The Common Mucosal System Fifty Years on: From Cell Traffic in the Rabbit to Immune Resilience to SARS-CoV-2 Infection by Shifting Risk within Normal and Disease Populations
Abstract
:1. Introduction
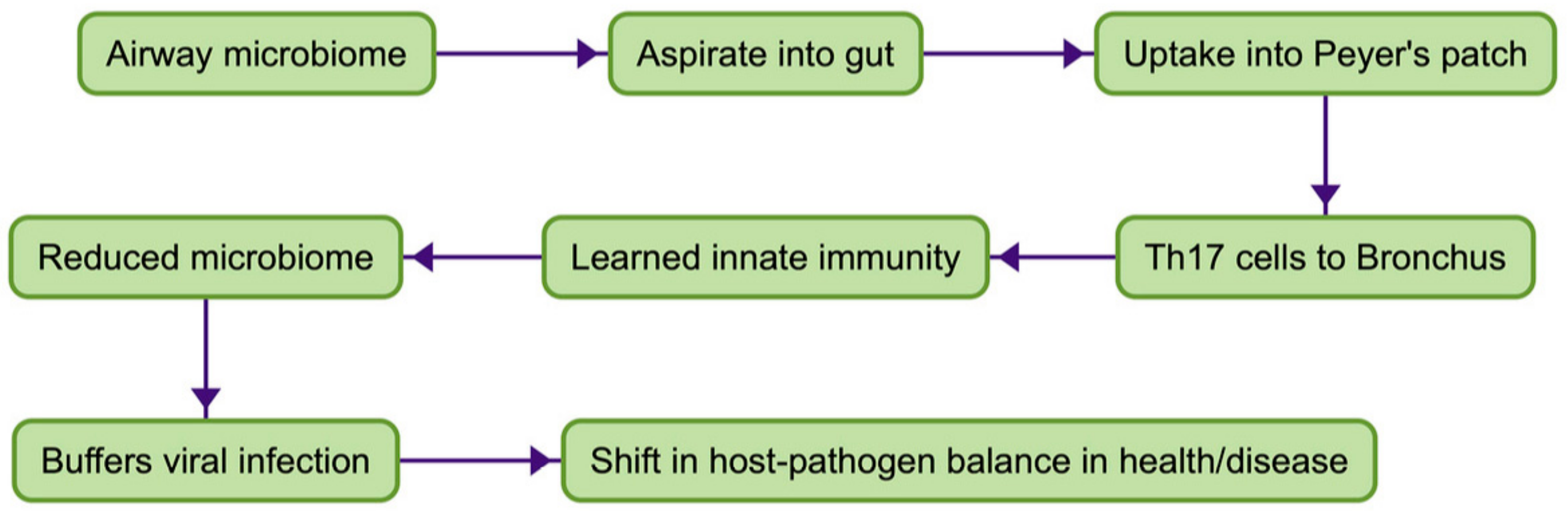
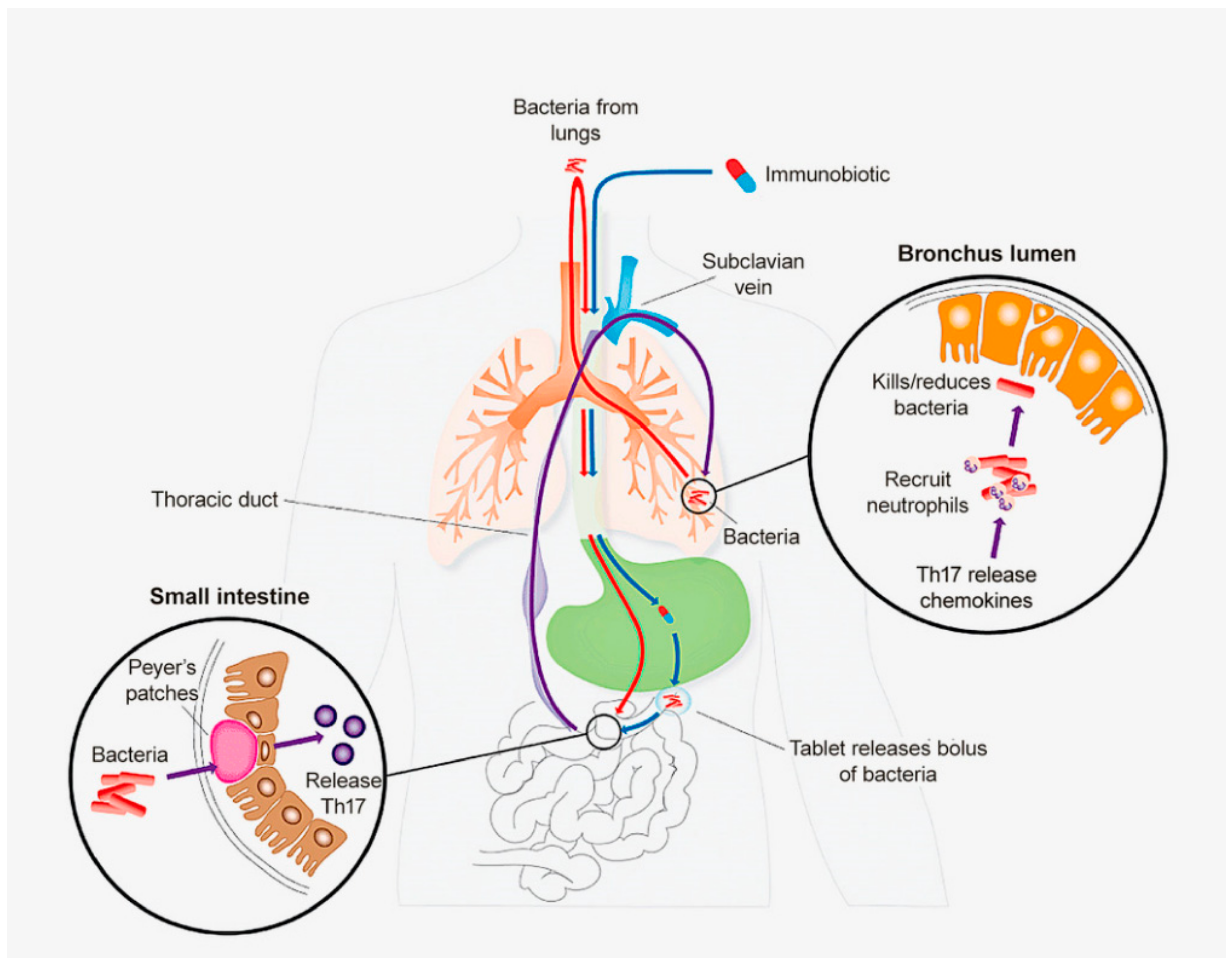
2. The Common Mucosal System (CMS)
3. The Common Mucosal System in Man
4. The CMS in Normal Subjects: Does Oral NTHi Have a Role in Reducing Risk from Viral Infection?
5. Discussion
6. Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shrestha, N.; Burke, C.; Nowacki, A. Risk of Coronavirus Disease (COVID-19) among Those Up-to-Date and Not-Up-to-Date on COVID-19 Vaccination. medRxiv 2023. Available online: https://www.medrxiv.org/content/10.1101/2023.06.09.23290893v1 (accessed on 3 July 2023).
