Hybrid Immunity from Gam-COVID-Vac Vaccination and Natural SARS-CoV-2 Infection Confers Broader Neutralizing Activity against Omicron Lineage VOCs Than Revaccination or Reinfection
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Serum Collection and Ethics Approval
2.3. ELISA-Based Detection of IgG, IgM, and IgA Antibodies Specific to SARS-CoV-2 Proteins
2.4. Plasmids
2.5. Production of S-Pseudotyped Lentiviral Particles
2.6. SARS-CoV-2 S-Pseudotyped Lentivirus Neutralization Assay
2.7. Statistics
3. Results
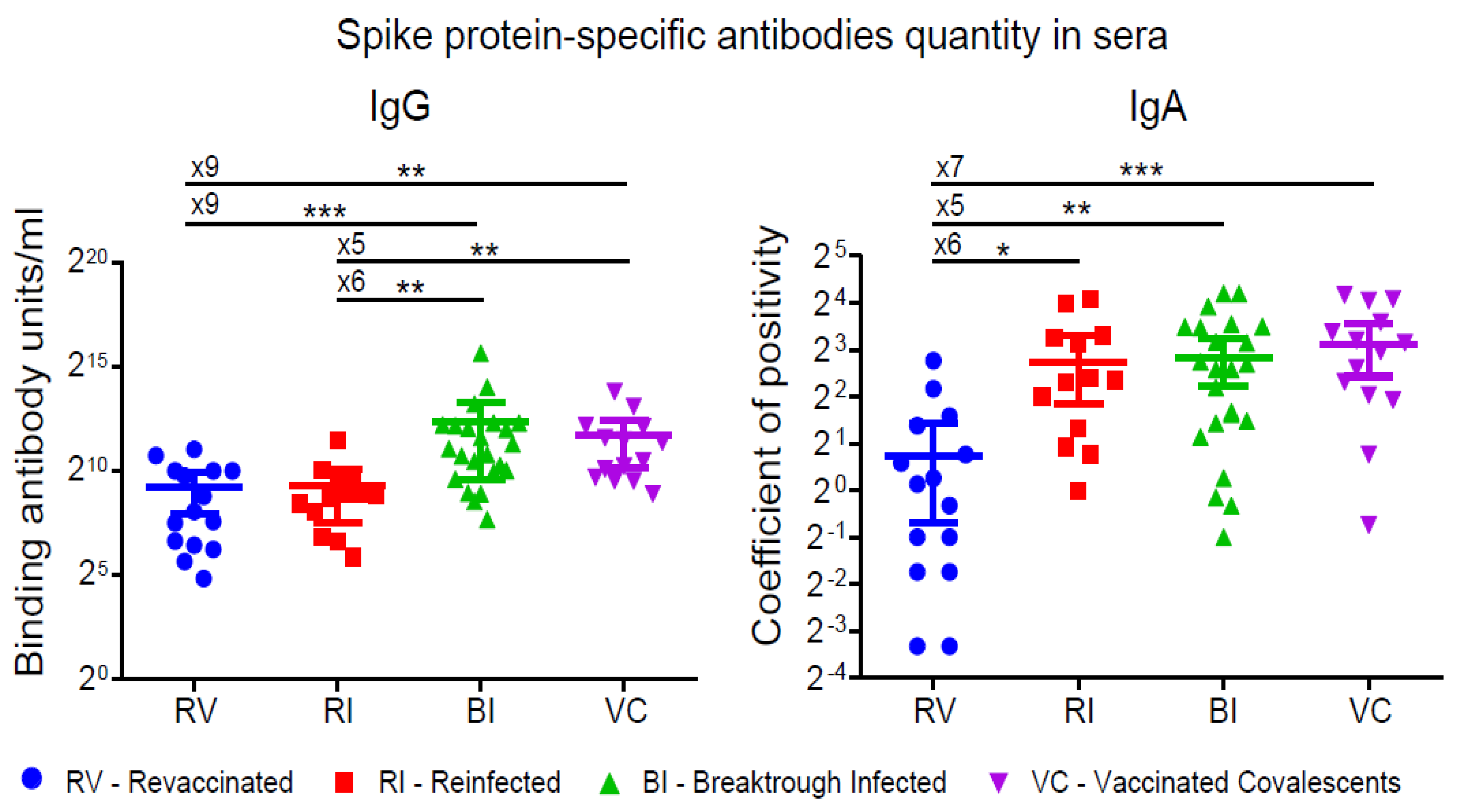
3.1. S-protein-Specific Antibody Levels
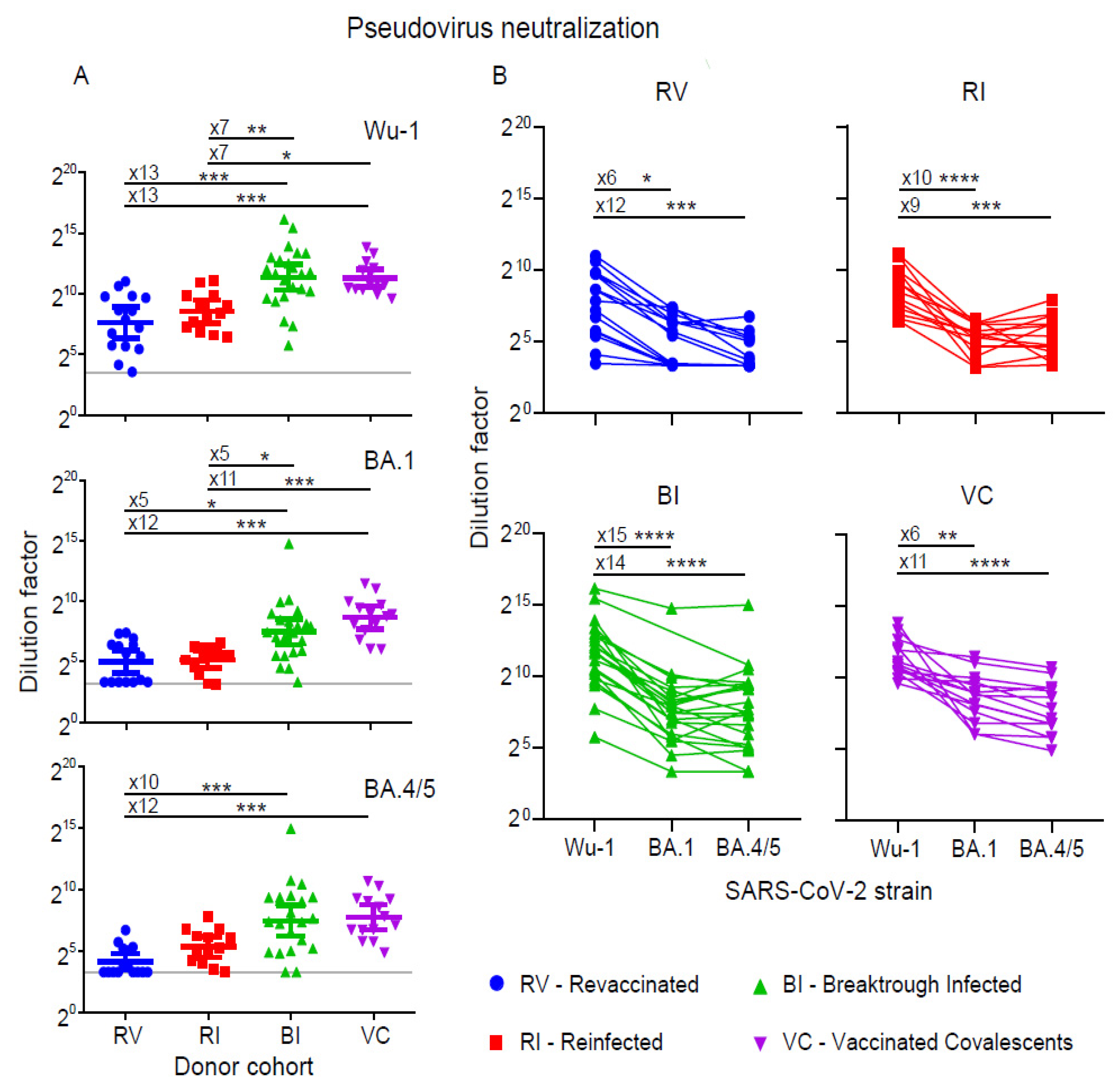
3.2. Neutralization of Pseudoviruses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Huo, J.; Zhou, D.; Zahradník, J.; Supasa, P.; Liu, C.; Duyvesteyn, H.M.E.; Ginn, H.M.; Mentzer, A.J.; Tuekprakhon, A.; et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022, 185, 467–484.e15. [Google Scholar] [CrossRef] [PubMed]
- VanBlargan, L.A.; Errico, J.M.; Halfmann, P.J.; Zost, S.J.; Crowe, J.E.; Purcell, L.A.; Kawaoka, Y.; Corti, D.; Fremont, D.H.; Diamond, M.S. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat. Med. 2022, 28, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yisimayi, A.; Jian, F.; Song, W.; Xiao, T.; Wang, L.; Du, S.; Wang, J.; Li, Q.; Chen, X.; et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature 2022, 608, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Ju, B.; Fan, Q.; Liu, C.; Shen, S.; Wang, M.; Guo, H.; Zhou, B.; Ge, X.; Zhang, Z. Omicron BQ.1.1 and XBB.1 unprecedentedly escape broadly neutralizing antibodies elicited by prototype vaccination. Cell Rep. 2023, 42, 112532. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Iketani, S.; Li, Z.; Liu, L.; Guo, Y.; Huang, Y.; Bowen, A.D.; Liu, M.; Wang, M.; Yu, J.; et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 2023, 186, 279–286.e8. [Google Scholar] [CrossRef]
