DNA Vaccines: Their Formulations, Engineering and Delivery
Abstract
1. Introduction
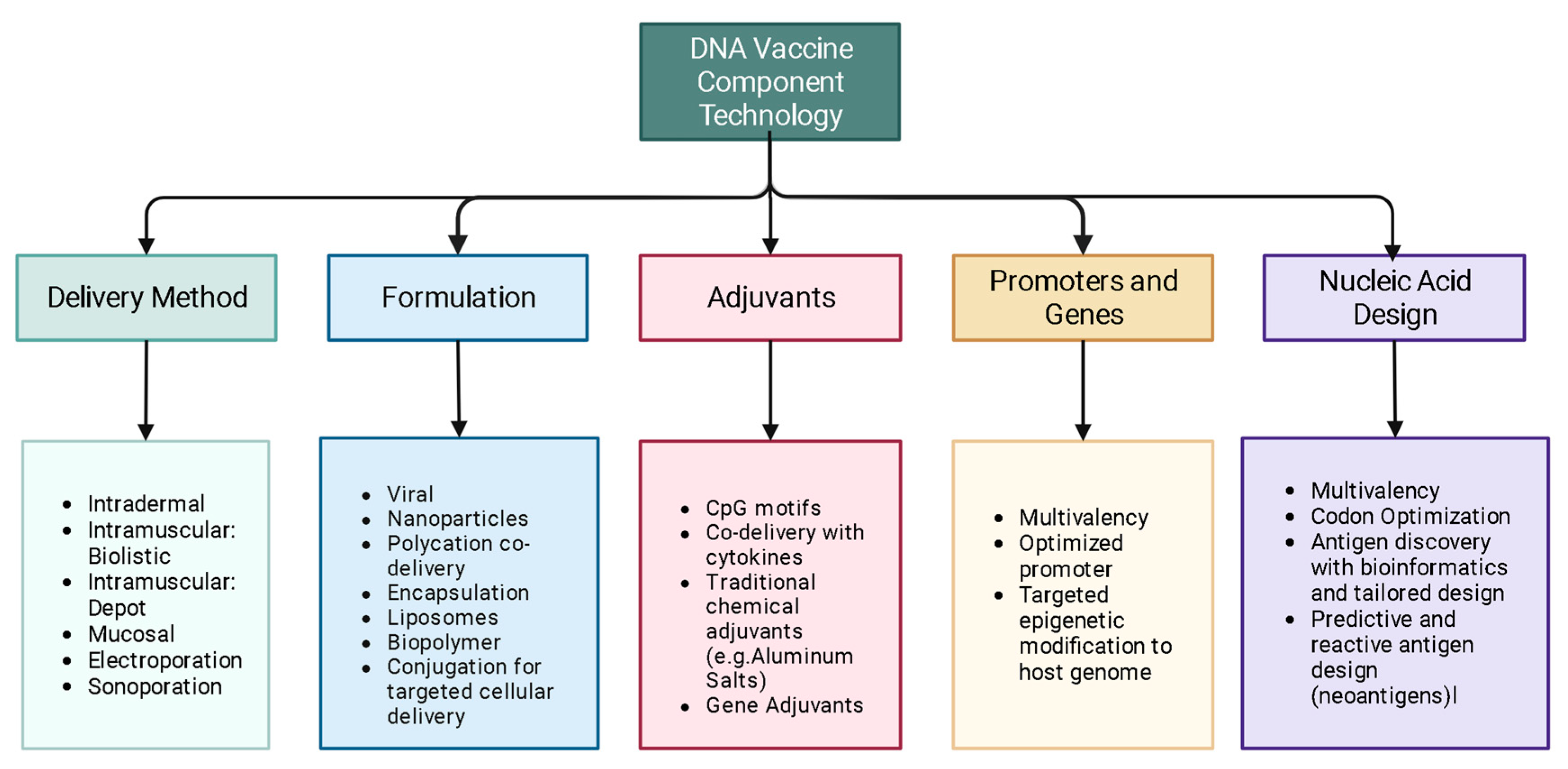
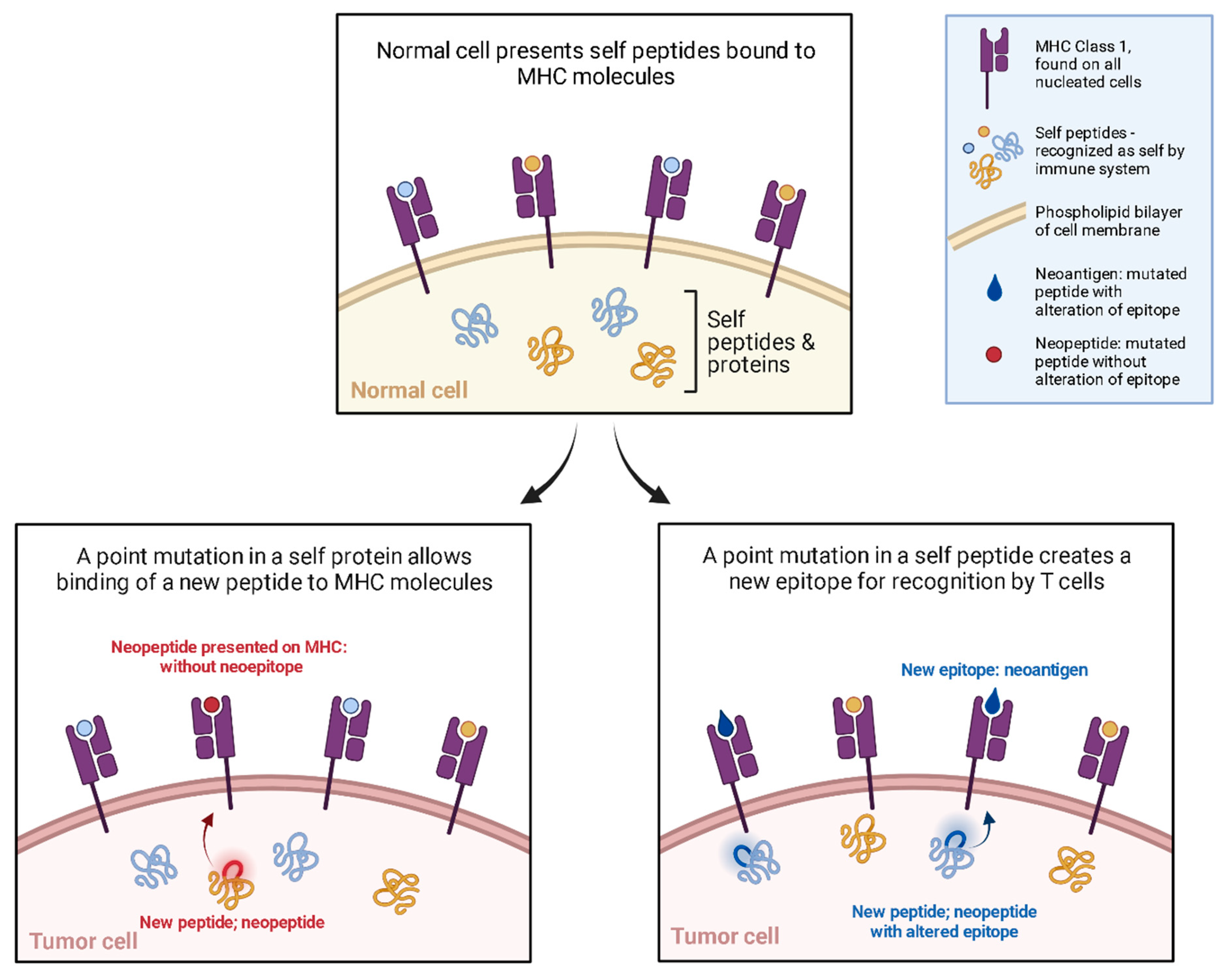
2. Mechanism
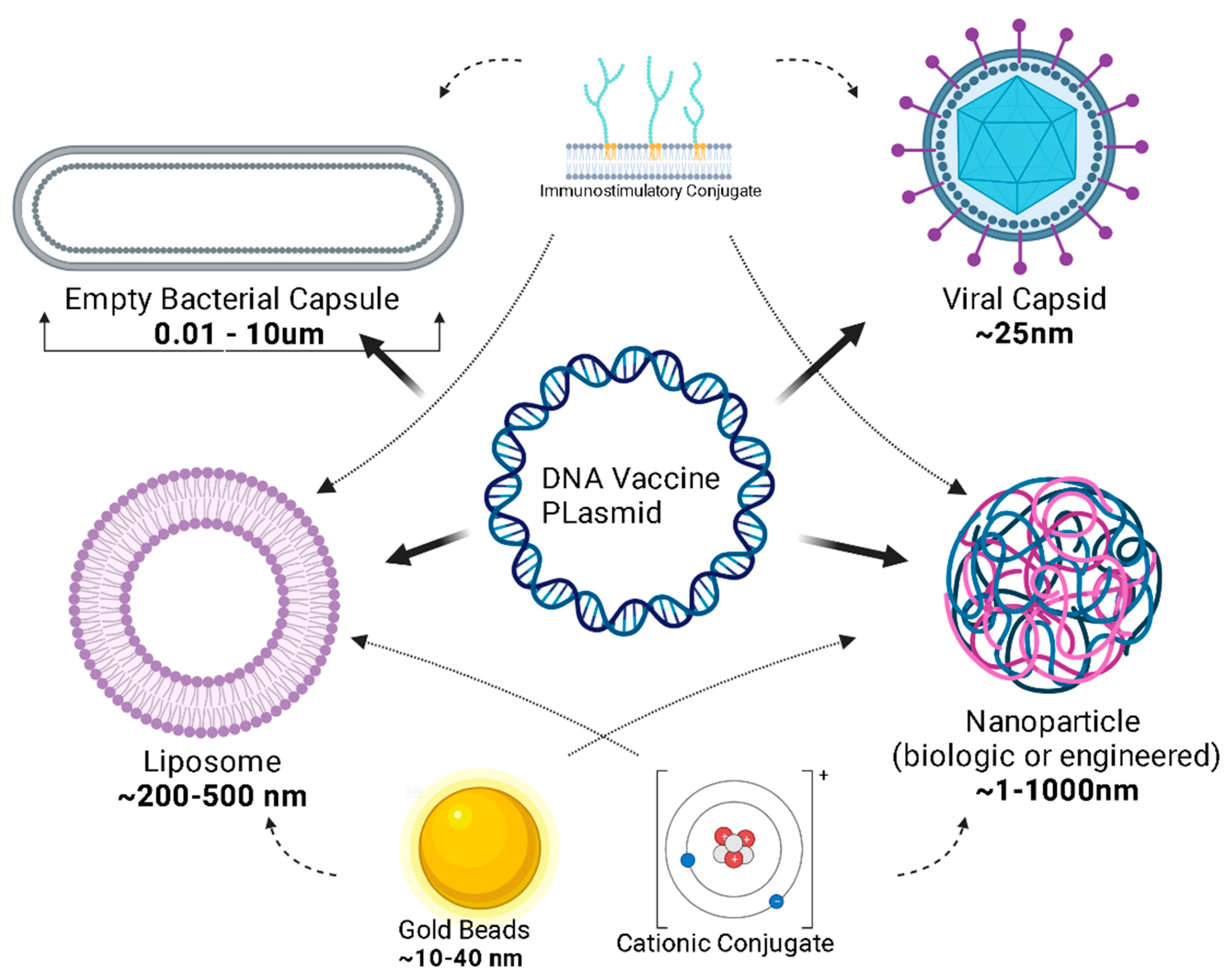
3. Design
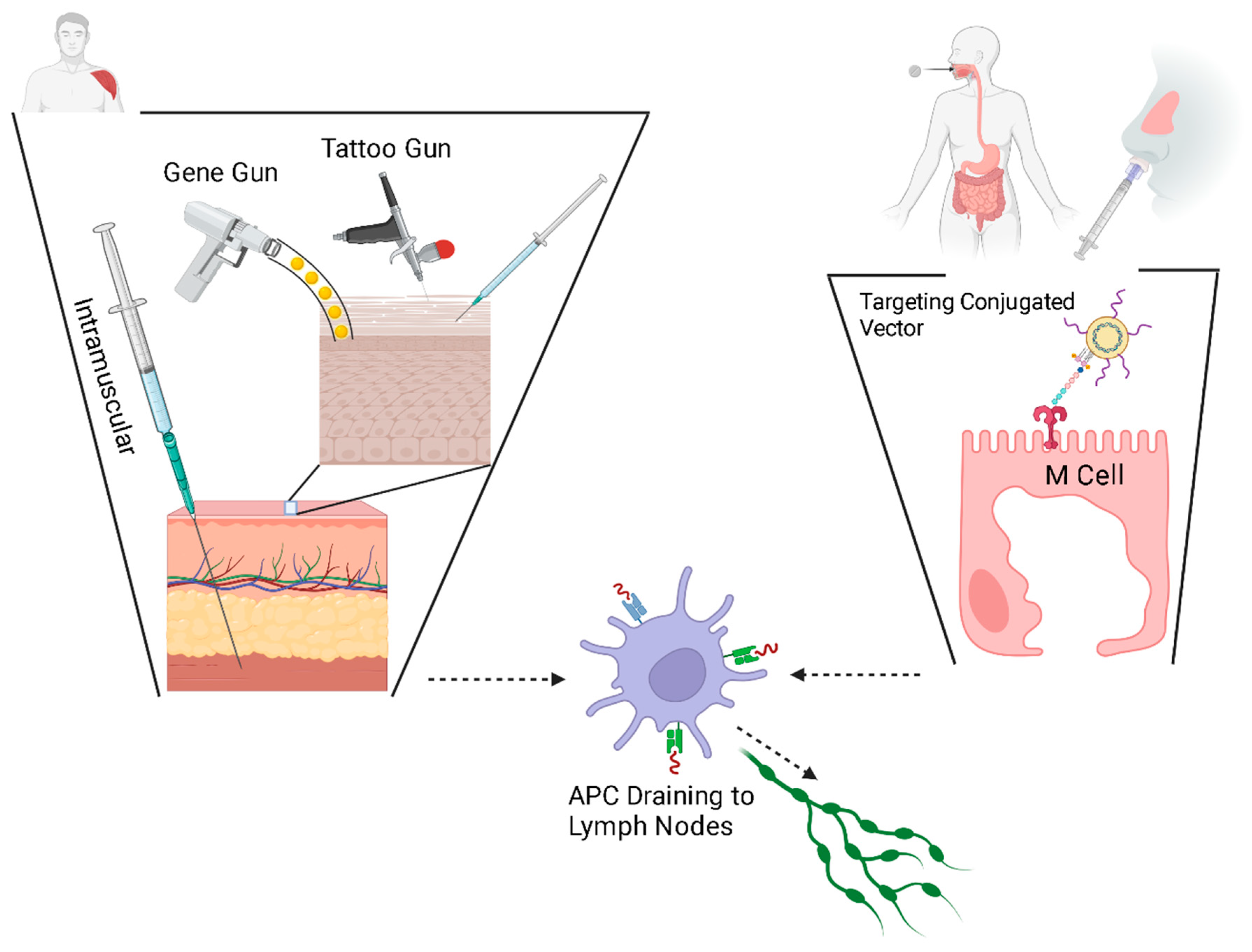
4. Delivery
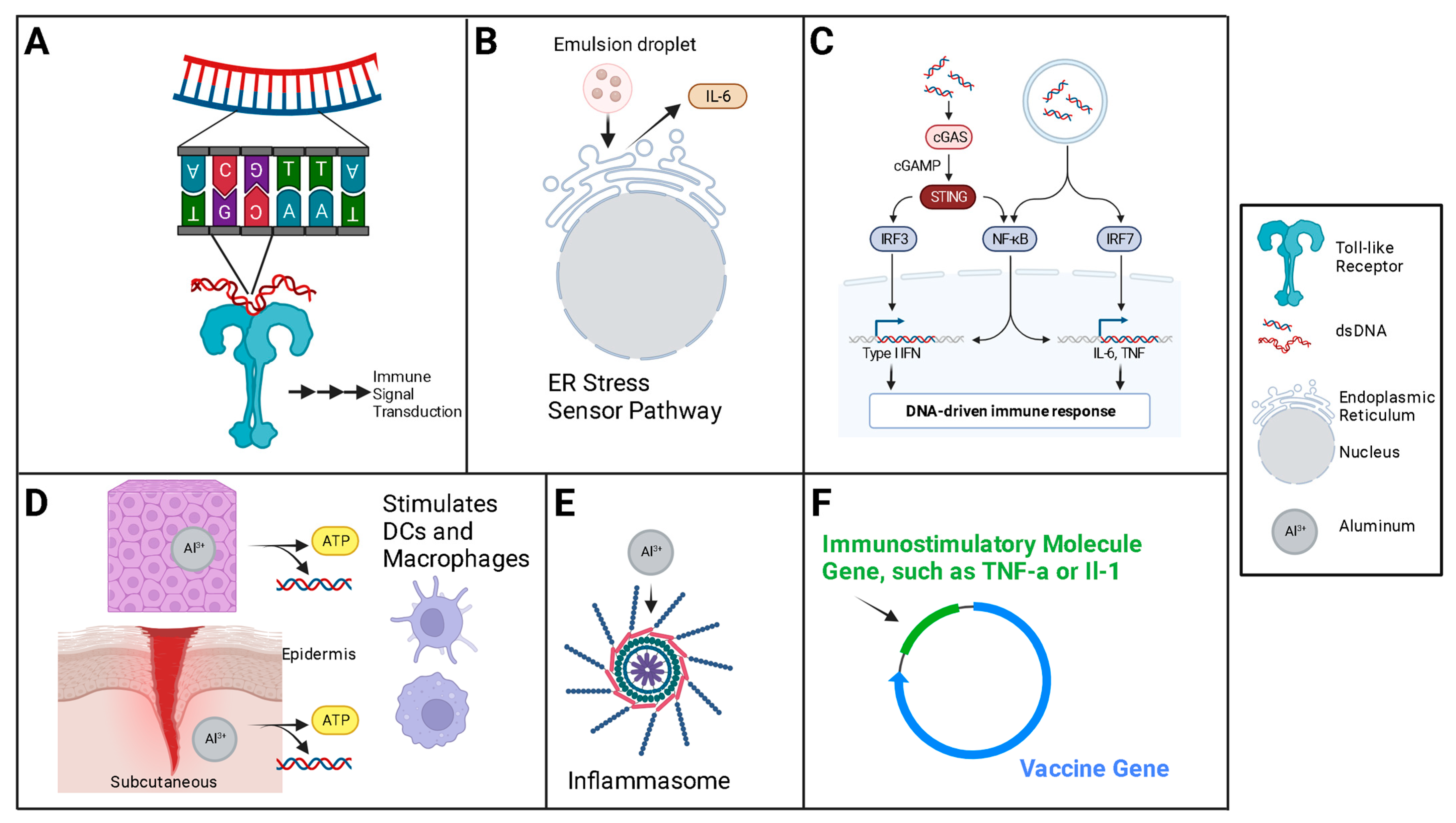
5. Adjuvants
6. Limitations/Concerns
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jedrzejczak-Silicka, M. History of Cell Culture. In New Insights into Cell Culture Technology; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- The Development of the Electron Microscope|American Association for the Advancement of Science (AAAS). Available online: https://www.aaas.org/development-electron-microscope (accessed on 11 September 2023).
