An Analysis of Linker-Dependent Effects on the APC Activation and In Vivo Immunogenicity of an R848-Conjugated Influenza Vaccine
Abstract
:1. Introduction
2. Materials and Methods
3. Results
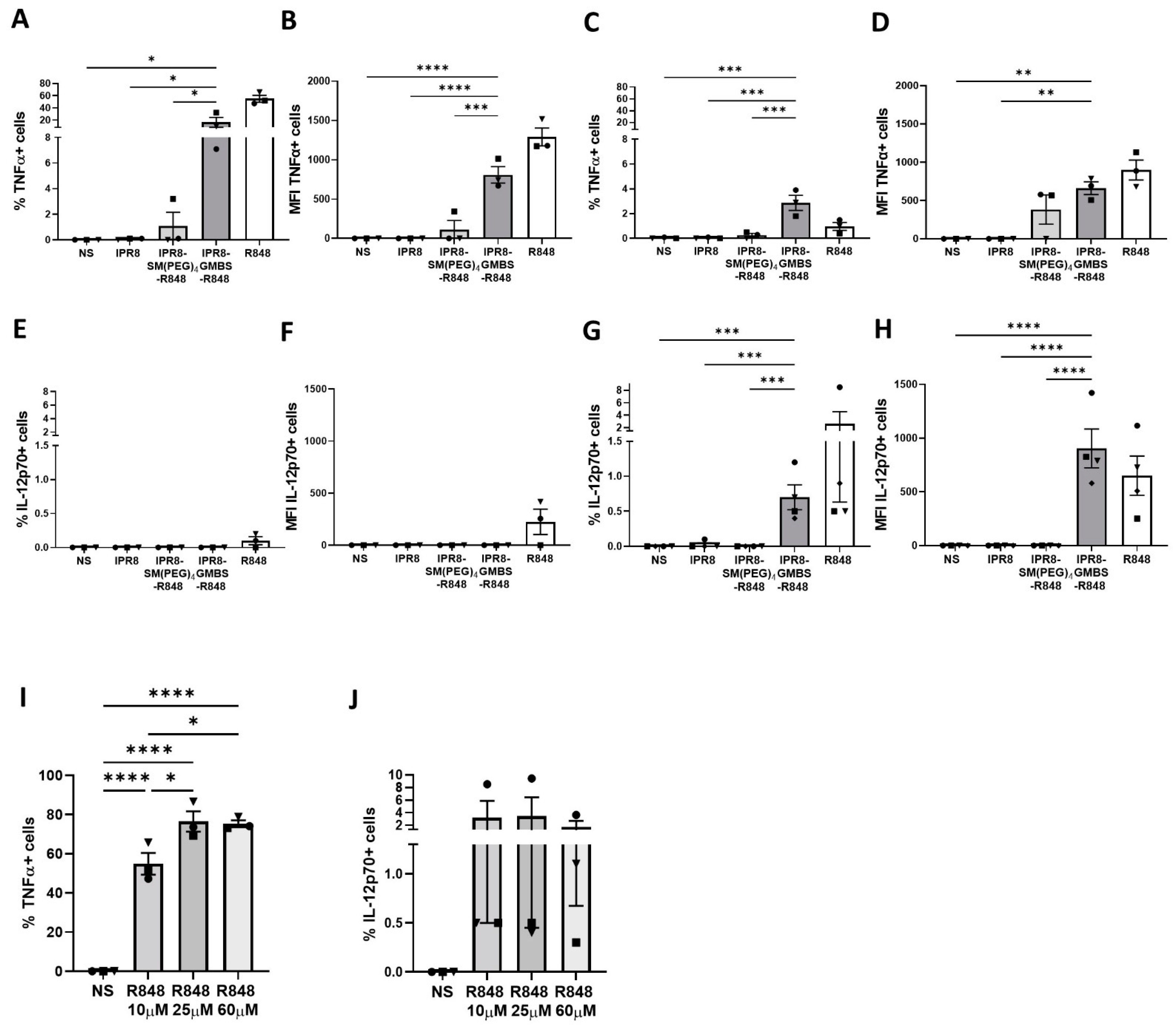
3.1. The GMBS-Containing Vaccine Promotes Proinflammatory Cytokine Production to a Higher Level and in an Increased Percentage of Human moDCs Compared to the SM(PEG)4-Containing Vaccine
3.2. GMBS and SM(PEG)4 Vaccine Constructs Have Similar Amounts of R848 Conjugated to the Inactivated Virion
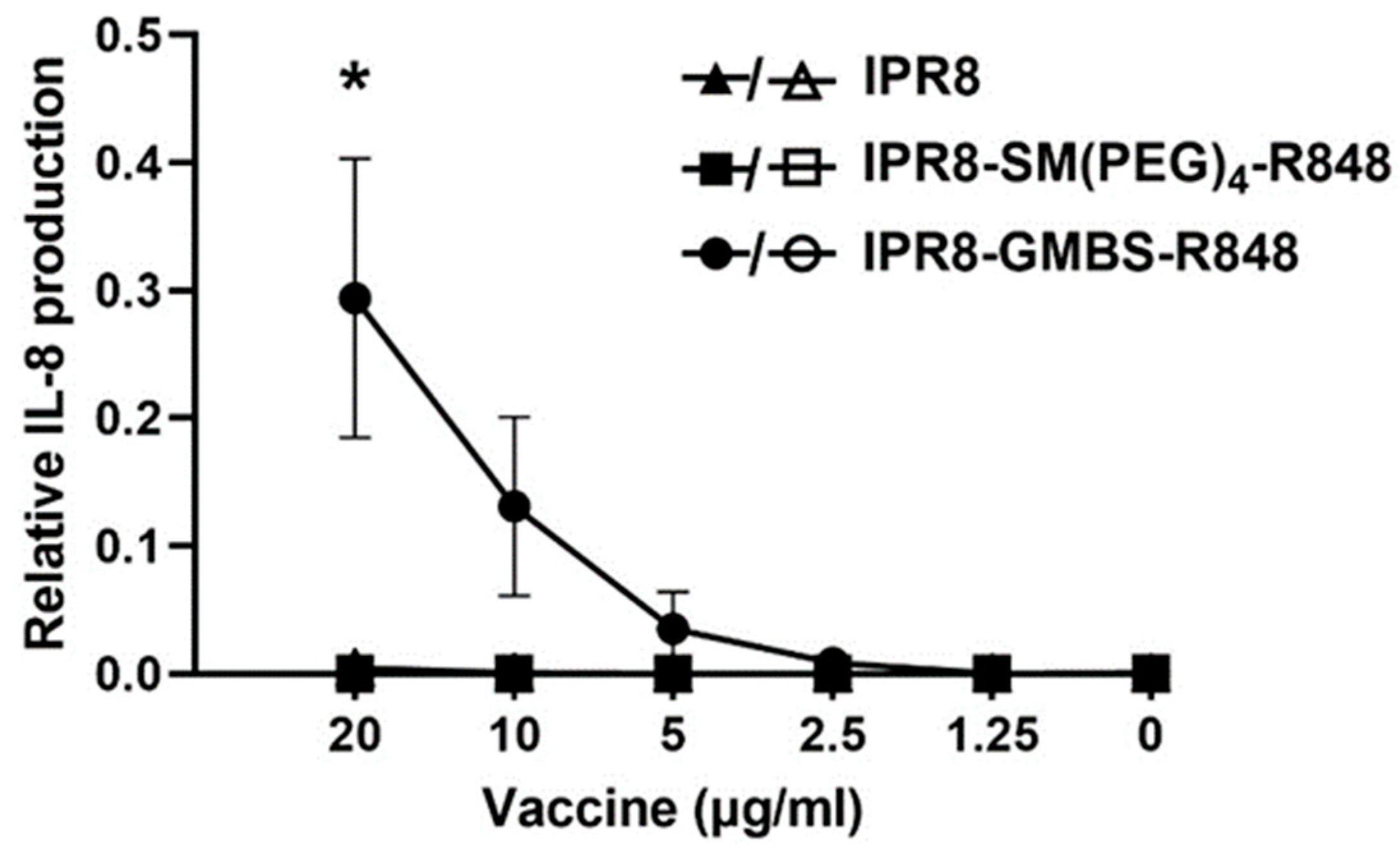
3.3. Differential Aggregation Does Not Account for the Stimulatory Activity Observed with the Vaccine Constructs
3.4. The Linker-Dependent Differences in Stimulatory Activity Are Dependent on TLR7/8 Engagement
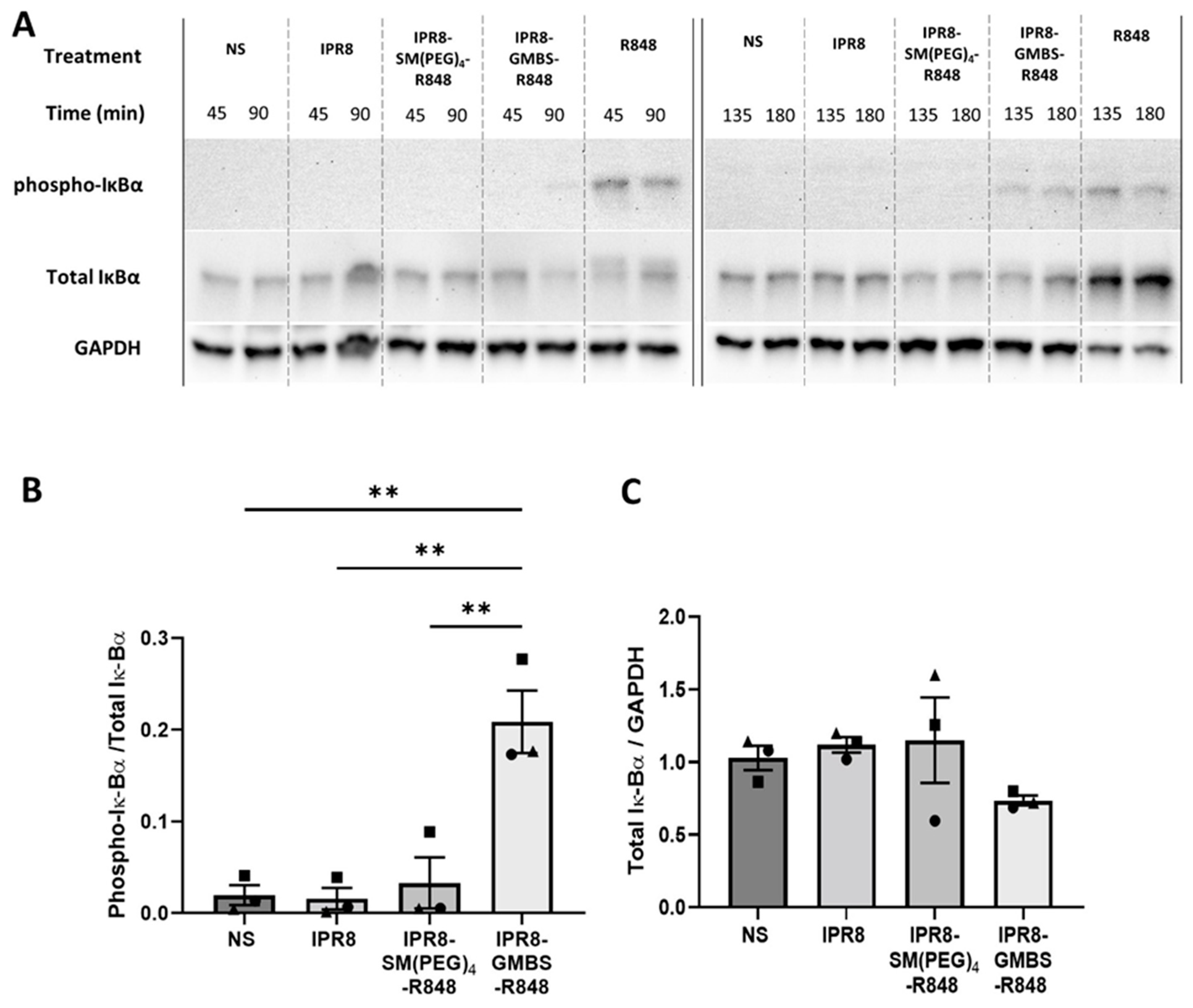
3.5. IPR8-GMBS-R848 Promotes Increases in IκBα Phosphorylation Compared to IPR8-SM(PEG)4-R848
3.6. The GMBS Vaccine Construct Promotes Proinflammatory Cytokine Production from APC within the PBMCs
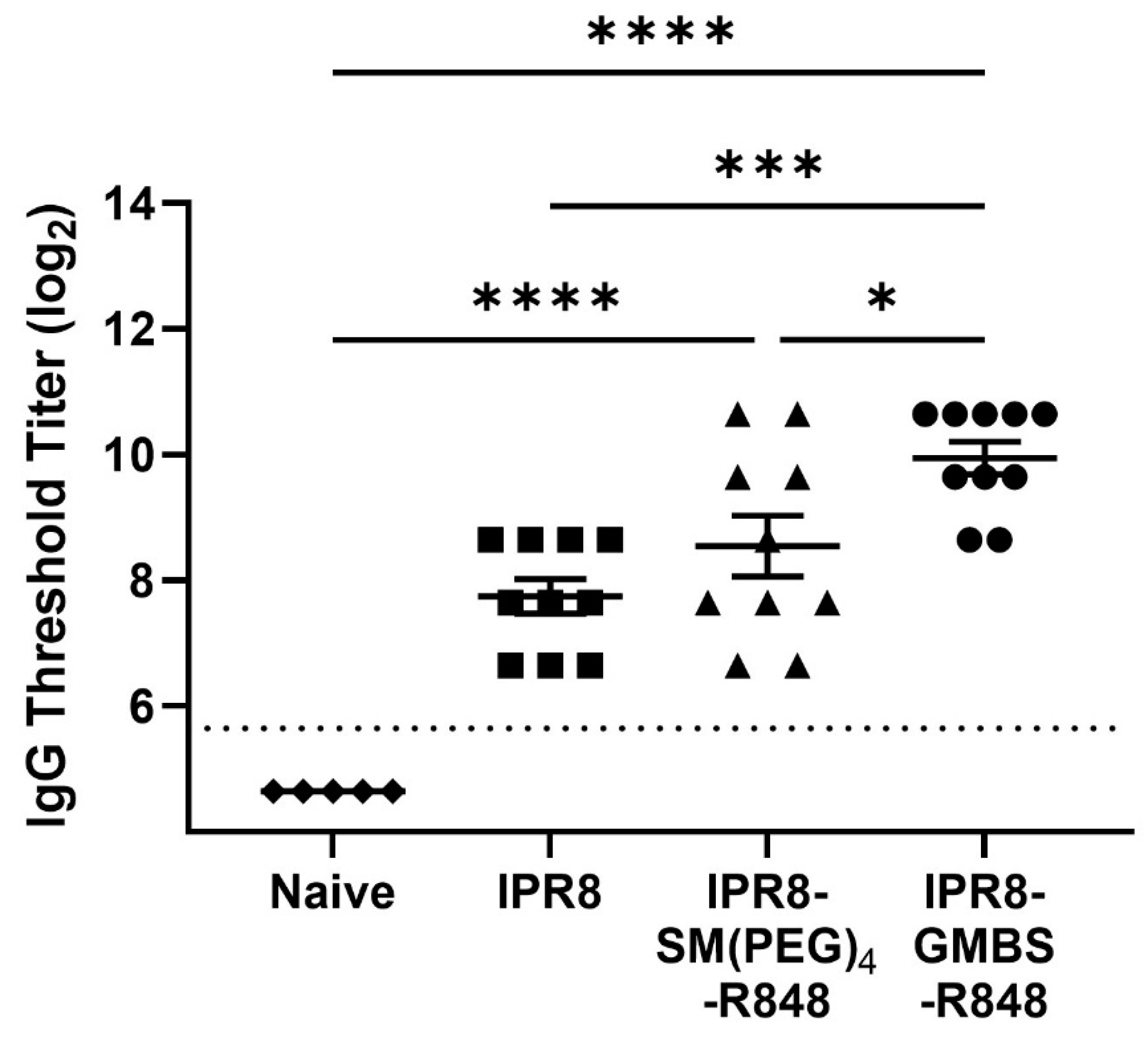
3.7. The GMBS-Conjugated Vaccine Promotes Increased PR8-Specific IgG Antibody Compared to the SM(PEG)4-Conjugated and IPR8 Vaccines
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pulendran, B.; SArunachalam, P.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.M.; Cybulski, V.; Whitacre, M.; Bess, L.S.; Livesay, M.T.; Walsh, L.; Burkhart, D.; Bazin, H.G.; Evans, J.T. Novel lipidated imidazoquinoline TLR7/8 adjuvants elicit influenza-specific Th1 immune responses and protect against heterologous H3N2 influenza challenge in mice. Front. Immunol. 2020, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, C.; Bertholet, S.; Philpott, D.J.; De Gregorio, E. Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proc. Natl. Acad. Sci. USA 2014, 111, 12294–12299. [Google Scholar] [CrossRef] [PubMed]
- Dowling, D.J. Recent advances in the discovery and delivery of TLR7/8 agonists as vaccine adjuvants. Immunohorizons 2018, 2, 185–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Pierce, S.K. How location governs toll-like receptor signaling. Traffic 2009, 10, 621–628. [Google Scholar] [CrossRef] [Green Version]