- Gorchakov, A.A.; Kulemzin, S.V.; Guselnikov, S.V.; Baranov, K.O.; Belovezhets, T.N.; Mechetina, L.V.; Volkova, O.Y.; Najakshin, A.M.; Chikaev, N.A.; Chikaev, A.N.; et al. Isolation of a panel of ultra-potent human antibodies neutralizing SARS-CoV-2 and viral variants of concern. Cell Discov. 2021, 7, 96. [Google Scholar] [CrossRef]
- Kulemzin, S.V.; Sergeeva, M.V.; Baranov, K.O.; Gorchakov, A.A.; Guselnikov, S.V.; Belovezhets, T.N.; Volkova, O.Y.; Najakshin, A.M.; Chikaev, N.A.; Danilenko, D.M.; et al. VH3-53/66-Class RBD-Specific Human Monoclonal Antibody iB20 Displays Cross-Neutralizing Activity against Emerging SARS-CoV-2 Lineages. J. Pers. Med. 2022, 12, 895. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Lai, L.; Samaha, H.; Feng, Y.; Hu, M.; Hui, H.S.-Y.; Wali, B.; Ellis, M.; Davis-Gardner, M.E.; Huerta, C.; et al. Durability of immune responses to mRNA booster vaccination against COVID-19. J. Clin. Investig. 2023, 133, e167955. [Google Scholar] [CrossRef]
- Wratil, P.R.; Stern, M.; Priller, A.; Willmann, A.; Almanzar, G.; Vogel, E.; Feuerherd, M.; Cheng, C.-C.; Yazici, S.; Christa, C.; et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat. Med. 2022, 28, 496–503. [Google Scholar] [CrossRef]
- Nakagama, Y.; Candray, K.; Kaku, N.; Komase, Y.; Rodriguez-Funes, M.-V.; Dominguez, R.; Tsuchida, T.; Kunishima, H.; Nagai, E.; Adachi, E.; et al. Antibody Avidity Maturation Following Recovery From Infection or the Booster Vaccination Grants Breadth of SARS-CoV-2 Neutralizing Capacity. J. Infect. Dis. 2023, 227, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-H.; Huang, C.-G.; Huang, C.-T.; Chen, Y.-C.; Kung, Y.-A.; Chen, C.-J.; Chuang, T.-C.; Liu, C.-C.; Huang, P.-W.; Yang, S.-L.; et al. Titers and breadth of neutralizing antibodies against SARS-CoV-2 variants after heterologous booster vaccination in health care workers primed with two doses of ChAdOx1 nCov-19: A single-blinded, randomized clinical trial. J. Clin. Virol. 2022, 157, 105328. [Google Scholar] [CrossRef] [PubMed]
- Luczkowiak, J.; Rivas, G.; Labiod, N.; Lasala, F.; Rolo, M.; Lora-Tamayo, J.; Mancheno-Losa, M.; Rial-Crestelo, D.; Pérez-Rivilla, A.; Folgueira, M.D.; et al. Cross neutralization of SARS-CoV-2 omicron subvariants after repeated doses of COVID-19 mRNA vaccines. J. Med. Virol. 2023, 95, e28268. [Google Scholar] [CrossRef] [PubMed]
