CrossRef] [PubMed]
- Master, R.O.; Chisale; Ramazanu, S.; Mwale, S.E.; Kumwenda, P.; Chipeta, M.; Kaminga, A.C.; Nkhata, O.; Nyambalo, B.; Mbakaya, B.C. Seroprevalence of anti-SARS-CoV-2 antibodies in Africa: A systematic review. Rev. Med. Virol. 2022, e2271. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/rmv.2271 (accessed on 18 June 2023).
- Rostami, A.; Sepidarkish, M.; Leeflang, M.M.G.; Riahi, S.M.; Shiadeh, N.M.; Esfandyari, S.; Mokdad, A.H.; Hotez, P.J.; Gasser, R.B. SARS-CoV-2 seroprevalence worldwide: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Posfay-Barbe, K.M.; Andrey, D.O.; Virzi, J.; Cohen, P.; Pigny, F.; Goncalves, A.R.; Pinosch, S.; Lacroix, L.; Stringhini, S.; Kaiser, L.; et al. Prevalence of Immunoglobulin G (IgG) Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Evaluation of a Rapid MEDsan IgG Test in Children Seeking Medical Care. Clin. Infect. Dis. 2021, 72, e192–e195. [Google Scholar] [CrossRef]
- Franceschi, V.B.; Santos, A.S.; Glaeser, A.B.; Paiz, J.C.; Caldana, G.D.; Lessa, C.L.M.; Mayer, A.d.M.; Kucle, J.G.; Zen, P.R.G.; Vigo, A.; et al. Population-based prevalence surveys during the COVID-19 pandemic: A systematic review. Rev. Med. Virol. 2021, 31, e2200. [Google Scholar] [CrossRef]
- George, C.E.; Inbaraj, L.R.; Chandrasingh, S.; de Witte, L.P. High seroprevalence of COVID-19 infection in a large slum in South India; what does it tell us about managing a pandemic and beyond? Epidemiol. Infect. 2021, 149, e39. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.P.; Piñera, C.; De La Maza, V.; Lagomarcino, A.J.; Simian, D.; Torres, B.; Urquidi, C.; Valenzuela, M.T.; O’ryan, M. Severe Acute Respiratory Syndrome Coronavirus 2 Antibody Prevalence in Blood in a Large School Community Subject to a Coronavirus Disease 2019 Outbreak: A Cross-sectional Study. Clin. Infect. Dis. 2020, 73, e458–e465. [Google Scholar] [CrossRef]
- Murhekar, M.V.; Bhatnagar, T.; Selvaraju, S.; Saravanakumar, V.; Wesley, J.; Thangaraj, V.; Kumar, M.S.; Rade, K.; Sabarinathan, R.; Asthana, S.; et al. SARS-CoV-2 antibody seroprevalence in India, August–September, 2020: Findings from the second nationwide household serosurvey. Lancet Glob. Health 2021, 9, e257–e266. [Google Scholar] [CrossRef]
- Sheehan, M.M.; Reddy, A.J.; Rothberg, M.B. Reinfection Rates among Patients Who Previously Tested Positive for Coronavirus Disease 2019: A Retrospective Cohort Study. Clin. Infect. Dis. 2021, 73, 1882–1886. [Google Scholar] [CrossRef] [PubMed]
- Gussarow, D.; Bonifacius, A.; Cossmann, A.; Stankov, M.V.; Mausberg, P.; Tischer-Zimmermann, S.; Fodecke, N.; Kalinke, U.; Behrens, G.M.N.; Blasczyk, R.; et al. Long-Lasting Immunity Against SARS-CoV-2: Dream or Reality? Front. Med. 2021, 8, 770381. [Google Scholar] [CrossRef]
- Hansen, C.H.; Michlmayr, D.; Gubbels, S.M.; Mølbak, K.; Ethelberg, S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: A population-level observational study. Lancet 2021, 397, 1204–1212. [Google Scholar] [CrossRef]
- Sanyang, B.; Kanteh, A.; Usuf, E.; Nadjm, B.; Jarju, S.; Bah, A.; Bojang, A.; Johnson, M.G.; Jones, J.C.; Gai, A.; et al. Correspondence in The Gambia by phylogenetically variants—First two. Lancet Glob. Health 2021, 9, e905–e907. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.C.; Figueiredo, R. Letter SARS-CoV-2 reinfection: A case report from Portugal. Rev. Soc. Brad. Med. Trop. 2021, 54. [Google Scholar]
- Vitale, J.; Mumoli, N.; Clerici, P.; de Paschale, M.; Evangelista, I.; Cei, M.A.M. Assessment of SARS-CoV-2 Reinfection 1 Year after Primary Infection in a Population in Lombardy, Italy. JAMA Intern. Med. 2021, 181, 1407–1408. [Google Scholar] [CrossRef]
- Abbott. COVID-19 Ag Rapid Test Device. Biomed. Saf. Stand. 2022, 52, 171–172. [Google Scholar]
- Abbott. Abbott Real Time SARS-CoV-2. 2020; 10, p. 608256. Available online: https://www.fda.gov/media/136258/download (accessed on 20 January 2023).