- Holbrook, B.C.; D’Agostino, R.B., Jr.; Tyler Aycock, S.; Jorgensen, M.J.; Hadimani, M.B.; Bruce King, S.; Alexander-Miller, M.A. Adjuvanting an inactivated influenza vaccine with conjugated R848 improves the level of antibody present at 6 months in a nonhuman primate neonate model. Vaccine 2017, 35, 6137–6142. [Google Scholar] [CrossRef]
- Holbrook, B.C.; Aycock, S.T.; Machiele, E.; Clemens, E.; Gries, D.; Jorgensen, M.J.; Hadimani, M.B.; King, S.B.; Alexander-Miller, M.A. An R848 adjuvanted influenza vaccine promotes early activation of B cells in the draining lymph nodes of non-human primate neonates. Immunology 2018, 153, 357–367. [Google Scholar] [CrossRef]
- Holbrook, B.C.; Kim, J.R.; Blevins, L.K.; Jorgensen, M.J.; Kock, N.D.; D’Agostino, R.B., Jr.; Aycock, S.T.; Hadimani, M.B.; King, S.B.; Parks, G.D.; et al. A novel R848-conjugated inactivated influenza virus vaccine is efficacious and safe in a neonate nonhuman primate model. J. Immunol. 2016, 197, 555–564. [Google Scholar] [CrossRef] [Green Version]
- Clemens, E.A.; Holbrook, B.C.; Kanekiyo, M.; Yewdell, J.W.; Graham, B.S.; Alexander-Miller, M.A. An R848 conjugated influenza virus vaccine elicits robust IgG to hemagglutinin stem in a newborn nonhuman primate model. J. Infect. Dis. 2020, 224, 351–359. [Google Scholar] [CrossRef]
- Dowling, D.J.; Tan, Z.; Prokopowicz, Z.M.; Palmer, C.D.; Matthews, M.A.; Dietsch, G.N.; Hershberg, R.M.; Levy, O. The ultra-potent and selective TLR8 agonist VTX-294 activates human newborn and adult leukocytes. PLoS ONE 2013, 8, e58164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wille-Reece, U.; Flynn, B.J.; Lore, K.; Koup, R.A.; Kedl, R.M.; Mattapallil, J.J.; Weiss, W.R.; Roederer, M.; Seder, R.A. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc. Natl. Acad. Sci. USA 2005, 102, 15190–15194. [Google Scholar] [CrossRef] [PubMed]