- Shaber, L. The History of the Electron Microscope. Advancing Materials. Available online: https://www.thermofisher.com/blog/materials/the-history-of-the-electron-microscope/ (accessed on 11 September 2023).
- Cobb, M. 60 years ago, Francis Crick changed the logic of biology. PLoS Biol. 2017, 15, e2003243. [Google Scholar] [CrossRef] [PubMed]
- The Sequence of Sequencers: The History of Sequencing DNA—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4727787/ (accessed on 11 September 2023).
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef] [PubMed]
- Vaccine Timeline|History of Vaccines. The College of Physicians of Philadelphia. Available online: https://historyofvaccines.org/history/vaccine-timeline/overview (accessed on 18 January 2023).
- Vaccine History Timeline. Immunize.org. Available online: https://www.immunize.org/vaccines/vaccine-timeline/ (accessed on 11 December 2023).
- Srivastava, I.K.; Liu, M.A. Gene vaccines. Ann. Intern. Med. 2003, 138, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M.; Hu, J. The Integrated Consideration of Vaccine Platforms, Adjuvants, and Delivery Routes for Successful Vaccine Development. Vaccines 2023, 11, 695. [Google Scholar] [CrossRef]
- Cui, Z. DNA vaccine. Adv. Genet. 2005, 54, 257–289. [Google Scholar] [CrossRef]
- Jazayeri, S.D.; Poh, C.L. Recent advances in delivery of veterinary DNA vaccines against avian pathogens. Vet. Res. 2019, 50, 78. [Google Scholar] [CrossRef]
- Grimmett, E.; Al-Share, B.; Alkassab, M.B.; Zhou, R.W.; Desai, A.; Rahim, M.M.; Woldie, I. Cancer vaccines: Past, present and future; a review article. Discov. Oncol. 2022, 13, 31. [Google Scholar] [CrossRef]
- Liu, M.A. A Comparison of Plasmid DNA and mRNA as Vaccine Technologies. Vaccines 2019, 7, 37. [Google Scholar] [CrossRef]
- Leitner, W.W.; Ying, H.; Restifo, N.P. DNA and RNA-based vaccines: Principles, progress and prospects. Vaccine 1999, 18, 765–777. [Google Scholar] [CrossRef]
- Decades in the Making: mRNA COVID-19 Vaccines. NIH COVID-19 Research. Available online: https://covid19.nih.gov/nih-strategic-response-covid-19/decades-making-mrna-covid-19-vaccines (accessed on 11 September 2023).
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA vaccine development. Sig. Transduct Target Ther. 2022, 7, 94. [Google Scholar] [CrossRef]
- Perez-Rueda, E.; Hernandez-Guerrero, R.; Martinez-Nuñez, M.A.; Armenta-Medina, D.; Sanchez, I.; Ibarra, J.A. Abundance, diversity and domain architecture variability in prokaryotic DNA-binding transcription factors. PLoS ONE 2018, 13, e0195332. [Google Scholar] [CrossRef]
- Margaliot, M.; Huleihel, W.; Tuller, T. Variability in mRNA translation: A random matrix theory approach. Sci. Rep. 2021, 11, 5300. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Hossain, M.K.; Beladiya, J.; Apostolopoulos, V. Nucleic Acid Vaccines for COVID-19: A Paradigm Shift in the Vaccine Development Arena. Biologics 2021, 1, 337–356. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247 Pt 1, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.C.; DeVit, M.; Johnston, S.A. Genetic immunization is a simple method for eliciting an immune response. Nature 1992, 356, 152–154. [Google Scholar] [CrossRef]
- Osborn, M.J.; McElmurry, R.T.; Lees, C.J.; DeFeo, A.P.; Chen, Z.Y.; Kay, M.A.; Naldini, L.; Freeman, G.; Tolar, J.; Blazar, B.R. Minicircle DNA-based gene therapy coupled with immune modulation permits long-term expression of α-L-iduronidase in mice with mucopolysaccharidosis type I. Mol. Ther. 2011, 19, 450–460. [Google Scholar] [CrossRef]
- Lechardeur, D.; Verkman, A.S.; Lukacs, G.L. Intracellular routing of plasmid DNA during non-viral gene transfer. Adv. Drug Deliv. Rev. 2005, 57, 755–767. [Google Scholar] [CrossRef]
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.D.F.R.E.; Blasi, M. The use of viral vectors in vaccine development. npj Vaccines 2022, 7, 75. [Google Scholar] [CrossRef]
- Hanke, T. New vector and vaccine platforms: mRNA, DNA, viral vectors. Curr. Opin. HIV AIDS 2022, 17, 338. [Google Scholar] [CrossRef]
- Mallapaty, S. India’s DNA COVID Vaccine Is a World First—More Are Coming. 2 September 2021. Available online: https://www.nature.com/articles/d41586-021-02385-x (accessed on 20 January 2023).
- Khobragade, A.; Bhate, S.; Ramaiah, V.; Deshpande, S.; Giri, K.; Phophle, H.; Supe, P.; Godara, I.; Revanna, R.; Nagarkar, R.; et al. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): The interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. Lancet 2022, 399, 1313–1321. [Google Scholar] [CrossRef]
- Blakney, A.K.; Bekker, L.-G. DNA vaccines join the fight against COVID-19. Lancet 2022, 399, 1281–1282. [Google Scholar] [CrossRef]
- Kutzler, M.A.; Weiner, D.B. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, R.R.; Boyer, J.D.; Ugen, K.E.; Lacy, K.E.; Gluckman, S.J.; Bagarazzi, M.L.; Chattergoon, M.A.; Baine, Y.; Higgins, T.J.; Ciccarelli, R.B.; et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: Safety and host response. J. Infect Dis. 1998, 178, 92–100. [Google Scholar] [CrossRef]
- Andrews, S.M.; Rowland-Jones, S. Recent advances in understanding HIV evolution. F1000Research 2017, 6, 597. [Google Scholar] [CrossRef]
- Sekaly, R.-P. The failed HIV Merck vaccine study: A step back or a launching point for future vaccine development? J. Exp. Med. 2008, 205, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.E. The repeated setbacks of HIV vaccine development laid the groundwork for SARS-CoV-2 vaccines. Health Policy Technol. 2022, 11, 100619. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.W.; Fiore-Gartland, A.; Walsh, S.R.; Yusim, K.; Frahm, N.; Elizaga, M.L.; Maenza, J.; Scott, H.; Mayer, K.H.; Goepfert, P.A.; et al. Trivalent mosaic or consensus HIV immunogens prime humoral and broader cellular immune responses in adults. J. Clin. Investig. 2023, 133, e163338. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.; Xu, W.; Liu, S. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 9. [Google Scholar] [CrossRef]
- Home—ClinicalTrials.gov. Available online: https://classic.clinicaltrials.gov/ct2/home (accessed on 28 September 2023).