- Tomasi, T.; Zieglbaum, S. The selective occurrence of gamma1 A globulins in certain body fluids. J. Clin. Investig. 1963, 42, 1553–1560. [Google Scholar] [CrossRef]
- Craig, S.; Cebra, J. Peyers patches: An enriched source of precursors for IgA-producing immunocytes in the rabbit. J. Exp. Med. 1971, 134, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Bienenstock, J.; Rudzik, O.; Clancy, R. Bronchial lymphoid tissue. Adv. Exp. Med. Biol. 1974, 45, 47–56. [Google Scholar]
- Cripps, A.W.; Dunkley, M.L.; Clancy, R.L. Mucosal and systemic immunisations with killed Pseudomonas aeruginosa protect against acute respiratory infection in rats. Infect. Immun. 1994, 62, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Clancy, R.; Bienenstock, J. The proliferative response of bronchus-associated lymphoid tissue after local and systemic immunization. J. Immunol. 1974, 112, 1997–2001. [Google Scholar] [CrossRef] [PubMed]
- Clancy, R.L.; Pucci, A.; Jelihovsky, T.; Bye, P. Immunologic “memory” for microbial antigens in lymphocytes obtained from human bronchial mucosa. Am. Rev. Respir. Dis. 1978, 117, 513–518. [Google Scholar]
- Holt, P.; Stumbles, P. Regulation of immunologic homeostasis in peripheral tissues by dendritic cells: The respiratory tract as a paradigm. J. Allergy Clin. Immunol. 2000, 105, 421–429. [Google Scholar] [CrossRef]
- Palomares, O. The role of regulatory T cells in IgE-mediated food allergy. J. Investig. Allergol. Clin. Immunol. 2013, 23, 371–382. [Google Scholar]
- Verhagen, J.; Blaser, K.; Akdis, C. Mechanisms of allergen-specific immunotherapy: T-regulatory cells and more. Immunol. Allergy Clin. N. Am. 2006, 26, 207–231. [Google Scholar] [CrossRef]
- UK Health Security Agency. COVID-19 Vaccine Surveillance Report; UK Health Security Agency: London, UK, 2021; Volume 42. [Google Scholar]
- Barberis, I.; Myles, P.; Ault, S. History and evolution of influenza control through vaccination: From the first monovalent vaccine to universal vaccines. J. Prev. Med. Hyg. 2016, 57, E115–E120. [Google Scholar]
- Wallace, F.; Cripps, A.; Clancy, R. A role for intestinal T lymphocytes in bronchus mucosal immunity. Immunology 1991, 74, 68–73. [Google Scholar] [PubMed]
- Clancy, R.; Dunkley, M. Acute Exacerbations in COPD and their control with oral immunization with non-typable Haemophilus influenzae. Front. Immunol. 2011, 2, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, F.; Cripps, A.; Clancy, R. Haemophilus influenzae immunity in the respiratory tract. In Advances in Mucosal Immunology; MacDonald, T., Challacombe, S., Bland, P., Eds.; Kluwer Academic Publishers: London, UK, 1990; Volume 74, pp. 859–860. [Google Scholar]
- Pang, G.; Ortega, M.; Zighang, R. Autocrine modulation of IL-8 production by sputum meutrophils in chronic bronchial sepsis. Am. J. Respir. Crit. Care Med. 1997, 155, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.; Dominguez-Andres, J.; Barreiro, L. Defining trained immunity and its role in health and disease Nature Reviews. Immunology 2020, 20, 375–388. [Google Scholar]
- Husband, A.; Dunkley, M.; Cripps, A. Antigen-specific response among T lymphocytes following intestinal administration of alloantigens. Aust. J. Exp. Biol. Med. Sci. 1984, 62, 687–699. [Google Scholar] [CrossRef]
- Clarke, J. Regulation of Cytokines and Chemokines during Lung Infection with Non-Typable Haemophilus Influenzae. Ph.D. Thesis, University of Canberra, Canberra, Australia, 2008. [Google Scholar]
- Chen, Y.; Li, F.; Hua, M.; Liang, M.; Song, C. Role of GM-CSF in Lung Balance and disease. Front. Immunol. 2023, 14, 1158859. [Google Scholar] [CrossRef] [PubMed]