- van Haren, S.D.; Dowling, D.J.; Foppen, W.; Christensen, D.; Andersen, P.; Reed, S.G.; Hershberg, R.M.; Baden, L.R.; Levy, O. Age-specific adjuvant synergy: Dual TLR7/8 and Mincle activation of human newborn dendritic cells enables Th1 polarization. J. Immunol. 2016, 197, 4413–4424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganapathi, L.; Van Haren, S.; Dowling, D.J.; Bergelson, I.; Shukla, N.M.; Malladi, S.S.; Balakrishna, R.; Tanji, H.; Ohto, U.; Shimizu, T.; et al. The Imidazoquinoline Toll-Like Receptor-7/8 agonist hybrid-2 potently induces cytokine production by human newborn and adult leukocytes. PLoS ONE 2015, 10, e0134640. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Suter, E.E.; Miller, R.L.; Wessels, M.R. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood 2006, 108, 1284–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, I.F.; Zhang, A.J.; To, K.K.; Chan, J.F.; Li, C.; Zhu, H.S.; Li, P.; Li, C.; Chan, T.C.; Cheng, V.C.; et al. Immunogenicity of intradermal trivalent influenza vaccine with topical imiquimod: A double blind randomized controlled trial. Clin. Infect. Dis. 2014, 59, 1246–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philbin, V.J.; Dowling, D.J.; Gallington, L.C.; Cortes, G.; Tan, Z.; Suter, E.E.; Chi, K.W.; Shuckett, A.; Stoler-Barak, L.; Tomai, M.; et al. Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J. Allergy Clin. Immunol. 2012, 130, 195–204.e199. [Google Scholar] [CrossRef] [Green Version]
- Clemens, E.A.; Holbrook, B.C.; McNeilly, B.; Kanekiyo, M.; Graham, B.S.; Alexander-Miller, M.A. TLR agonists induce sustained IgG to hemagglutinin stem and modulate T cells following newborn vaccination. NPJ Vaccines 2022, 7, 102. [Google Scholar] [CrossRef]
- Pockros, P.J.; Guyader, D.; Patton, H.; Tong, M.J.; Wright, T.; McHutchison, J.G.; Meng, T.C. Oral resiquimod in chronic HCV infection: Safety and efficacy in 2 placebo-controlled, double-blind phase IIa studies. J. Hepatol. 2007, 47, 174–182. [Google Scholar] [CrossRef]
- Dowling, D.J.; van Haren, S.D.; Scheid, A.; Bergelson, I.; Kim, D.; Mancuso, C.J.; Foppen, W.; Ozonoff, A.; Fresh, L.; Theriot, T.B.; et al. TLR7/8 adjuvant overcomes newborn hyporesponsiveness to pneumococcal conjugate vaccine at birth. JCI Insight 2017, 2, e91020. [Google Scholar] [CrossRef] [Green Version]