- Yahav, D.; Yelin, D.; Eckerle, I.; Eberhardt, C.S.; Wang, J.; Cao, B.; Kaiser, L. Definitions for coronavirus disease 2019 reinfection, relapse and PCR re-positivity. Clin. Microbiol. Infect. 2021, 27, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Chisale, M.R.O.; Nyambalo, B.W.; Mitambo, C.; Kumwenda, P.; Mwale, S.E.; Mbakaya, B.C. Comparative characterisation of COVID-19 patients with hypertension comorbidity in Malawi: A 1:2 matched retrospective case-control study. IJID Reg. 2022, 2, 25–29. Available online: https://www.sciencedirect.com/science/article/pii/S277270762100031X (accessed on 18 June 2023). [CrossRef]
- Chisale, M.; Mwale, S.; Mbakaya, B.; Kumwenda, P.; Regina Makhamba, C.B. Knowledge, Attitudes and Practices on COVID-19 Vaccinations among General Population in Malawi: A Country Wide Cross-Sectional Survey, Faculty of Sciences, Technology and Innovations, Biological Sciences, Mzuzu University: Mzuzu, Malawi, 2023; unpublished.
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef]
- Lumley, S.F.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Phil, D.; Cox, S.; James, T.; et al. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N. Engl. J. Med. 2021, 384, 533–540. [Google Scholar] [CrossRef]
- Ren, X.; Zhou, J.; Guo, J.; Hao, C.; Zheng, M.; Zhang, R.; Huang, Q.; Yao, X.; Li, R.; Jin, Y. Reinfection in patients with COVID-19: A systematic review. Glob. Health Res. Policy 2022, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Okhuese, A.V. Estimation of the probability of reinfection with COVID-19 by the susceptible-exposed-infectious-removed-undetectable-susceptible model. JMIR Public Health Surveill. 2020, 6, e19097. [Google Scholar] [CrossRef] [PubMed]
- Flacco, M.E.; Soldato, G.; Acuti Martellucci, C.; Di Martino, G.; Carota, R.; Caponetti, A.; Manzoli, L. Risk of SARS-CoV-2 Reinfection 18 Months After Primary Infection: Population-Level Observational Study. Front. Public Health 2022, 10, 2020–2023. [Google Scholar] [CrossRef] [PubMed]
- Finch, E.; Lowe, R.; Fischinger, S.; Aubin, M.D.S.; Siddiqui, S.M.; Dayal, D.; Loesche, M.A.; Rhee, J.; Beger, S.; Hu, Y.; et al. SARS-CoV-2 antibodies protect against reinfection for at least 6 months in a multicentre seroepidemiological workplace cohort. PLoS Biol. 2022, 20, e3001531. [Google Scholar] [CrossRef] [PubMed]
- Medić, S.; Anastassopoulou, C.; Lozanov-Crvenković, Z.; Vuković, V.; Dragnić, N.; Petrović, V.; Ristic, M.; Pustahija, T.; Gojkovic, Z.; Tsakris, A.; et al. Risk and severity of SARS-CoV-2 reinfections during 2020–2022 in Vojvodina, Serbia: A population-level observational study. Lancet Reg. Health Eur. 2022, 20, 100453. [Google Scholar] [CrossRef]
