PD-L1 Is Involved in the Development of Non-Hodgkin’s Lymphoma by Mediating Circulating Lymphocyte Apoptosis
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Clinical Data
2.3. Antibodies
2.4. Flow Cytometry Analysis
2.5. Apoptosis Measurements Using Flow Cytometry
2.6. Statistical Analyses
3. Results
3.1. Demographic and Clinical Criteria
3.2. Circulating Lymphocytes in NHL
3.3. PD-L1+/PD-1 Upregulation in Circulating Lymphocytes in NHL Patients
3.4. Expression of PD-L1/PD-1 in Apoptotic Lymphocytes
3.5. Study of Peripheral Lymphocytes and PD-L1 Expression
3.6. Diagnostic Performance of Circulating Cells and PD-L1 Expression for the Identification of NHL Patents
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Padala, S.A.; Barsouk, A.; Rawla, P. Epidemiology of Non-Hodgkin lymphoma. Med. Sci. 2021, 9, 5. [Google Scholar]
- De Level, L.; Jaffe, E.S. Lymphoma classification. Cancer J. 2020, 26, 176–185. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Liang, A.B.; Ansell, S.M. T-cell-mediated antitumor immunity in B-cell non-Hodgkin lymphoma: Activation, suppression, and exhaustion. Leuk. Lymphoma 2015, 56, 2498–2504. [Google Scholar] [CrossRef]
- Biernat, M.M.; Wróbel, T. Bacterial Infection and Non-Hodgkin B-Cell Lymphoma: Interactions between Pathogen, Host and the Tumor Environment. Int. J. Mol. Sci. 2021, 22, 7372. [Google Scholar] [CrossRef]
- Roufaiel, M.E.K.N.; Wells, J.W.; Steptoe, R.J. Impaired T-Cell Function in B-Cell Lymphoma: A Direct Consequence of Events at the Immunological Synapse? Front. Immunol. 2015, 6, 258. [Google Scholar]
- Menter, T.; Tzankov, A. Mechanisms of Immune Evasion and Immune Modulation by Lymphoma Cells. Front. Oncol. 2018, 8, 54. [Google Scholar] [CrossRef]
- Friman, V.; Winqvist, O.; Blimark, C.; Langerbeins, P.; Chapel, H.; Dhalla, F. Secondary immunodeficiency in lymphoproliferative malignancies. Hematol. Oncol. 2016, 34, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S.; Horn, L.A.; Haile, S.T. The programmed death-1 immune suppressive pathway: Barrier to anti-tumor immunity. J. Immunol. 2014, 193, 3835–3841. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Colin, E.; Saldmann, A.; Gey, A.; Oudard, S.; Tartour, E. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open 2017, 2, e000213. [Google Scholar]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Butte, M.J.; Pena-Cruz, V.; Kim, M.J.; Freeman, G.J.; Sharpe, A.H. Interaction of human PD-L1 and B7-1. Mol. Immunol. 2008, 45, 3567–3572. [Google Scholar] [CrossRef]