- Van Hoeven, N.; Fox, C.B.; Granger, B.; Evers, T.; Joshi, S.W.; Nana, G.I.; Evans, S.C.; Lin, S.; Liang, H.; Liang, L.; et al. A Formulated TLR7/8 Agonist is a Flexible, Highly Potent and Effective Adjuvant for Pandemic Influenza Vaccines. Sci. Rep. 2017, 7, 46426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowling, D.J.; Scott, E.A.; Scheid, A.; Bergelson, I.; Joshi, S.; Pietrasanta, C.; Brightman, S.; Sanchez-Schmitz, G.; Van Haren, S.D.; Ninković, J.; et al. Toll-like receptor 8 agonist nanoparticles mimic immunomodulating effects of the live BCG vaccine and enhance neonatal innate and adaptive immune responses. J. Allergy Clin. Immunol. 2017, 140, 1339–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortez, A.; Li, Y.; Miller, A.T.; Zhang, X.; Yue, K.; Maginnis, J.; Hampton, J.; Hall, D.S.; Shapiro, M.; Nayak, B.; et al. Incorporation of Phosphonate into Benzonaphthyridine Toll-like Receptor 7 Agonists for Adsorption to Aluminum Hydroxide. J. Med. Chem. 2016, 59, 5868–5878. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Du, J.L.; Huang, J.; Wu, C.Y. Additive effects of CpG ODN and R-848 as adjuvants on augmenting immune responses to HBsAg vaccination. Biochem. Biophys. Res. Commun. 2007, 361, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Bhagchandani, S.; Johnson, J.A.; Irvine, D.J. Evolution of Toll-like receptor 7/8 agonist therapeutics and their delivery approaches: From antiviral formulations to vaccine adjuvants. Adv. Drug Deliv. Rev. 2021, 175, 113803. [Google Scholar] [CrossRef]
- Francica, J.R.; Lynn, G.M.; Laga, R.; Joyce, M.G.; Ruckwardt, T.J.; Morabito, K.M.; Chen, M.; Chaudhuri, R.; Zhang, B.; Sastry, M.; et al. Thermoresponsive polymer nanoparticles co-deliver RSV F trimers with a TLR-7/8 adjuvant. Bioconjug. Chem. 2016, 27, 2372–2385. [Google Scholar] [CrossRef]
- Lynn, G.M.; Laga, R.; Darrah, P.A.; Ishizuka, A.S.; Balaci, A.J.; Dulcey, A.E.; Pechar, M.; Pola, R.; Gerner, M.Y.; Yamamoto, A.; et al. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat. Biotechnol. 2015, 33, 1201–1210. [Google Scholar] [CrossRef]