- Aida, V.; Pliasas, V.C.; Neasham, P.J.; North, J.F.; McWhorter, K.L.; Glover, S.R.; Kyriakis, C.S. Novel Vaccine Technologies in Veterinary Medicine: A Herald to Human Medicine Vaccines. Front. Vet. Sci. 2021, 8, 654289. Available online: https://www.frontiersin.org/articles/10.3389/fvets.2021.654289 (accessed on 16 October 2023). [CrossRef]
- Li, L.; Petrovsky, N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev. Vaccines 2016, 15, 313–329. [Google Scholar] [CrossRef]
- Disis, M.L.; Guthrie, K.A.; Liu, Y.; Coveler, A.L.; Higgins, D.M.; Childs, J.S.; Dang, Y.; Salazar, L.G. Safety and Outcomes of a Plasmid DNA Vaccine Encoding the ERBB2 Intracellular Domain in Patients with Advanced-Stage ERBB2-Positive Breast Cancer: A Phase 1 Nonrandomized Clinical Trial. JAMA Oncol. 2023, 9, 71–78. [Google Scholar] [CrossRef]
- Shafaati, M.; Saidijam, M.; Soleimani, M.; Hazrati, F.; Mirzaei, R.; Amirheidari, B.; Tanzadehpanah, H.; Karampoor, S.; Kazemi, S.; Yavari, B.; et al. A brief review on DNA vaccines in the era of COVID-19. Future Virol. 2022, 17, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, B.; Morrow, M.P.; Hutnick, N.A.; Shin, T.H.; Lucke, C.E.; Weiner, D.B. Clinical Applications of DNA Vaccines: Current Progress. Clin. Infect Dis. 2011, 53, 296–302. [Google Scholar] [CrossRef]
- Lopes, A.; Vandermeulen, G.; Préat, V. Cancer DNA vaccines: Current preclinical and clinical developments and future perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 146. [Google Scholar] [CrossRef]
- Search for: DNA Vaccine|List Results|ClinicalTrials.gov. Available online: https://clinicaltrials.gov/search?intr=DNA%20Vaccine&viewType=Table&limit=100&page=1 (accessed on 1 January 2024).
- Gary, E.N.; Weiner, D.B. DNA vaccines: Prime time is now. Curr. Opin. Immunol. 2020, 65, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Maslow, J.N.; Kwon, I.; Kudchodkar, S.B.; Kane, D.; Tadesse, A.; Lee, H.; Park, Y.K.; Muthumani, K.; Roberts, C.C. DNA Vaccines for Epidemic Preparedness: SARS-CoV-2 and Beyond. Vaccines 2023, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, E.W. The MHC class I antigen presentation pathway: Strategies for viral immune evasion. Immunology 2003, 110, 163–169. [Google Scholar] [CrossRef]
- Blum, J.S.; Wearsch, P.A.; Cresswell, P. Pathways of Antigen Processing. Annu. Rev. Immunol. 2013, 31, 443–473. [Google Scholar] [CrossRef]
- Delamarre, L.; Holcombe, H.; Mellman, I. Presentation of Exogenous Antigens on Major Histocompatibility Complex (MHC) Class I and MHC Class II Molecules Is Differentially Regulated during Dendritic Cell Maturation. J. Exp. Med. 2003, 198, 111–122. [Google Scholar] [CrossRef]
- Harding, C.V. Class I MHC presentation of exogenous antigens. J. Clin. Immunol. 1996, 16, 90–96. [Google Scholar] [CrossRef]
- Khan, K.H. DNA vaccines: Roles against diseases. Germs 2013, 3, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Leifert, J.A.; Whitton, J.L. Immune Responses to DNA Vaccines: Induction of CD8 T Cells. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK6523/ (accessed on 28 September 2023).
- Daniels, M.A.; Devine, L.; Miller, J.D.; Moser, J.M.; Lukacher, A.E.; Altman, J.D.; Kavathas, P.; Hogquist, K.A.; Jameson, S.C. CD8 binding to MHC class I molecules is influenced by T cell maturation and glycosylation. Immunity 2001, 15, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.J.; Wu, M.S.; Barr, L.J.; Fuller, J.T.; Tussey, L.G.; Speller, S.; Culp, J.; Burkholder, J.K.; Swain, W.F.; Dixon, R.M.; et al. Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine 2000, 19, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Hobernik, D.; Bros, M. DNA Vaccines—How Far From Clinical Use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yao, Y.; Zhang, X.; Liu, H.; Gao, G.; Peng, Y.; Chen, M.; Zhao, J.; Zhang, X.; Yin, C.; et al. Both chimpanzee adenovirus-vectored and DNA vaccines induced long-term immunity against Nipah virus infection. npj Vaccines 2023, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Embgenbroich, M.; Burgdorf, S. Current Concepts of Antigen Cross-Presentation. Front. Immunol. 2018, 9, 1643. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2018.01643 (accessed on 28 September 2023). [CrossRef] [PubMed]
- Gilbert, S.C. T-cell-inducing vaccines—what’s the future. Immunology 2012, 135, 19–26. [Google Scholar] [CrossRef]
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 2011, 10, 499–511. [Google Scholar] [CrossRef]
- Krieg, A.M.; Yi, A.K.; Matson, S.; Waldschmidt, T.J.; Bishop, G.A.; Teasdale, R.; Koretzky, G.A.; Klinman, D.M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 1995, 374, 546–549. [Google Scholar] [CrossRef]
- Hartl, A.; Kiesslich, J.; Weiss, R.; Bernhaupt, A.; Mostböck, S.; Scheiblhofer, S.; Ebner, C.; Ferreira, F.; Thalhamer, J. Immune responses after immunization with plasmid DNA encoding Bet v 1, the major allergen of birch pollen. J. Allergy Clin. Immunol. 1999, 103 Pt 1, 107–113. [Google Scholar] [CrossRef]
- Janeway, J.C.; Travers, P.; Walport, M.; Shlomchik, M.J. Antigen Recognition by B-cell and T-cell Receptors. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK10770/ (accessed on 28 September 2023).
- Szeto, C.; Lobos, C.A.; Nguyen, A.T.; Gras, S. TCR Recognition of Peptide–MHC-I: Rule Makers and Breakers. Int. J. Mol. Sci. 2020, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Amanna, I.J.; Slifka, M.K. Contributions of humoral and cellular immunity to vaccine-induced protection in humans. Virology 2011, 411, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Shedlock, D.J.; Weiner, D.B. DNA vaccination: Antigen presentation and the induction of immunity. J. Leukoc. Biol. 2000, 68, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Koup, R.A.; Douek, D.C. Vaccine Design for CD8 T Lymphocyte Responses. Cold Spring Harb. Perspect. Med. 2011, 1, a007252. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.K.; Lam, K.T.; Liu, Y.; Fang, V.J.; Mu, X.; Leung, N.H.; Peiris, J.M.; Leung, G.M.; Cowling, B.J.; Tu, W. Investigation of CD4 and CD8 T cell-mediated protection against influenza A virus in a cohort study. BMC Med. 2022, 20, 230. [Google Scholar] [CrossRef]
- Reinscheid, M.; Luxenburger, H.; Karl, V.; Graeser, A.; Giese, S.; Ciminski, K.; Reeg, D.B.; Oberhardt, V.; Roehlen, N.; Lang-Meli, J. COVID-19 mRNA booster vaccine induces transient CD8+ T effector cell responses while conserving the memory pool for subsequent reactivation. Nat. Commun. 2022, 13, 4631. [Google Scholar] [CrossRef]
- Liu, J.; Yu, J.; McMahan, K.; Jacob-Dolan, C.; He, X.; Giffin, V.; Wu, C.; Sciacca, M.; Powers, O.; Nampanya, F.; et al. CD8 T cells contribute to vaccine protection against SARS-CoV-2 in macaques. Sci. Immunol. 2022, 7, eabq7647. [Google Scholar] [CrossRef]
- Dolina, J.S.; Lee, J.; Brightman, S.E.; McArdle, S.; Hall, S.M.; Thota, R.R.; Zavala, K.S.; Lanka, M.; Premlal, A.L.; Greenbaum, J.A.; et al. Linked CD4+/CD8+ T cell neoantigen vaccination overcomes immune checkpoint blockade resistance and enables tumor regression. J. Clin. Investig. 2023, 133, e164258. [Google Scholar] [CrossRef]
- Fierer, J.; Waters, C.; Walls, L. Both CD4+ and CD8+ T Cells Can Mediate Vaccine-Induced Protection against Coccidioides immitis Infection in Mice. J. Infect. Dis. 2006, 193, 1323–1331. [Google Scholar] [CrossRef]
- Oh, D.Y.; Fong, L.; Newell, E.W.; Turk, M.J.; Chi, H.; Chang, H.Y.; Satpathy, A.T.; Fairfax, B.; Silva-Santos, B.; Lantz, O. Toward a better understanding of T cells in cancer. Cancer Cell 2021, 39, 1549–1552. [Google Scholar] [CrossRef]
- Franck, C.O.; Fanslau, L.; Popov, A.B.; Tyagi, P.; Fruk, L. Biopolymer-based Carriers for DNA Vaccine Design. Angew. Chem. Int. Ed. 2021, 60, 13225–13243. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Leitner, W.W.; Scheiblhofer, S.; Chen, D.; Bernhaupt, A.; Mostböck, S.; Thalhamer, J.; Lyon, J.A. Genetic Vaccination against Malaria Infection by Intradermal and Epidermal Injections of a Plasmid Containing the Gene Encoding the Plasmodium berghei Circumsporozoite Protein. Infect. Immun. 2000, 68, 5914–5919. [Google Scholar] [CrossRef] [PubMed]
- Feltquate, D.M.; Heaney, S.; Webster, R.G.; Robinson, H.L. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J. Immunol. 1997, 158, 2278–2284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, Z.; Wang, Y.; Wu, S.; Wang, B.; Zhang, J.; Song, X.; Chen, Y.; Lv, P.; Hou, L. Comparative immunogenicity analysis of intradermal versus intramuscular immunization with a recombinant human adenovirus type 5 vaccine against Ebola virus. Front. Immunol. 2022, 13, 963049. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2022.963049 (accessed on 12 October 2023). [CrossRef] [PubMed]
- Fynan, E.F.; Webster, R.G.; Fuller, D.H.; Haynes, J.R.; Santoro, J.C.; Robinson, H.L. DNA vaccines: Protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc. Natl. Acad. Sci. USA 1993, 90, 11478–11482. [Google Scholar] [CrossRef] [PubMed]
- Black, M.; Trent, A.; Tirrell, M.; Olive, C. Advances in the design and delivery of peptide subunit vaccines with a focus on Toll-like receptor agonists. Expert Rev. Vaccines 2010, 9, 157–173. [Google Scholar] [CrossRef]
- Sarkar, B.; Ullah, M.A.; Araf, Y.; Rahman, M.S. Engineering a novel subunit vaccine against SARS-CoV-2 by exploring immunoinformatics approach. Inform. Med. Unlocked 2020, 21, 100478. [Google Scholar] [CrossRef]
- Krauson, A.J.; Casimero, F.V.C.; Siddiquee, Z.; Stone, J.R. Duration of SARS-CoV-2 mRNA vaccine persistence and factors associated with cardiac involvement in recently vaccinated patients. npj Vaccines 2023, 8, 141. [Google Scholar] [CrossRef]
- Klinman, D.M.; Klaschik, S.; Tross, D.; Shirota, H.; Steinhagen, F. FDA guidance on prophylactic DNA vaccines: Analysis and recommendations. Vaccine 2010, 28, 2801–2805. [Google Scholar] [CrossRef]
- Correia, B.E.; Bates, J.T.; Loomis, R.J.; Baneyx, G.; Carrico, C.; Jardine, J.G.; Rupert, P.; Correnti, C.; Kalyuzhniy, O.; Vittal, V.; et al. Proof of principle for epitope-focused vaccine design. Nature 2014, 507, 201–206. [Google Scholar] [CrossRef]
- Antonopoulou, T.; Athanassakis, I. SARS-CoV-2 immunogenicity: Is S protein the best target for vaccination? Vaccine 2022, 40, 3093–3095. [Google Scholar] [CrossRef]
- Yurina, V.; Adianingsih, O.R. Predicting epitopes for vaccine development using bioinformatics tools. Ther. Adv. Vaccines Immunother. 2022, 10, 25151355221100218. [Google Scholar] [CrossRef]
- Yurina, V.; Yudani, T.; Raras, M. Design and construction of DNA vaccine expressing lectin-like oxidize-LDL receptor-1 (LOX-1) as atherosclerosis vaccine candidate. J. Biotech. Res. 2017, 1, 103–112. [Google Scholar]
- Home—Protein—NCBI. Available online: https://www.ncbi.nlm.nih.gov/protein (accessed on 3 October 2023).