- Bellusci, L.; Grubbs, G.; Zahra, F.T.; Forgacs, D.; Golding, H.; Ross, T.M.; Khurana, S. Antibody affinity and cross-variant neutralization of SARS-CoV-2 Omicron BA.1, BA.2 and BA.3 following third mRNA vaccination. Nat. Commun. 2022, 13, 4617. [Google Scholar] [CrossRef] [PubMed]
- Pušnik, J.; Monzon-Posadas, W.O.; Zorn, J.; Peters, K.; Baum, M.; Proksch, H.; Schlüter, C.B.; Alter, G.; Menting, T.; Streeck, H. SARS-CoV-2 humoral and cellular immunity following different combinations of vaccination and breakthrough infection. Nat. Commun. 2023, 14, 572. [Google Scholar] [CrossRef] [PubMed]
- Tauzin, A.; Nicolas, A.; Ding, S.; Benlarbi, M.; Medjahed, H.; Chatterjee, D.; Dionne, K.; Gong, S.Y.; Gendron-Lepage, G.; Bo, Y.; et al. Spike recognition and neutralization of SARS-CoV-2 Omicron subvariants elicited after the third dose of mRNA vaccine. Cell Rep. 2023, 42, 111998. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Astakhova, E.A.; Byazrova, M.G.; Yusubalieva, G.M.; Kulemzin, S.V.; Kruglova, N.A.; Prilipov, A.G.; Baklaushev, V.P.; Gorchakov, A.A.; Taranin, A.V.; Filatov, A.V. Functional Profiling of In Vitro Reactivated Memory B Cells Following Natural SARS-CoV-2 Infection and Gam-COVID-Vac Vaccination. Cells 2022, 11, 1991. [Google Scholar] [CrossRef]
- Byazrova, M.G.; Kulemzin, S.V.; Astakhova, E.A.; Belovezhets, T.N.; Efimov, G.A.; Chikaev, A.N.; Kolotygin, I.O.; Gorchakov, A.A.; Taranin, A.V.; Filatov, A.V. Memory B Cells Induced by Sputnik V Vaccination Produce SARS-CoV-2 Neutralizing Antibodies Upon Ex Vivo Restimulation. Front. Immunol. 2022, 13, 840707. [Google Scholar] [CrossRef]
- Komissarov, A.B.; Safina, K.R.; Garushyants, S.K.; Fadeev, A.V.; Sergeeva, M.V.; Ivanova, A.A.; Danilenko, D.M.; Lioznov, D.; Shneider, O.V.; Shvyrev, N.; et al. Genomic epidemiology of the early stages of the SARS-CoV-2 outbreak in Russia. Nat. Commun. 2021, 12, 649. [Google Scholar] [CrossRef]