- Roth, G.A.; Saouaf, O.M.; Smith, A.A.A.; Gale, E.C.; Hernández, M.A.; Idoyaga, J.; Appel, E.A. Prolonged Codelivery of Hemagglutinin and a TLR7/8 Agonist in a Supramolecular Polymer-Nanoparticle Hydrogel Enhances Potency and Breadth of Influenza Vaccination. ACS Biomater. Sci. Eng. 2021, 7, 1889–1899. [Google Scholar] [CrossRef]
- Lynn, G.M.; Sedlik, C.; Baharom, F.; Zhu, Y.; Ramirez-Valdez, R.A.; Coble, V.L.; Tobin, K.; Nichols, S.R.; Itzkowitz, Y.; Zaidi, N.; et al. Peptide-TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens. Nat. Biotechnol. 2020, 38, 320–332. [Google Scholar] [CrossRef]
- Wille-Reece, U.; Flynn, B.J.; Lore, K.; Koup, R.A.; Miles, A.P.; Saul, A.; Kedl, R.M.; Mattapallil, J.J.; Weiss, W.R.; Roederer, M.; et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J. Exp. Med. 2006, 203, 1249–1258. [Google Scholar] [CrossRef] [Green Version]
- Wille-Reece, U.; Wu, C.Y.; Flynn, B.J.; Kedl, R.M.; Seder, R.A. Immunization with HIV-1 Gag protein conjugated to a TLR7/8 agonist results in the generation of HIV-1 Gag-specific Th1 and CD8+ T cell responses. J. Immunol. 2005, 174, 7676–7683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.Z.; Kurche, J.S.; Burchill, M.A.; Kedl, R.M. TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway. Blood 2011, 118, 3028–3038. [Google Scholar] [CrossRef] [Green Version]
- Westcott, M.M.; Clemens, E.A.; Holbrook, B.C.; King, S.B.; Alexander-Miller, M.A. The choice of linker for conjugating R848 to inactivated influenza virus determines the stimulatory capacity for innate immune cells. Vaccine 2018, 36, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Arimilli, S.; Johnson, J.B.; Clark, K.M.; Graff, A.H.; Alexander-Miller, M.A.; Mizel, S.B.; Parks, G.D. Engineered expression of the TLR5 ligand flagellin enhances paramyxovirus activation of human dendritic cell function. J. Virol. 2008, 82, 10975–10985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratanji, K.D.; Derrick, J.P.; Dearman, R.J.; Kimber, I. Immunogenicity of therapeutic proteins: Influence of aggregation. J. Immunotoxicol. 2014, 11, 99–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Singh, S.K.; Li, N.; Toler, M.R.; King, K.R.; Nema, S. Immunogenicity of protein aggregates--concerns and realities. Int. J. Pharm. 2012, 431, 1–11. [Google Scholar] [CrossRef]