- Ishida, M.; Iwai, Y.; Tanaka, Y.; Okazaku, T.; Freeman, G.J.; Minato, N.; Honjo, T. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol. Lett. 2002, 84, 57–62. [Google Scholar] [CrossRef]
- Gravelle, P.; Burroni, B.; Péricart, S.; Rossi, C.; Bezombes, C.; Tosolini, M.; Damotte, D.; Brousset, P.; Fournié, J.-J.; Laurent, C. Mechanisms of PD-1/PD-L1 expression and prognostic relevance in non-Hodgkin lymphoma: A summary of immunohistochemical studies. Oncotarget 2017, 8, 44960–44975. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, M.; Zhang, Y.; Meng, H.; Wang, Y.; Liu, Y.; Jing, J.; Huang, L.; Sun, M.; Zhang, Y.; et al. The prognostic value of programmed cell death ligand 1 expression in non-Hodgkin lymphoma: A meta-analysis. Cancer Biol. Med. 2018, 15, 290–298. [Google Scholar]
- Armand, P. Immune checkpoint blockade in hematologic malignancies. Blood 2015, 125, 3393–3400. [Google Scholar] [CrossRef]
- Ostroumov, D.; Fekete-Drimusz, N.; Saborowski, M.; Kühnel, F.; Woller, N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell. Mol. Life Sci. 2018, 75, 689–713. [Google Scholar] [CrossRef] [PubMed]
- Pavlasova, G.; Mraz, M. The regulation and function of CD20: An “enigma” of B-cell biology and targeted therapy. Haematologica 2020, 105, 1494–1506. [Google Scholar] [CrossRef] [PubMed]
- Pedoeem, A.; Azoulay-Alfaguter, I.; Strazza, M.; Silverman, G.J.; Mor, A. Programmed death-1 pathway in cancer and autoimmunity. Clin. Immunol. 2014, 153, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Jang, R.W.; Caraiscos, V.B.; Swami, N.; Banerjee, S.; Mak, E.; Kaya, E.; Rodin, G.; Bryson, J.; Ridley, J.Z.; Le, L.W. Simple prognostic model for patients with advanced cancer based on performance status. J. Oncol. Pract. 2014, 10, e335–e341. [Google Scholar] [CrossRef]
- Green, M.R.; Rodig, S.; Juszczynski, P.; Ouyang, J.; Sinha, P.; O’Donnell, E.; Neuberg, D.; Shipp, M.A. Constitutive AP-1 activity, and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: Implications for targeted therapy. Clin. Cancer Res. 2012, 18, 1611–1618. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, J.-F.; Zuo, M.-X.; Cao, X.-X.; Chen, M.; Zhang, Y.; Han, X.; Zhong, D.-R.; Zhou, D.-B. PD-1 expression on the surface of peripheral blood CD4+ T cell and its association with the prognosis of patients with diffuse large B-cell lymphoma. Cancer Med. 2016, 11, 3077–3084. [Google Scholar] [CrossRef]
- Zodelava, M.; Datikashvili-David, I. Prognostic significance of apoptosis-related CD95 antigen expression in patients with non-Hodgkin’s lymphoma. Georgian Med. 2005, 120, 37–39. [Google Scholar]