- Butt, H.L.; Clancy, R.L.; Cripps, A.W.; Murree-Allen, K.; Saunders, N.A.; Sutherland, D.C.; Hensley, M.J. Bacterial colonisation of the respiratory tract in chronic bronchitis. Aust. N. Z. J. Med. 1990, 20, 35–38. [Google Scholar] [CrossRef]
- Dunkley, M.; Husband, A.; Beagley, K. A rodent model of concurrent respiratory infection with influenza virus and gram-negative bacteria:synergistic infection and protection by oral immunisation. In Mucosal Solutions: Advances in Mucosal Immunology; University of Sydney: Sydney, Australia, 1997; Volume 1, pp. 261–268. [Google Scholar]
- Iwanaga, N.; Kolls, J. Updates on T helper type 17 immunity in respiratory disease. Immunology 2019, 156, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Mvaya, L.; Mvaya, A.; Hummel, A. Airway CD8+CD161++TCRva7.2+ T cell depletion during untreated HIV infection targets CD103 expressing cells. Front. Immunol. 2019, 10, 2003. [Google Scholar] [CrossRef] [Green Version]
- Clancy, R.; Murree-Allen, K.; Cripps, A. Oral immunisation with killed haemophilus influenza for protection against acute bronchitis in chronic obstructive lung disease. Lancet 1985, 326, 1395–1397. [Google Scholar] [CrossRef]
- Tandon, M.; Gebsky, V. A controlled trial of a killed haemophilus influenzae vaccine for prevention of acute exacerbations of chronic bronchitis. Intern. Med. J. 1991, 21, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, D.; Coakley, K.; Coakley, C. Reduction in the incidence of acute bronchitis by an haemophilus influenzae vaccine in patients with chronic bronchitis in the highlands of Papua New Guinea. Am. J. Resp. Crit. Care Med. 1991, 144, 324–330. [Google Scholar]
- Tandon, M.; Phillips, M.; Waterer, G. Oral immunotherapy with inactivated nontypable haemophilus influenzae reduces severity of acute exacerbations in severe COPD. Chest 2010, 137, 805–811. [Google Scholar] [CrossRef]
- Clancy, R.; Dunkley, M.; Sockler, J. Multi-site placebo-controlled randomised clinical trial to assess protection following oral immunisation with inactivated non-typable haemophilus influenzae in chronic obstructive pulmonary disease. Int. Med. J. 2016, 46, 684–693. [Google Scholar] [CrossRef]
- Clancy, R.; Cripps, A. An Oral Whole-Cell Killed Nontypable Haemophilus influenzae Immunotherapeutic for the Prevention of Acute Exacerbations of Chronic Airway Disease. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 2423–2431. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Morales, A. Assessing the effectiveness of COVID-19 vaccines in older people in Latin America. Lancet Healthy Longev. 2022, 3, E219–E220. [Google Scholar] [CrossRef] [PubMed]
- Clancy, R.; Dunkley, M. Oral nontypable haemophilus influenzae enhances physiological mechanism of airways protection. Clin. Exp. Immunol. 2010, 161, 127–133. [Google Scholar] [CrossRef]
- Clancy, R.; Cripps, A.; Gebski, V. Protection against recurrent bronchitis after oral immunisation with Killed haemophilus influenzae. Med. J. Aust. 1990, 152, 413–416. [Google Scholar] [CrossRef]
- Wallace, F.; Clancy, R.; Cripps, A. An animal model demonstration of enhanced clearance of nontypable haemophilus influenzae from the respiratory tract after antigen stimulation of gut-associated lymphoid tissue. Am. Rev. Respir. Dis. 1989, 140, 311–316. [Google Scholar] [CrossRef]
- Clancy, R.; Cripps, A.; Pang, G. The paradox of immunisation against Haemophilus influenzae-related endobronchitis: Protection restricted to ingestion of “non-adjuvenated” vaccine. Adv. Exp. Med. Biol. 1987, 216B, 1759–1764. [Google Scholar] [PubMed]