- Hemmi, H.; Kaisho, T.; Takeuchi, O.; Sato, S.; Sanjo, H.; Hoshino, K.; Horiuchi, T.; Tomizawa, H.; Takeda, K.; Akira, S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 2002, 3, 196–200. [Google Scholar] [CrossRef]
- Larange, A.; Antonios, D.; Pallardy, M.; Kerdine-Romer, S. TLR7 and TLR8 agonists trigger different signaling pathways for human dendritic cell maturation. J. Leukoc. Biol. 2009, 85, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Cervantes, J.L.; Weinerman, B.; Basole, C.; Salazar, J.C. TLR8: The forgotten relative revindicated. Cell Mol. Immunol. 2012, 9, 434–438. [Google Scholar] [CrossRef] [Green Version]
- Ernst, O.; Vayttaden, S.J.; Fraser, I.D.C. Measurement of NF-κB activation in TLR-activated macrophages. Methods Mol. Biol. 2018, 1714, 67–78. [Google Scholar] [CrossRef]
- Hay, R.T.; Vuillard, L.; Desterro, J.M.; Rodriguez, M.S. Control of NF-kappa B transcriptional activation by signal induced proteolysis of I kappa B alpha. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1999, 354, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, D.Y.; Gudjonsson, A.; Bogen, B.; Fossum, E. Targeting conventional dendritic cells to fine-tune antibody responses. Front. Immunol. 2019, 10, 1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, S.T.; Rehor, A.; Schmoekel, H.G.; Hubbell, J.A.; Swartz, M.A. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control. Release 2006, 112, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Petes, C.; Odoardi, N.; Gee, K. The toll for trafficking: Toll-like receptor 7 delivery to the endosome. Front. Immunol. 2017, 8, 1075. [Google Scholar] [CrossRef] [Green Version]
- Ishii, N.; Funami, K.; Tatematsu, M.; Seya, T.; Matsumoto, M. Endosomal localization of TLR8 confers distinctive proteolytic processing on human myeloid cells. J. Immunol. 2014, 193, 5118–5128. [Google Scholar] [CrossRef] [Green Version]