- Pilz, S.; Chakeri, A.; Ioannidis, J.P.A.; Richter, L.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Allerberger, F. SARS-CoV-2 reinfection risk in Austria. Eur. J. Clin. Invest. 2021, 51, e13520. [Google Scholar] [CrossRef]
- Harvey, R.A.; Rassen, J.A.; Kabelac, C.A.; Turenne, W.; Leonard, S.; Klesh, R.; Meyer, W.A.; Kaufman, H.W.; Anderson, S.; Cohen, O.; et al. Association of SARS-CoV-2 Seropositive Antibody Test with Risk of Future Infection. JAMA Intern. Med. 2021, 181, 672–679. [Google Scholar] [CrossRef]
- Babiker, A.; Marvil, C.E.; Waggoner, J.J.; Collins, M.H.; Piantadosia, A. The Importance and Challenges of Identifying SARS-CoV-2 Reinfections. J. Clin. Microbiol. 2021, 59, e02769-20. [Google Scholar] [CrossRef] [PubMed]
- Amanna, I.J.; Slifka, M.K.; Crotty, S. Immunity and immunological memory following smallpox vaccination. Immunol. Rev. 2006, 211, 320–337. [Google Scholar] [CrossRef] [PubMed]
- WHO. COVID-19 Vaccine Efficacy, Effectiveness and Protection. 2021. Available online: https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection (accessed on 20 January 2023).
- Wu, N.; Joyal-Desmarais, K.; Ribeiro, P.A.B.; Vieira, A.M.; Stojanovic, J.; Sanuade, C.; Yip, D.; Bacon, S.L. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: Findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir. Med. 2023, 11, 439–452. [Google Scholar] [CrossRef]
- Tavakoli, A.; Lotfi, F. COVID-19 Reinfection Rate and Related Risk Factors in Fars Province, Iran: A Retrospective Cohort Study. Iran. J. Med. Sci. 2022, 48, 302–312. [Google Scholar]
- Islam, Z.; Riazb, B.K.; Ashrafic, S.A.A.; Farjanad, S.; Efae, S.S.; Khan, M.A. Severity of COVID-19 reinfection and associated risk factors: Findings of a cross-sectional study in Bangladesh. medRxiv 2022, 2021, 12. [Google Scholar]
- Kumar, V.; Iyengar, K.; Garg, R.; Vaishya, R. Diabetes & Metabolic Syndrome: Clinical Research & Reviews Elucidating reasons of COVID-19 reinfection and its management strategies. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 1001–1006. [Google Scholar] [CrossRef]
- Piazza, M.F.; Amicizia, D.; Marchini, F.; Astengo, M.; Grammatico, F.; Battaglini, A.; Sticchi, C.; Paganino, C.; Lavieri, R.; Battaglini, A.; et al. Who Is at Higher Risk of SARS-CoV-2 Reinfection? Results from a Northern Region of Italy. Vaccines 2022, 10, 1885. [Google Scholar] [CrossRef] [PubMed]
- Grad, Y.H.; Larremore, D.B. Model-informed COVID-19 vaccine prioritisation strategies by age and serostatus. Science 2021, 921, 916–921. [Google Scholar]
- Asri, A.; Asri, V.; Renerte, B.; Föllmi-Heusi, F.; Leuppi, J.D.; Muser, J.; Nüesch, R.; Schuler, D.; Fischbacher, U. Wearing a mask-For yourself or for others? Behavioral correlates of mask wearing among COVID-19 frontline workers. PLoS ONE 2021, 16, e0253621. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Joshi, A.D.; Lo, C.-H.; Drew, D.A.; Nguyen, L.H.; Guo, C.-G.; Ma, W.; Mehta, R.S.; Shebl, F.M.; Warner, E.T.; et al. Association of social distancing and face mask use with risk of COVID-19. Nat. Commun. 2021, 12, 3737. [Google Scholar] [CrossRef]