- Rich, R.; Mollick, J.; Cook, R. Superantigens: Interaction of staphylococcal enterotoxins with MHC class II molecules. Trans. Am. Clin. Climatol. Assoc. 1990, 101, 195–206. [Google Scholar] [PubMed]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef]
- Stertz, S.; Hale, B. Interferon system deficiencies exacerbating severe pandemic virus infections. Trends Microbiol. 2021, 29, 973–982. [Google Scholar] [CrossRef]
- Drzmalla, E.; Green, R.; Knuth, M. COVID-19-related health outcomes in people with primary immunodeficiency: A systematic review. Clin. Immunol. 2022, 243, 109097. [Google Scholar] [CrossRef]
- Patanavanich, R.; Glantz, S. Smoking is associated with worse outcomes of COVID-19 particularly among younger adults. BMC Public. Health 2021, 21, 1554. [Google Scholar] [CrossRef]
- Morens, D.; Taubenberger, J.; Fauci, A. Rethinking next-generation vaccines for coronaviruses, influenza viruses, and other respiratory viruses. Cell Host Microbe 2023, 31, 146–157. [Google Scholar] [CrossRef]
- Clancy, R.; Cripps, A.; Husband, A. Specific Immune Response in the Respiratory Tract after Administration of an Oral Polyvalent bacterial vaccine. Infect. Immunol. 1983, 39, 491–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, M.; Moldoveanu, Z.; Ogra, P. Mucosal Immunity in COVID-19: A Neglected but Critical Aspect of SARS-CoV-2 Infection. Front. Immunol. 2020, 11, 611337. [Google Scholar] [CrossRef]
- Mestecky, J. The Common Mucosal Immune System and Current Strategies for Induction of Immune Responses in External Secretions. J. Clin. Immunol. 1987, 7, 265–276. [Google Scholar] [CrossRef]
- Holmgren, J.; Czevrkinsky, C. Mucosal Immunity and Vaccines. Nat. Med. 2005, 11 (Suppl. S4), S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Lovelle, E.; Ward, R. Mucosal Vaccines-fortifying the Frontiers. Nat. Rev. Immunol. 2022, 22, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Fraser, R.; Orta-Resendiz, A.; Mazein, A. Upper Respiratory Tract Mucosal Immunity for SARS-CoV-2 Vaccines. Trends Mol. Med. 2023, 29, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.; Manoharen, M.; Lee, G. Immune resilience despite inflammatory stress promotes longevity and favourable health outcomes including resistance to infection. Nat. Commun. 2023, 14, 3286. [Google Scholar] [CrossRef]
- Zimmerman, P.; Curtis, N. The Influence of Probiotics on Vaccine responses—A Systemic Review. Vaccine 2018, 36, 207–213. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clancy AM, R. The Common Mucosal System Fifty Years on: From Cell Traffic in the Rabbit to Immune Resilience to SARS-CoV-2 Infection by Shifting Risk within Normal and Disease Populations. Vaccines 2023, 11, 1251. https://doi.org/10.3390/vaccines11071251
Clancy AM R. The Common Mucosal System Fifty Years on: From Cell Traffic in the Rabbit to Immune Resilience to SARS-CoV-2 Infection by Shifting Risk within Normal and Disease Populations. Vaccines. 2023; 11(7):1251. https://doi.org/10.3390/vaccines11071251
Chicago/Turabian StyleClancy AM, Robert. 2023. "The Common Mucosal System Fifty Years on: From Cell Traffic in the Rabbit to Immune Resilience to SARS-CoV-2 Infection by Shifting Risk within Normal and Disease Populations" Vaccines 11, no. 7: 1251. https://doi.org/10.3390/vaccines11071251
APA StyleClancy AM, R. (2023). The Common Mucosal System Fifty Years on: From Cell Traffic in the Rabbit to Immune Resilience to SARS-CoV-2 Infection by Shifting Risk within Normal and Disease Populations. Vaccines, 11(7), 1251. https://doi.org/10.3390/vaccines11071251