- Adianingsih, O.R.; Kharisma, V.D. Study of B cell epitope conserved region of the Zika virus envelope glycoprotein to develop multi-strain vaccine. J. Appl. Pharm. Sci. 2019, 9, 98–103. [Google Scholar]
- Sitompul, L.S.; Widodo, N.; Djati, M.S.; Utomo, D.H. Epitope mapping of gp350/220 conserved domain of epstein barr virus to develop nasopharyngeal carcinoma (npc) vaccine. Bioinformation 2012, 8, 479–482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- He, Y.; Racz, R.; Sayers, S.; Lin, Y.; Todd, T.; Hur, J.; Li, X.; Patel, M.; Zhao, B.; Chung, M.; et al. Updates on the web-based VIOLIN vaccine database and analysis system. Nucl. Acids Res. 2014, 42, D1124–D1132. [Google Scholar] [CrossRef]
- Immunotherapy for Cancer—NCI. Available online: https://www.cancer.gov/about-cancer/treatment/types/immunotherapy (accessed on 3 October 2023).
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- Melenhorst, J.J.; Chen, G.M.; Wang, M.; Porter, D.L.; Chen, C.; Collins, M.A.; Gao, P.; Bandyopadhyay, S.; Sun, H.; Zhao, Z.; et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 2022, 602, 7897. [Google Scholar] [CrossRef]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef]
- Yaddanapudi, K.; Mitchell, R.A.; Eaton, J.W. Cancer vaccines. Oncoimmunology 2013, 2, e23403. [Google Scholar] [CrossRef]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N. Engl. J. Med. 2014, 371, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: Promising targets for cancer therapy. Sig. Transduct Target Ther. 2023, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiao, Z.; Wang, D.; Jia, L.; Nie, S.; Zeng, X.; Hu, W. The screening, identification, design and clinical application of tumor-specific neoantigens for TCR-T cells. Mol. Cancer 2023, 22, 141. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.J.; Svensson-Arvelund, J.; Lubitz, G.S.; Marabelle, A.; Melero, I.; Brown, B.D.; Brody, J.D. Cancer vaccines: The next immunotherapy frontier. Nat. Cancer 2022, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Finn, O.J. Cancer vaccines: Between the idea and the reality. Nat. Rev. Immunol. 2003, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Biswas, N.; Chakrabarti, S.; Padul, V.; Jones, L.D.; Ashili, S. Designing neoantigen cancer vaccines, trials, and outcomes. Front Immunol. 2023, 14, 1105420. [Google Scholar] [CrossRef]
- Fritah, H.; Rovelli, R.; Chiang, C.L.-L.; Kandalaft, L.E. The current clinical landscape of personalized cancer vaccines. Cancer Treat. Rev. 2022, 106, 102383. [Google Scholar] [CrossRef]
- Mattos-Arruda, L.D.; Blanco-Heredia, J.; Aguilar-Gurrieri, C.; Carrillo, J.; Blanco, J. New emerging targets in cancer immunotherapy: The role of neoantigens. ESMO Open 2019, 4, e000684. [Google Scholar] [CrossRef]
- Niemi, J.V.L.; Sokolov, A.V.; Schiöth, H.B. Neoantigen Vaccines; Clinical Trials, Classes, Indications, Adjuvants and Combinatorial Treatments. Cancers 2022, 14, 5163. [Google Scholar] [CrossRef]
- Eggleton, J.S.; Nagalli, S. Highly Active Antiretroviral Therapy (HAART). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK554533/ (accessed on 2 November 2023).
- Report of the NIH Panel to Define Principles of Therapy of HIV Infection. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00052295.htm (accessed on 2 November 2023).
- Maggiolo, F.; Leone, S. Is HAART modifying the HIV epidemic? Lancet 2010, 376, 492–493. [Google Scholar] [CrossRef] [PubMed]
- Baylin, S.B.; Jones, P.A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect Biol. 2016, 8, a019505. [Google Scholar] [CrossRef] [PubMed]
- Grønbæk, K.; Hother, C.; Jones, P.A. Epigenetic changes in cancer. APMIS 2007, 115, 1039–1059. [Google Scholar] [CrossRef]
- Agrawal, B. Heterologous Immunity: Role in Natural and Vaccine-Induced Resistance to Infections. Front Immunol. 2019, 10, 2631. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, H.S.; Ahmed, S.S.; Curtis, N.; Kollmann, T.R.; Levy, O.; Netea, M.G.; Pollard, A.J.; van Crevel, R.; Wilson, C.B. Harnessing the beneficial heterologous effects of vaccination. Nat. Rev. Immunol. 2016, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Saadatian-Elahi, M.; Aaby, P.; Shann, F.; Netea, M.G.; Levy, O.; Louis, J.; Picot, V.; Greenberg, M.; Warren, W. Heterologous vaccine effects. Vaccine 2016, 34, 3923–3930. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Finn, A.; Curtis, N. Non-specific effects of vaccines: Plausible and potentially important, but implications uncertain. Arch. Dis. Child 2017, 102, 1077–1081. [Google Scholar] [CrossRef]
- Oriol-Tordera, B.; Esteve-Codina, A.; Berdasco, M.; Rosás-Umbert, M.; Goncalves, E.; Duran-Castells, C.; Catala-Moll, F.; Llano, A.; Cedeno, S.; Puertas, M.C.; et al. Epigenetic landscape in the kick-and-kill therapeutic vaccine BCN02 clinical trial is associated with antiretroviral treatment interruption (ATI) outcome. EBioMedicine 2022, 78, 103956. [Google Scholar] [CrossRef]
- Takahashi, H.; Kühtreiber, W.M.; Keefe, R.C.; Lee, A.H.; Aristarkhova, A.; Dias, H.F.; Ng, N.; Nelson, K.J.; Bien, S.; Scheffey, D.; et al. BCG vaccinations drive epigenetic changes to the human T cell receptor: Restored expression in type 1 diabetes. Sci. Adv. 2022, 8, eabq7240. [Google Scholar] [CrossRef]
- Faustman, D.L.; Wang, L.; Okubo, Y.; Burger, D.; Ban, L.; Man, G.; Zheng, H.; Schoenfeld, D.; Pompei, R.; Avruch, J.; et al. Proof-of-concept, randomized, controlled clinical trial of Bacillus-Calmette-Guerin for treatment of long-term type 1 diabetes. PLoS ONE 2012, 7, e41756. [Google Scholar] [CrossRef]
- Kühtreiber, W.M.; Faustman, D.L. BCG Therapy for Type 1 Diabetes: Restoration of Balanced Immunity and Metabolism. Trends Endocrinol. Metab. 2019, 30, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Katzmarski, N.; Domínguez-Andrés, J.; Cirovic, B.; Renieris, G.; Ciarlo, E.; Le Roy, D.; Lepikhov, K.; Kattler, K.; Gasparoni, G.; Händler, K.; et al. Transmission of trained immunity and heterologous resistance to infections across generations. Nat. Immunol. 2021, 22, 11. [Google Scholar] [CrossRef]
- Ledwith, B.J.; Manam, S.; Troilo, P.J.; Barnum, A.B.; Pauley, C.J.; Griffiths, I.I.T.G.; Harper, L.B.; Beare, C.M.; Bagdon, W.J.; Nichols, W.W. Plasmid DNA vaccines: Investigation of integration into host cellular DNA following intramuscular injection in mice. Intervirology 2000, 43, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Ledwith, B.J.; Manam, S.; Troilo, P.J.; Barnum, A.B.; Pauley, C.J.; Griffiths, T.G., 2nd; Harper, L.B.; Schock, H.B.; Zhang, H.; Faris, J.E.; et al. Plasmid DNA vaccines: Assay for integration into host genomic DNA. Dev. Biol. 2000, 104, 33–43. [Google Scholar]
- Kang, K.K.; Choi, S.M.; Choi, J.H.; Lee, D.S.; Kim, C.Y.; Ahn, B.O.; Kim, B.M.; Kim, W.B. Safety evaluation of GX-12, a new HIV therapeutic vaccine: Investigation of integration into the host genome and expression in the reproductive organs. Intervirology 2003, 46, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Iurescia, S.; Fioretti, D.; Rinaldi, M. A blueprint for DNA vaccine design. Methods Mol. Biol. 2014, 1143, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Shahin, V. Gatekeepers of the nucleus. Nat. Nanotechnol. 2016, 11, 8. [Google Scholar] [CrossRef]