- Han, X.; Ruan, J.; Zhang, W.; Zhou, D.; Xu, D.; Pei, Q.; Quuyang, M.; Zuo, M. Prognostic implication of leucocyte subpopulations in diffuse large B-cell lymphoma. Oncotarget 2017, 8, 47790–47800. [Google Scholar] [CrossRef]
- Bruna, R.; Geuna, M.; Scalabrini, D.R.; Cignetti, A.; Santoro, N.; Gottardi, D.; Passera, R.; Tarella, C. Low Circulating B Lymphocytes in Newly Diagnosed Follicular and Diffuse Large B Cell Lymphoma: Differences Compared to Normal Subjects and Possible Prognostic Role. Blood 2017, 130 (Suppl. S1), 1482. [Google Scholar]
- Hou, H.; Luo, Y.; Tang, G.; Zhang, B.; Quyang, R.; Wang, T.; Huang, M.; Wu, S.; Li, D.; Wang, F. Dynamic changes in peripheral blood lymphocyte subset counts and functions in patients with diffuse large B cell lymphoma during chemotherapy. Cancer Cell Int. 2021, 21, 282. [Google Scholar] [CrossRef]
- Judd, J.; Dulaimi, E.; Li, T.; Millenson, M.M.; Borghaei, H.; Smith, M.R.; Al-Saleem, T. Low level of blood CD4+ T cells is an independent predictor of inferior progression-free survival in diffuse large B-cell lymphoma. Clin. Lymphoma Myeloma Leuk. 2017, 17, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.C.; Nofroni, I.; Ruco, L.; Amodeo, R.; Ferrari, A.; La Verde, G.; Cardelli, P.; Montefusco, E.; Conte, E.; Monarca, B.; et al. Low absolute lymphocyte count is a poor prognostic factor in diffuse-large-B-cell-lymphoma. Leuk. Lymphoma 2008, 49, 1745–1751. [Google Scholar] [CrossRef]
- Protti, M.P.; De Monte, L.; Di Lullo, G. Tumor antigen-specific CD4+ T cells in cancer immunity: From antigen identification to tumor prognosis and development of therapeutic strategies. Tissue Antigens 2014, 83, 237–246. [Google Scholar] [CrossRef]
- Saber, M.M. Diagnostic performance of PD-L1 versus PD-1 in circulating CD20 cells in diffuse large B cell lymphoma. Antibodies 2022, 11, 15. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, W.; Wang, S.; Zhou, X.; Liu, S.; Ma, S. Peripheral blood lymphocyte subsets of newly diagnosed DLBCL patients and their dynamic changes with rituximab-based immunochemotherapy. Leuk. Lymphoma 2019, 60, 2909–2916. [Google Scholar] [CrossRef]
- Schwartz, M.; Zhang, Y.; Rosenblatt; J. D. B cell regulation of the anti-tumor response and role in carcinogenesis. J. Immunother. Cancer 2016, 4, 40. [Google Scholar] [CrossRef]