| Variable | Frequency | Percent |
|---|---|---|
| Country of residence | ||
| Malawi | 43,859 | 99.37 |
| South Africa | 13 | 0.03 |
| Other African Countries | 102 | 0.23 |
| Non-African Countries | 165 | 0.37 |
| Age | ||
| 0–19 | 6697 | 15.39 |
| 20–39 | 21,040 | 48.36 |
| 40–59 | 11,053 | 25.41 |
| ≥60 | 4713 | 10.83 |
| Sex/Gender | ||
| Male | 25,310 | 55.76 |
| Female | 20,081 | 44.24 |
| Presentation | ||
| Symptomatic | 29,645 | 65.17 |
| Asymptomatic | 11,696 | 25.71 |
| Not Documented | 4145 | 9.11 |
| Hospitalised | ||
| Yes | 2156 | 4.74 |
| No | 36,595 | 80.45 |
| Not documented | 6735 | 14.81 |
| Reinfection | ||
| Not Reinfected | 45,404 | 99.82 |
| Reinfected | 82 | 0.18 |
| Variable | Unadjusted OR (95% CI) † | p-Value | Adjusted OR (95% CI) ǂ | p-Value | |
|---|---|---|---|---|---|
| Sex | Female (ref) | - | - | - | - |
| Male | 1.62 (1.02–2.56) | 0.04 | 1.47 (0.92–2.33) | 0.11 | |
| Age (years) | 0–19 (ref) | - | - | - | - |
| 20–39 | 3.12 (1.24–7.84) | 0.02 | 2.83 (1.13–7.13) | 0.03 | |
| 40–59 | 2.79 (1.06–7.34) | 0.04 | 2.58 (0.98–6.81) | 0.06 | |
| ≥60 | 1.42 (0.41–4.91) | 0.58 | 1.39 (0.40–4.84) | 0.60 | |
| Clinical presentation | Asymptomatic (ref) | - | - | - | - |
| Symptomatic | 0.56 (0.35–0.91) | 0.02 | 0.59 (0.36–0.95) | 0.03 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chisale, M.R.O.; Sinyiza, F.W.; Kaseka, P.U.; Chimbatata, C.S.; Mbakaya, B.C.; Wu, T.-S.J.; Nyambalo, B.W.; Chauma-Mwale, A.; Chilima, B.; Yu, K.-L.J.; et al. Coronavirus Disease 2019 (COVID-19) Reinfection Rates in Malawi: A Possible Tool to Guide Vaccine Prioritisation and Immunisation Policies. Vaccines 2023, 11, 1185. https://doi.org/10.3390/vaccines11071185
Chisale MRO, Sinyiza FW, Kaseka PU, Chimbatata CS, Mbakaya BC, Wu T-SJ, Nyambalo BW, Chauma-Mwale A, Chilima B, Yu K-LJ, et al. Coronavirus Disease 2019 (COVID-19) Reinfection Rates in Malawi: A Possible Tool to Guide Vaccine Prioritisation and Immunisation Policies. Vaccines. 2023; 11(7):1185. https://doi.org/10.3390/vaccines11071185
Chicago/Turabian StyleChisale, Master R. O., Frank Watson Sinyiza, Paul Uchizi Kaseka, Chikondi Sharon Chimbatata, Balwani Chingatichifwe Mbakaya, Tsung-Shu Joseph Wu, Billy Wilson Nyambalo, Annie Chauma-Mwale, Ben Chilima, Kwong-Leung Joseph Yu, and et al. 2023. "Coronavirus Disease 2019 (COVID-19) Reinfection Rates in Malawi: A Possible Tool to Guide Vaccine Prioritisation and Immunisation Policies" Vaccines 11, no. 7: 1185. https://doi.org/10.3390/vaccines11071185