- Warrington, K.H.; Gorbatyuk, O.S.; Harrison, J.K.; Opie, S.R.; Zolotukhin, S.; Muzyczka, N. Adeno-Associated Virus Type 2 VP2 Capsid Protein Is Nonessential and Can Tolerate Large Peptide Insertions at Its N Terminus. J. Virol. 2004, 78, 6595–6609. [Google Scholar] [CrossRef]
- Panté, N.; Kann, M. Nuclear Pore Complex Is Able to Transport Macromolecules with Diameters of ∼39 nm. Mol. Biol. Cell 2002, 13, 425–434. [Google Scholar] [CrossRef]
- Tran, E.J.; Wente, S.R. Dynamic nuclear pore complexes: Life on the edge. Cell 2006, 125, 1041–1053. [Google Scholar] [CrossRef]
- Azzam, I.; Liashkovich, I.; Luchtefeld, I.; Kouzel, I.U.; Shahin, V. Facilitating plasmid nuclear delivery by interfering with the selective nuclear pore barrier. Bioeng. Transl. Med. 2019, 4, e10136. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, T.; Zhang, B.; Liu, S.; Song, W.; Qiao, J.; Ruan, H. Types of nuclear localization signals and mechanisms of protein import into the nucleus. Cell Commun. Signal. 2021, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Qi, X.; Hu, Z.; Tang, Q. Mechanisms Mediating Nuclear Trafficking Involved in Viral Propagation by DNA Viruses. Viruses 2019, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Alderton, M.R.; Gray, P.J.; Prolf, D.F. Genetic Vaccination: Can Plasmid DNA deliver its expectations? JMVH 2001, 10, 59–65. [Google Scholar]
- Garmory, H.S.; Brown, K.A.; Titball, R.W. DNA vaccines: Improving expression of antigens. Genet. Vaccines Ther. 2003, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef]
- Finer, M.; Glorioso, J. A brief account of viral vectors and their promise for gene therapy. Gene Ther. 2017, 24, 1. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral Vectors in Gene Therapy. Diseases 2018, 6, 42. [Google Scholar] [CrossRef]
- Ghosh, S.; Brown, A.M.; Jenkins, C.; Campbell, K. Viral Vector Systems for Gene Therapy: A Comprehensive Literature Review of Progress and Biosafety Challenges. Appl. Biosaf. 2020, 25, 7–18. [Google Scholar] [CrossRef]
- Mingozzi, F.; High, K.A. Immune responses to AAV vectors: Overcoming barriers to successful gene therapy. Blood 2013, 122, 23–36. [Google Scholar] [CrossRef]
- Samulski, R.J.; Muzyczka, N. AAV-Mediated Gene Therapy for Research and Therapeutic Purposes. Annu. Rev. Virol. 2014, 1, 427–451. [Google Scholar] [CrossRef]
- Chen, Y.H.; Keiser, M.S.; Davidson, B.L. Viral Vectors for Gene Transfer. Curr. Protoc. Mouse Biol. 2018, 8, e58. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Z.; Tian, H.; Qi, M.; Zhai, Z.; Li, S.; Li, R.; Zhang, H.; Wang, W.; Fu, S.; et al. Biodistribution and Safety Assessment of Bladder Cancer Specific Recombinant Oncolytic Adenovirus in Subcutaneous Xenografts Tumor Model in Nude Mice. Curr. Gene Ther. 2012, 12, 67–76. [Google Scholar] [CrossRef]
- Liu, M.A.; Ulmer, J.B. Human clinical trials of plasmid DNA vaccines. Adv. Genet. 2005, 55, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials Using pTVG-HP Plasmid DNA Vaccine—NCI. Available online: https://www.cancer.gov/research/participate/clinical-trials/intervention/ptvg-hp-plasmid-dna-vaccine (accessed on 30 December 2023).
- Lim, K.L.; Jazayeri, S.D.; Yeap, S.K.; Alitheen, N.B.; Bejo, M.H.; Ideris, A.; Omar, A.R. Antibody and T cell responses induced in chickens immunized with avian influenza virus N1 and NP DNA vaccine with chicken IL-15 and IL-18. Res. Vet. Sci. 2013, 95, 1224–1234. [Google Scholar] [CrossRef]
- Capua, I.; Terregino, C.; Cattoli, G.; Mutinelli, F.; Rodriguez, J.F. Development of a DIVA (Differentiating Infected from Vaccinated Animals) strategy using a vaccine containing a heterologous neuraminidase for the control of avian influenza. Avian Pathol. 2003, 32, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wu, X.; Li, L.; Ge, S.; Liu, Z.; Wang, Z. Virus-like particles: Promising platforms with characteristics of DIVA for veterinary vaccine design. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 343–352. [Google Scholar] [CrossRef]
- Mandal, D.; Maran, A.; Yaszemski, M.J.; Bolander, M.E.; Sarkar, G. Cellular Uptake of Gold Nanoparticles Directly Cross-linked with Carrier Peptides by Osteosarcoma Cells. J. Mater. Sci. Mater. Med. 2009, 20, 347–350. [Google Scholar] [CrossRef][Green Version]
- Rossi, G.; Monticelli, L. Gold nanoparticles in model biological membranes: A computational perspective. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2016, 1858, 2380–2389. [Google Scholar] [CrossRef]
- Van Lehn, R.C.; Atukorale, P.U.; Carney, R.P.; Yang, Y.S.; Stellacci, F.; Irvine, D.J.; Alexander-Katz, A. Effect of Particle Diameter and Surface Composition on the Spontaneous Fusion of Monolayer-Protected Gold Nanoparticles with Lipid Bilayers. Nano Lett. 2013, 13, 4060–4067. [Google Scholar] [CrossRef]
- Ledesma-Feliciano, C.; Chapman, R.; Hooper, J.W.; Elma, K.; Zehrung, D.; Brennan, M.B.; Spiegel, E.K. Improved DNA Vaccine Delivery with Needle-Free Injection Systems. Vaccines 2023, 11, 280. [Google Scholar] [CrossRef]
- Walker, M. Ground-breaking Approach to Vaccine Delivery Using Ultrasound Technology. Available online: https://medriva.com/breaking-news/revolutionizing-vaccine-delivery-needle-free-and-painless-ultrasound-technology/ (accessed on 10 December 2023).
- Jenner, E. On the Origin of the Vaccine Inoculation. Med. Phys. J. 1801, 5, 505–508. [Google Scholar]
- Fulginiti, V.A.; Papier, A.; Lane, J.M.; Neff, J.M.; Henderson, D.A.; Henderson, D.A.; Inglesby, T.V., Jr.; O’Toole, T. Smallpox Vaccination: A Review, Part I. Background, Vaccination Technique, Normal Vaccination and Revaccination, and Expected Normal Reactions. Clin. Infect. Dis. 2003, 37, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.M.; Babuadze, G.; Plourde-Campagna, M.A.; Azizi, H.; Berger, A.; Kozak, R.; de La Vega, M.A.; Xiii, A.; Naghibosadat, M.; Nepveu-Traversy, M.E.; et al. A novel intradermal tattoo-based injection device enhances the immunogenicity of plasmid DNA vaccines. npj Vaccines 2022, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Sunada, Y. Plasmid DNA gene therapy by electroporation: Principles and recent advances. Curr. Gene Ther. 2011, 11, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Pokorna, D.; Rubio, I.; Müller, M. DNA-vaccination via tattooing induces stronger humoral and cellular immune responses than intramuscular delivery supported by molecular adjuvants. Genet. Vaccines Ther. 2008, 6, 4. [Google Scholar] [CrossRef]
- Otten, G.; Schaefer, M.; Doe, B.; Liu, H.; Srivastava, I.; zur Megede, J.; O’Hagan, D.; Donnelly, J.; Widera, G.; Rabussay, D.; et al. Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine 2004, 22, 2489–2493. [Google Scholar] [CrossRef]
- Xu, R. Sonoporation as a Novel Delivery Platform for an HIV DNA Vaccine. Available online: https://grantome.com/grant/NIH/R03-AI141031-01A1 (accessed on 5 October 2023).