- Georgiou, K.; Chen, L.; Berglund, M.; Ren, W.; de Miranda, N.F.C.C.; Lisboa, S.; Fangazio, M.; Zhu, S.; Hou, Y.; Wu, K.; et al. Genetic basis of PD-L1 overexpression in diffuse large B-cell lymphomas. Blood 2016, 127, 3026–3034. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced non-squamous non-small-cell lung cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R. Pembrolizumab for the treatment of non-small cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Zajac, M.; Ye, J.; Mukhopadhyay, P.; Jin, X.; Ben, Y.; Antel, J.; Gupta, A.K.; Rebelatto, M.C.; Williams, J.A.; Walker, J. Optimal PD-L1-high cutoff for association with overall survival in patients with urothelial cancer treated with durvalumab monotherapy. PLoS ONE 2020, 15, e0231936. [Google Scholar] [CrossRef]

| NHL | Normal Controls | p-Value | |
|---|---|---|---|
| (N = 20) | (N = 20) | ||
| Age (mean ± SD) | 39.25 ± 16.2 | 40.3 ± 15.9 | 0.86 |
| Sex | 1.00 | ||
| Male | 10 (50.0%) | 10 (50.0%) | |
| Female | 10 (50.0%) | 10 (50.0%) | |
| Hepatomegaly | 7 (35.0%) | 0 (0.0%) | 0.008 * |
| Splenomegaly | 9 (45.0%) | 0 (0.0%) | 0.001 ** |
| Enlarged lymph node | 20 (100.0%) | 0 (0.0%) | <0.001 ** |
| NHL | Normal Controls | ZMWU | p-Value | ||
|---|---|---|---|---|---|
| (N = 20) | (N = 20) | ||||
| CD3+, % | Median | 57 (42–68.2) | 71.5 (67.5–77) | −4.061 | <0.001 ** |
| IQR | |||||
| CD3+CD4+, % | Median | 39.5 (32.5–46.25) | 44.9 (43–47.5) | −2.207 | 0.027 * |
| IQR | |||||
| CD3+CD8+, % | Median | 17 (14–21) | 22 (20–24.5) | −3.36 | 0.001 * |
| IQR | |||||
| CD20+, % | Median | 22 (18.5–25.5) | 24 (21–25.5) | −0.952 | 0.341 |
| IQR | |||||
| NHL | Controls | p-Value | ||
|---|---|---|---|---|
| (N = 20) | (N = 20) | |||
| PD-L1+CD3+, % | IQR | 53 (33.8–58.85) | 2.7 (1.5–3.6) | <0.001 ** |
| Median | ||||
| PD-L1+CD3+CD4+, % | IQR | 20.5 (16.5–22.5) | 0.95 (0.4–1.25) | <0.001 ** |
| Median | ||||
| PD-L1+ CD3+CD8+, % | IQR | 8.1 (6–11.2) | 0.5 (0.4–0.8) | <0.001 ** |
| Median | ||||
| PD-L1+CD20+, % | IQR | 16 (14–19) | 1 (0.8–1) | <0.001 ** |
| Median | ||||
| PD-1+CD3+, % | IQR | 9.5 (8–11.5) | 2 (1.2–2.5) | <0.001 ** |
| Median | ||||
| PD-1+CD3+CD4+, % | IQR | 7 (6–7.5) | 0.8 (0.55–1.1) | <0.001 ** |
| Median | ||||
| PD-1+ CD3+CD8+, % | IQR | 5 (4–6) | 0.45 (0.35–0.8) | <0.001 ** |
| Median | ||||
| PD-1+CD20+, % | IQR | 8 (7–9) | 0.7 (0.6–0.9) | <0.001 ** |
| Median | ||||
| NHL | Controls | p-Value | ||
|---|---|---|---|---|
| (N = 20) | (N = 20) | |||
| Annexin-V+CD3+, % | IQR | 35 (25.5–45.5) | 0.1 (0.05–0.15) | <0.001 ** |
| Median | ||||
| Annexin-V+CD3+CD4+, % | IQR | 27.5 (23.5–39) | 0.1 (0–0.1) | <0.001 ** |
| Median | ||||
| Annexin-V+CD3+CD8+, % | IQR | 10.5 (9–12.5) | 0.1 (0–0.1) | <0.001 ** |
| Median | ||||
| Annexin-V+CD20+, % | IQR | 17 (16–22) | 0.1 (0–0.1) | <0.001 ** |
| Median | ||||
| Annexin-V+CD3+, % | Annexin-V+CD4+, % | Annexin-V+CD8+, % | Annexin-V+CD20+, % | |||||
|---|---|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | r | p-Value | |