- Matsvay, A.; Klink, G.V.; Safina, K.R.; Nabieva, E.; Garushyants, S.K.; Biba, D.; Bazykin, G.A.; Mikhaylov, I.M.; Say, A.V.; Zakamornaya, A.I.; et al. Genomic epidemiology of SARS-CoV-2 in Russia reveals recurring cross-border transmission throughout 2020. PLoS ONE 2023, 18, e0285664. [Google Scholar] [CrossRef] [PubMed]
- Congrave-Wilson, Z.; Cheng, W.A.; Lee, Y.; Perez, S.; Turner, L.; Marentes Ruiz, C.J.; Mendieta, S.; Skura, A.; Jumarang, J.; Del Valle, J.; et al. Twelve-Month Longitudinal Serology in SARS-CoV-2 Naïve and Experienced Vaccine Recipients and Unvaccinated COVID-19-Infected Individuals. Vaccines 2022, 10, 813. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Shenai, M.B.; Rahme, R.; Noorchashm, H. Equivalency of Protection From Natural Immunity in COVID-19 Recovered Versus Fully Vaccinated Persons: A Systematic Review and Pooled Analysis. Cureus 2021, 13, e19102. [Google Scholar] [CrossRef] [PubMed]
- Gazit, S.; Shlezinger, R.; Perez, G.; Lotan, R.; Peretz, A.; Ben-Tov, A.; Herzel, E.; Alapi, H.; Cohen, D.; Muhsen, K.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Naturally Acquired Immunity versus Vaccine-induced Immunity, Reinfections versus Breakthrough Infections: A Retrospective Cohort Study. Clin. Infect. Dis. 2022, 75, e545–e551. [Google Scholar] [CrossRef]
- Pilz, S.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Ioannidis, J.P.A. SARS-CoV-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Environ. Res. 2022, 209, 112911. [Google Scholar] [CrossRef]
- Fraser, R.; Orta-Resendiz, A.; Mazein, A.; Dockrell, D.H. Upper respiratory tract mucosal immunity for SARS-CoV-2 vaccines. Trends Mol. Med. 2023, 29, 255. [Google Scholar] [CrossRef]
- Byazrova, M.; Yusubalieva, G.; Spiridonova, A.; Efimov, G.; Mazurov, D.; Baranov, K.; Baklaushev, V.; Filatov, A. Pattern of circulating SARS-CoV-2-specific antibody-secreting and memory B-cell generation in patients with acute COVID-19. Clin. Transl. Immunol. 2021, 10, e1245. [Google Scholar] [CrossRef]