- Hipp, M.M.; Shepherd, D.; Gileadi, U.; Aichinger, M.C.; Kessler, B.M.; Edelmann, M.J.; Essalmani, R.; Seidah, N.G.; Reis e Sousa, C.; Cerundolo, V. Processing of human toll-like receptor 7 by furin-like proprotein convertases is required for its accumulation and activity in endosomes. Immunity 2013, 39, 711–721. [Google Scholar] [CrossRef] [Green Version]
- Ilarregui, J.M.; van Beelen, A.J.; Fehres, C.M.; Bruijns, S.C.; Garcia-Vallejo, J.J.; van Kooyk, Y. New roles for CD14 and IL-beta linking inflammatory dendritic cells to IL-17 production in memory CD4(+) T cells. Immunol. Cell Biol. 2016, 94, 907–916. [Google Scholar] [CrossRef]
- De Koker, S.; Van Hoecke, L.; De Beuckelaer, A.; Roose, K.; Deswarte, K.; Willart, M.A.; Bogaert, P.; Naessens, T.; De Geest, B.G.; Saelens, X.; et al. Inflammatory monocytes regulate Th1 oriented immunity to CpG adjuvanted protein vaccines through production of IL-12. Sci. Rep. 2017, 7, 5986. [Google Scholar] [CrossRef] [Green Version]
- Jakubzick, C.V.; Randolph, G.J.; Henson, P.M. Monocyte differentiation and antigen-presenting functions. Nat. Rev. Immunol. 2017, 17, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.R.; Atif, S.M.; Gibbings, S.L.; Thomas, S.M.; Prabagar, M.G.; Danhorn, T.; Leach, S.M.; Henson, P.M.; Jakubzick, C.V. Ly6C(+) monocyte efferocytosis and cross-presentation of cell-associated antigens. Cell Death Differ. 2016, 23, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, R.; Ide, K.; Terahara, K.; Wagatsuma, R.; Iwaki, R.; Matsunaga, H.; Tsunetsugu-Yokota, Y.; Takeyama, H.; Takahashi, Y. Development of an inflammatory CD14+ dendritic cell subset in humanized mice. Front. Immunol. 2021, 12, 643040. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, T.R.; Crabtree, J.; Rein-Weston, A.; Blimkie, D.; Thommai, F.; Wang, X.Y.; Lavoie, P.M.; Furlong, J.; Fortuno, E.S., 3rd; Hajjar, A.M.; et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J. Immunol. 2009, 183, 7150–7160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Wit, D.; Tonon, S.; Olislagers, V.; Goriely, S.; Boutriaux, M.; Goldman, M.; Willems, F. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J. Autoimmun. 2003, 21, 277–281. [Google Scholar] [CrossRef]