| Annexin-V+CD3+CD4+, % | 0.386 | 0.093 | ||||||
| Annexin-V+CD3+CD8+, % | 0.69 | 0.001 * | 0.088 | 0.713 | ||||
| Annexin-V+CD20+, % | −0.336 | 0.147 | 0.132 | 0.579 | −0.33 | 0.156 | ||
| Annexin-V+PD-L1+, % | 0.678 | 0.001 * | 0.313 | 0.179 | 0.646 | 0.002 * | −0.172 | 0.468 |
| Annexin-V+PD-1+, % | 0.707 | <0.001 ** | 0.367 | 0.112 | 0.53 | 0.016 * | −0.32 | 0.169 |
| PD-L1+CD3+, % | 0.567 | 0.009 * | 0.149 | 0.531 | 0.317 | 0.174 | −0.167 | 0.482 |
| PD-L1+CD3+CD4+, % | −0.191 | 0.419 | 0.117 | 0.625 | −0.235 | 0.318 | 0.28 | 0.233 |
| PD-L1+CD3+CD8+, % | 0.615 | 0.004 * | 0.084 | 0.724 | 0.618 | 0.004 * | −0.205 | 0.386 |
| PD-L1+CD20+, % | −0.345 | 0.136 | 0.167 | 0.483 | −0.379 | 0.099 | 0.887 | <0.001 ** |
| PD-1+CD3+, % | 0.744 | <0.001 ** | 0.343 | 0.138 | 0.588 | 0.006 * | −0.388 | 0.091 |
| PD-1+CD3+CD4+, % | 0.565 | 0.009 * | 0.477 | 0.034 * | 0.602 | 0.005 * | 0.029 | 0.903 |
| PD-1+CD3+CD8+, % | −0.019 | 0.938 | 0.413 | 0.07 | 0.344 | 0.137 | 0.09 | 0.705 |
| PD-1+CD20+, % | −0.237 | 0.315 | 0.365 | 0.114 | −0.082 | 0.73 | −0.003 | 0.992 |
| CD3+, % | 0.945 | <0.001 ** | 0.373 | 0.105 | 0.655 | 0.002 * | −0.317 | 0.174 |
| CD3+CD4+, % | 0.66 | 0.002 * | 0.179 | 0.449 | 0.589 | 0.006 * | −0.218 | 0.356 |
| CD3+CD8+, % | 0.607 | 0.005 * | 0.085 | 0.721 | 0.936 | <0.001 ** | −0.264 | 0.26 |
| CD20+, % | −0.425 | 0.062 | 0.102 | 0.669 | −0.422 | 0.064 | 0.968 | <0.001 ** |
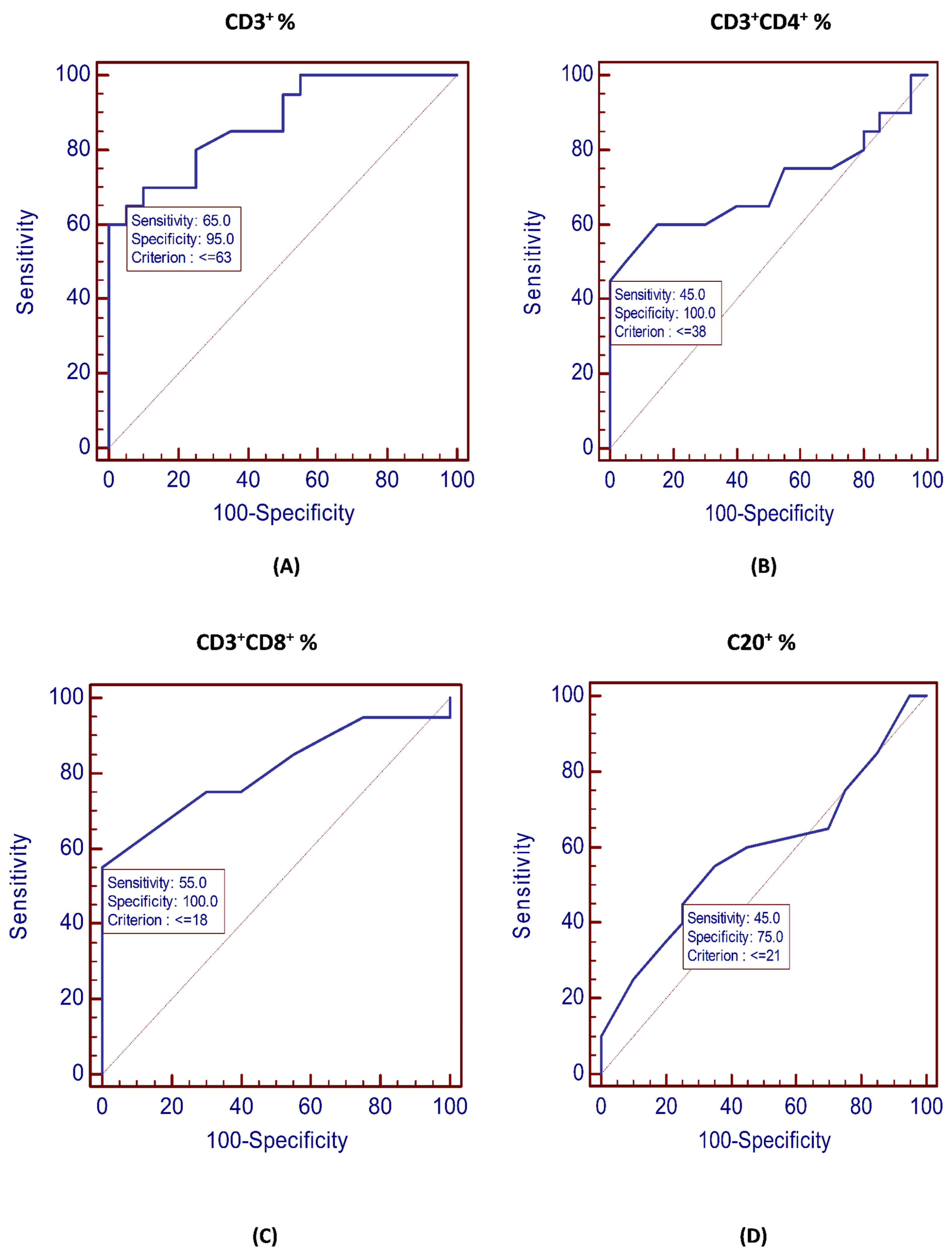
| Cutoff | AUC | p-Value | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| CD3+, % | ≤63 | 0.875 | <0.001 ** | 65% | 95% |
| CD3+CD4+, % | ≤38 | 0.704 | 0.022 * | 45% | 100% |
| CD3+CD8+, % | ≤18 | 0.808 | <0.001 ** | 55% | 100% |
| CD20+, % | ≤21 | 0.588 | 0.346 | 45% | 75% |
| PD-L1+CD3+CD4+, % | >1.6 | 1.0 | <0.001 ** | 100% | 100% |
| PD-L1+CD3+CD8+, % | >1.6 | 1.0 | <0.001 ** | 100% | 100% |
| PD-L1+CD20+, % | >1.1 | 1.0 | <0.001 ** | 100% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saber, M.M. PD-L1 Is Involved in the Development of Non-Hodgkin’s Lymphoma by Mediating Circulating Lymphocyte Apoptosis. Vaccines 2023, 11, 1474. https://doi.org/10.3390/vaccines11091474
Saber MM. PD-L1 Is Involved in the Development of Non-Hodgkin’s Lymphoma by Mediating Circulating Lymphocyte Apoptosis. Vaccines. 2023; 11(9):1474. https://doi.org/10.3390/vaccines11091474
Chicago/Turabian StyleSaber, Manal Mohamed. 2023. "PD-L1 Is Involved in the Development of Non-Hodgkin’s Lymphoma by Mediating Circulating Lymphocyte Apoptosis" Vaccines 11, no. 9: 1474. https://doi.org/10.3390/vaccines11091474