- Vanshylla, K.; Di Cristanziano, V.; Kleipass, F.; Dewald, F.; Schommers, P.; Gieselmann, L.; Gruell, H.; Schlotz, M.; Ercanoglu, M.S.; Stumpf, R.; et al. Kinetics and correlates of the neutralizing antibody response to SARS-CoV-2 infection in humans. Cell Host Microbe 2021, 29, 917–929.e4. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulemzin, S.V.; Guselnikov, S.V.; Nekrasov, B.G.; Molodykh, S.V.; Kuvshinova, I.N.; Murasheva, S.V.; Belovezhets, T.N.; Gorchakov, A.A.; Chikaev, A.N.; Chikaev, N.A.; et al. Hybrid Immunity from Gam-COVID-Vac Vaccination and Natural SARS-CoV-2 Infection Confers Broader Neutralizing Activity against Omicron Lineage VOCs Than Revaccination or Reinfection. Vaccines 2024, 12, 55. https://doi.org/10.3390/vaccines12010055
Kulemzin SV, Guselnikov SV, Nekrasov BG, Molodykh SV, Kuvshinova IN, Murasheva SV, Belovezhets TN, Gorchakov AA, Chikaev AN, Chikaev NA, et al. Hybrid Immunity from Gam-COVID-Vac Vaccination and Natural SARS-CoV-2 Infection Confers Broader Neutralizing Activity against Omicron Lineage VOCs Than Revaccination or Reinfection. Vaccines. 2024; 12(1):55. https://doi.org/10.3390/vaccines12010055
Chicago/Turabian StyleKulemzin, Sergey V., Sergey V. Guselnikov, Boris G. Nekrasov, Svetlana V. Molodykh, Irina N. Kuvshinova, Svetlana V. Murasheva, Tatyana N. Belovezhets, Andrey A. Gorchakov, Anton N. Chikaev, Nikolai A. Chikaev, and et al. 2024. "Hybrid Immunity from Gam-COVID-Vac Vaccination and Natural SARS-CoV-2 Infection Confers Broader Neutralizing Activity against Omicron Lineage VOCs Than Revaccination or Reinfection" Vaccines 12, no. 1: 55. https://doi.org/10.3390/vaccines12010055
APA StyleKulemzin, S. V., Guselnikov, S. V., Nekrasov, B. G., Molodykh, S. V., Kuvshinova, I. N., Murasheva, S. V., Belovezhets, T. N., Gorchakov, A. A., Chikaev, A. N., Chikaev, N. A., Volkova, O. Y., Yurina, A. A., Najakshin, A. M., & Taranin, A. V. (2024). Hybrid Immunity from Gam-COVID-Vac Vaccination and Natural SARS-CoV-2 Infection Confers Broader Neutralizing Activity against Omicron Lineage VOCs Than Revaccination or Reinfection. Vaccines, 12(1), 55. https://doi.org/10.3390/vaccines12010055