- Zhang, N.; Foiret, J.; Kheirolomoom, A.; Liu, P.; Feng, Y.; Tumbale, S.; Raie, M.; Wu, B.; Wang, J.; Fite, B.Z.; et al. Optimization of microbubble-based DNA vaccination with low-frequency ultrasound for enhanced cancer immunotherapy. Adv. Ther. 2021, 4, 2100033. [Google Scholar] [CrossRef]
- Wang, C.; Karlsson, A.; Oguin, I.I.I.T.H.; Macintyre, A.N.; Sempowski, G.D.; McCarthy, K.R.; Wang, Y.; Moody, M.A.; Yuan, F. Transient inhibition of lysosomal functions potentiates nucleic acid vaccines. Proc. Natl. Acad. Sci. USA 2023, 120, e2306465120. [Google Scholar] [CrossRef]
- Braathen, R.; Spång, H.C.; Hinke, D.M.; Blazevski, J.; Bobic, S.; Fossum, E.; Bogen, B. A DNA Vaccine That Encodes an Antigen-Presenting Cell-Specific Heterodimeric Protein Protects against Cancer and Influenza. Mol. Ther. Methods Clin. Dev. 2020, 17, 378–392. [Google Scholar] [CrossRef]
- Kawamura, H.; Berzofsky, J.A. Enhancement of antigenic potency in vitro and immunogenicity in vivo by coupling the antigen to anti-immunoglobulin. J. Immunol. 1986, 136, 58–65. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Csencsits, K.L.; Haddad, A.; Walters, N.; Pascual, D.W. M cell-targeted DNA vaccination. Proc. Natl. Acad. Sci. USA 2001, 98, 9318–9323. [Google Scholar] [CrossRef] [PubMed]
- Hillemanns, P.; Denecke, A.; Woelber, L.; Böhmer, G.; Jentschke, M.; Schjetne, K.W.; Bruins Slot, K.M.; Fredriksen, A.B. A Therapeutic Antigen-Presenting Cell-Targeting DNA Vaccine VB10.16 in HPV16-Positive High-Grade Cervical Intraepithelial Neoplasia: Results from a Phase I/IIa Trial. Clin. Cancer Res. 2022, 28, 4885–4892. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.D.; Ugen, K.E.; Wang, B.; Agadjanyan, M.; Gilbert, L.; Bagarazzi, M.L.; Chattergoon, M.; Frost, P.; Javadian, A.; Williams, W.V.; et al. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat. Med. 1997, 3, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Werninghaus, I.C.; Hinke, D.M.; Fossum, E.; Bogen, B.; Braathen, R. Neuraminidase delivered as an APC-targeted DNA vaccine induces protective antibodies against influenza. Mol. Ther. 2023, 31, 2188–2205. [Google Scholar] [CrossRef]
- Suschak, J.J.; Williams, J.A.; Schmaljohn, C.S. Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity. Hum. Vaccin. Immunother. 2017, 13, 2837–2848. [Google Scholar] [CrossRef]
- Putnam, D.; Gentry, C.A.; Pack, D.W.; Langer, R. Polymer-based gene delivery with low cytotoxicity by a unique balance of side-chain termini. Proc. Natl. Acad. Sci. USA 2001, 98, 1200–1205. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Jin, Y.; Wu, B.; Wu, Y. Advancements in the development of nucleic acid vaccines for syphilis prevention and control. Hum. Vaccines Immunother. 2023, 19, 2234790. [Google Scholar] [CrossRef]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, Bioapplications, and Toxicity Evaluation of Chitosan-Based Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef]
- Xu, Z.; Patel, A.; Tursi, N.J.; Zhu, X.; Muthumani, K.; Kulp, D.W.; Weiner, D.B. Harnessing Recent Advances in Synthetic DNA and Electroporation Technologies for Rapid Vaccine Development Against COVID-19 and Other Emerging Infectious Diseases. Front. Med. Technol. 2020, 2. Available online: https://www.frontiersin.org/articles/10.3389/fmedt.2020.571030 (accessed on 3 October 2023). [CrossRef]
- Stenler, S.; Blomberg, P.; Smith, C.E. Safety and efficacy of DNA vaccines. Hum. Vaccines Immunother. 2014, 10, 1306–1308. [Google Scholar] [CrossRef]
- Grunwald, T.; Ulbert, S. Improvement of DNA vaccination by adjuvants and sophisticated delivery devices: Vaccine-platforms for the battle against infectious diseases. Clin. Exp. Vaccine Res. 2015, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.H.; Chen, H.W.; Tao, M.H. Modulation of immune responses to DNA vaccines by codelivery of cytokine genes. J. Formos Med. Assoc. 1999, 98, 722–729. [Google Scholar] [PubMed]
- Scheerlinck, J.P.; Casey, G.; McWaters, P.; Kelly, J.; Woollard, D.; Lightowlers, M.W.; Tennent, J.M.; Chaplin, P.J. The immune response to a DNA vaccine can be modulated by co-delivery of cytokine genes using a DNA prime-protein boost strategy. Vaccine 2001, 19, 4053–4060. [Google Scholar] [CrossRef] [PubMed]
- Kwissa, M.; Kröger, A.; Hauser, H.; Reimann, J.; Schirmbeck, R. Cytokine-facilitated priming of CD8+ T cell responses by DNA vaccination. J. Mol. Med. 2003, 81, 91–101. [Google Scholar] [CrossRef]
- Flingai, S.; Czerwonko, M.; Goodman, J.; Kudchodkar, S.B.; Muthumani, K.; Weiner, D.B. Synthetic DNA Vaccines: Improved Vaccine Potency by Electroporation and Co-Delivered Genetic Adjuvants. Front. Immunol. 2013, 4, 54. [Google Scholar] [CrossRef]
- Cornelie, S.; Poulain-Godefroy, O.; Lund, C.; Vendeville, C.; Ban, E.; Capron, M.; Riveau, G. Methylated CpG-containing plasmid activates the immune system. Scand. J. Immunol. 2004, 59, 143–151. [Google Scholar] [CrossRef]
- Krieg, A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002, 20, 709–760. [Google Scholar] [CrossRef]
- Wahren, B.; Liu, M.A. DNA Vaccines: Recent Developments and the Future. Vaccines 2014, 2, 785–796. [Google Scholar] [CrossRef]
- Häcker, G.; Redecke, V.; Häcker, H. Activation of the immune system by bacterial CpG-DNA. Immunology 2002, 105, 245–251. [Google Scholar] [CrossRef]
- Zhao, B.G.; Vasilakos, J.P.; Tross, D.; Smirnov, D.; Klinman, D.M. Combination therapy targeting toll like receptors 7, 8 and 9 eliminates large established tumors. J. ImmunoTherapy Cancer 2014, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Sabbaghi, A.; Ghaemi, A. Molecular Adjuvants for DNA Vaccines: Application, Design, Preparation, and Formulation. Methods Mol. Biol. 2021, 2197, 87–112. [Google Scholar] [CrossRef]
- Kalams, S.A.; Parker, S.; Jin, X.; Elizaga, M.; Metch, B.; Wang, M.; Hural, J.; Lubeck, M.; Eldridge, J.; Cardinali, M.; et al. Safety and immunogenicity of an HIV-1 gag DNA vaccine with or without IL-12 and/or IL-15 plasmid cytokine adjuvant in healthy, HIV-1 uninfected adults. PLoS ONE 2012, 7, e29231. [Google Scholar] [CrossRef]
- Luchner, M.; Reinke, S.; Milicic, A. TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics 2021, 13, 142. [Google Scholar] [CrossRef]
- Kramer, K.; Shields, N.J.; Poppe, V.; Young, S.L.; Walker, G.F. Intracellular Cleavable CpG Oligodeoxynucleotide-Antigen Conjugate Enhances Anti-tumor Immunity. Mol. Ther. 2017, 25, 62–70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuo, T.Y.; Lin, M.Y.; Coffman, R.L.; Campbell, J.D.; Traquina, P.; Lin, Y.J.; Liu, L.T.; Cheng, J.; Wu, Y.C.; Wu, C.C.; et al. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci. Rep. 2020, 10, 20085. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.C.; Huang, M.S.; Chiu, F.F.; Chai, K.M.; Liao, C.L.; Wu, S.C.; Chen, H.W.; Liu, S.J. Co-delivery of a trimeric spike DNA and protein vaccine with aluminum hydroxide enhanced Th1-dominant humoral and cellular immunity against SARS-CoV-2. J. Med. Virol. 2023, 95, e29040. [Google Scholar] [CrossRef]
- Li, E.; Zhang, Y. DNA Methylation in Mammals. Cold Spring Harb. Perspect Biol. 2014, 6, a019133. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, G.-M. The role of bacterial DNA containing CpG motifs in diseases. J. Leukoc. Biol. 2021, 109, 991–998. [Google Scholar] [CrossRef]
- Faurez, F.; Dory, D.; Le Moigne, V.; Gravier, R.; Jestin, A. Biosafety of DNA vaccines: New generation of DNA vectors and current knowledge on the fate of plasmids after injection. Vaccine 2010, 28, 3888–3895. [Google Scholar] [CrossRef]
- Martin, T.; Parker, S.E.; Hedstrom, R.; Le, T.; Hoffman, S.L.; Norman, J.; Hobart, P.; Lew, D. Plasmid DNA malaria vaccine: The potential for genomic integration after intramuscular injection. Hum. Gene Ther. 1999, 10, 759–768. [Google Scholar] [CrossRef]
- Zhang, L.; Richards, A.; Barrasa, M.I.; Hughes, S.H.; Young, R.A.; Jaenisch, R. Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc. Natl. Acad. Sci. USA 2021, 118, e2105968118. [Google Scholar] [CrossRef] [PubMed]
- Integration Into the Human Genome? Available online: https://www.science.org/content/blog-post/integration-human-genome (accessed on 17 October 2023).