- Goriely, S.; Vincart, B.; Stordeur, P.; Vekemans, J.; Willems, F.; Goldman, M.; De Wit, D. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J. Immunol. 2001, 166, 2141–2146. [Google Scholar] [CrossRef]
- Langrish, C.L.; Buddle, J.C.; Thrasher, A.J.; Goldblatt, D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin. Exp. Immunol. 2002, 128, 118–123. [Google Scholar] [CrossRef]



| Vaccine | R848/mL for 100 µg Protein | Z-Average | PDI |
|---|---|---|---|
| IPR8 | - | 142 d.nm | 0.099 |
| IPR8-SM(PEG)4-R848 | 8.12 nmoL/mL | 138.3 d.nm | 0.137 |
| IPR8-GMBS-R848 | 6.05 nmoL/mL | 138.6 d.nm | 0.129 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crofts, K.F.; Page, C.L.; Swedik, S.M.; Holbrook, B.C.; Meyers, A.K.; Zhu, X.; Parsonage, D.; Westcott, M.M.; Alexander-Miller, M.A. An Analysis of Linker-Dependent Effects on the APC Activation and In Vivo Immunogenicity of an R848-Conjugated Influenza Vaccine. Vaccines 2023, 11, 1261. https://doi.org/10.3390/vaccines11071261
Crofts KF, Page CL, Swedik SM, Holbrook BC, Meyers AK, Zhu X, Parsonage D, Westcott MM, Alexander-Miller MA. An Analysis of Linker-Dependent Effects on the APC Activation and In Vivo Immunogenicity of an R848-Conjugated Influenza Vaccine. Vaccines. 2023; 11(7):1261. https://doi.org/10.3390/vaccines11071261
Chicago/Turabian StyleCrofts, Kali F., Courtney L. Page, Stephanie M. Swedik, Beth C. Holbrook, Allison K. Meyers, Xuewei Zhu, Derek Parsonage, Marlena M. Westcott, and Martha A. Alexander-Miller. 2023. "An Analysis of Linker-Dependent Effects on the APC Activation and In Vivo Immunogenicity of an R848-Conjugated Influenza Vaccine" Vaccines 11, no. 7: 1261. https://doi.org/10.3390/vaccines11071261
APA StyleCrofts, K. F., Page, C. L., Swedik, S. M., Holbrook, B. C., Meyers, A. K., Zhu, X., Parsonage, D., Westcott, M. M., & Alexander-Miller, M. A. (2023). An Analysis of Linker-Dependent Effects on the APC Activation and In Vivo Immunogenicity of an R848-Conjugated Influenza Vaccine. Vaccines, 11(7), 1261. https://doi.org/10.3390/vaccines11071261