- Rudolf, J. Eminent MIT Scientists Defend Controversial SARS-CoV-2 Genome Integration Results. GEN-Genetic Engineering and Biotechnology News. Available online: https://www.genengnews.com/insights/eminent-mit-scientists-defend-controversial-sars-cov-2-genome-integration-results/ (accessed on 17 October 2023).
- No, the COVID-19 Vaccine Will Not Change Your DNA-mlive.com. Available online: https://www.mlive.com/public-interest/2021/04/no-the-covid-19-vaccine-will-not-change-your-dna.html (accessed on 17 October 2023).
- Fox, A.; Martin, J.; Beilharz, T. Can the Pfizer or Moderna mRNA Vaccines Affect My Genetic Code? The Conversation. Available online: http://theconversation.com/can-the-pfizer-or-moderna-mrna-vaccines-affect-my-genetic-code-162590 (accessed on 17 October 2023).
- Araten, D.J.; Golde, D.W.; Zhang, R.H.; Thaler, H.T.; Gargiulo, L.; Notaro, R.; Luzzatto, L. A Quantitative Measurement of the Human Somatic Mutation Rate. Cancer Res. 2005, 65, 8111–8117. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.L.; Loeb, L.A. The mutation rate and cancer. Genetics 1998, 148, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Neely, S.R.; Eldredge, C.; Ersing, R.; Remington, C. Vaccine Hesitancy and Exposure to Misinformation: A Survey Analysis. J. Gen. Intern. Med. 2022, 37, 179–187. [Google Scholar] [CrossRef]
- Pertwee, E.; Simas, C.; Larson, H.J. An epidemic of uncertainty: Rumors, conspiracy theories and vaccine hesitancy. Nat. Med. 2022, 28, 3. [Google Scholar] [CrossRef]
- Banoun, H. mRNA: Vaccine or Gene Therapy? The Safety Regulatory Issues. Int. J. Mol. Sci. 2023, 24, 10514. [Google Scholar] [CrossRef]
- Spencer, J.P.; Pawlowski, R.H.T.; Thomas, S. Vaccine Adverse Events: Separating Myth from Reality. Am. Fam. Physician 2017, 95, 786–794. [Google Scholar]
- Mignon, C.; Sodoyer, R.; Werle, B. Antibiotic-Free Selection in Biotherapeutics: Now and Forever. Pathogens 2015, 4, 157–181. [Google Scholar] [CrossRef]
- Mairhofer, J.; Lara, A.R. Advances in host and vector development for the production of plasmid DNA vaccines. Methods Mol. Biol. 2014, 1139, 505–541. [Google Scholar] [CrossRef] [PubMed]
- Cranenburgh, R.M.; Hanak, J.A.J.; Williams, S.G.; Sherratt, D.J. Escherichia coli strains that allow antibiotic-free plasmid selection and maintenance by repressor titration. Nucleic Acids Res. 2001, 29, e26. [Google Scholar] [CrossRef] [PubMed]
- Garmory, H.S.; Leckenby, M.W.; Griffin, K.F.; Elvin, S.J.; Taylor, R.R.; Hartley, M.G.; Hanak, J.A.; Williamson, E.D.; Cranenburgh, R.M. Antibiotic-Free Plasmid Stabilization by Operator-Repressor Titration for Vaccine Delivery by Using Live Salmonella enterica Serovar Typhimurium. Infect. Immun. 2005, 73, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Mairhofer, J.; Pfaffenzeller, I.; Merz, D.; Grabherr, R. A novel antibiotic free plasmid selection system: Advances in safe and efficient DNA therapy. Biotechnol. J. 2008, 3, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Alcolea, P.J.; Larraga, J.; Rodríguez-Martín, D.; Alonso, A.; Loayza, F.J.; Rojas, J.M.; Ruiz-García, S.; Louloudes-Lázaro, A.; Carlón, A.B.; Sánchez-Cordón, P.J.; et al. Non-replicative antibiotic resistance-free DNA vaccine encoding S and N proteins induces full protection in mice against SARS-CoV-2. Front. Immunol. 2022, 13, 1023255. [Google Scholar] [CrossRef] [PubMed]
- Walters, A.A.; Kinnear, E.; Shattock, R.J.; McDonald, J.U.; Caproni, L.J.; Porter, N.; Tregoning, J.S. Comparative analysis of enzymatically produced novel linear DNA constructs with plasmids for use as DNA vaccines. Gene Ther. 2014, 21, 7. [Google Scholar] [CrossRef]
- Niezold, T.; Storcksdieck Genannt Bonsmann, M.; Maaske, A.; Temchura, V.; Heinecke, V.; Hannaman, D.; Buer, J.; Ehrhardt, C.; Hansen, W.; Überla, K.; et al. DNA vaccines encoding DEC205-targeted antigens: Immunity or tolerance? Immunology 2015, 145, 519–533. [Google Scholar] [CrossRef]
- Ho, P.P.; Higgins, J.P.; Kidd, B.A.; Tomooka, B.; DiGennaro, C.; Lee, L.Y.; De Vegvar, H.E.; Steinman, L.; Robinson, W.H. Tolerizing DNA vaccines for autoimmune arthritis. Autoimmunity 2006, 39, 675–682. [Google Scholar] [CrossRef]
- Moorman, C.D.; Sohn, S.J.; Phee, H. Emerging Therapeutics for Immune Tolerance: Tolerogenic Vaccines, T cell Therapy, and IL-2 Therapy. Front. Immunol. 2021, 12, 657768. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2021.657768 (accessed on 18 October 2023). [CrossRef]
- Parks, R.J.; Gussoni, E. Building immune tolerance through DNA vaccination. Proc. Natl. Acad. Sci. USA 2018, 115, 9652–9654. [Google Scholar] [CrossRef]
- Umeshappa, C.S.; Singha, S.; Blanco, J.; Shao, K.; Nanjundappa, R.H.; Yamanouchi, J.; Parés, A.; Serra, P.; Yang, Y.; Santamaria, P. Suppression of a broad spectrum of liver autoimmune pathologies by single peptide-MHC-based nanomedicines. Nat. Commun. 2019, 10, 2150. [Google Scholar] [CrossRef]
- Wolff, J.A.; Ludtke, J.J.; Acsadi, G.; Williams, P.; Jani, A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum. Mol. Genet. 1992, 1, 363–369. [Google Scholar] [CrossRef]
- Soucy, A.M.; Hurteau, G.J.; Metzger, D.W. Live Vaccination Generates Both Disease Tolerance and Host Resistance During Chronic Pulmonary Infection With Highly Virulent Francisella tularensis SchuS4. J. Infect. Dis. 2018, 218, 1802–1812. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Redwan, E.M.; Makis, W.; Rubio-Casillas, A. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines 2023, 11, 991. [Google Scholar] [CrossRef]
- Górecki, D.C.; Simons, J.P. The dangers of DNA vaccination. Nat. Med. 1999, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Williams, P.; Berg, R.K.; Hodgeman, B.A.; Liu, L.; Repetto, G.; Wolff, J.A. Direct gene transfer into nonhuman primate myofibers in vivo. Hum. Gene Ther. 1992, 3, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, C. First COVID-19 DNA vaccine approved, others in hot pursuit. Nat. Biotechnol. 2021, 39, 1479–1482. [Google Scholar] [CrossRef]
- Nucleotide BLAST: Search Nucleotide Databases Using a Nucleotide Query. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&BLAST_SPEC=GeoBlast&PAGE_TYPE=BlastSearch (accessed on 19 October 2023).
- Basic Local Alignment Search Tool (BLAST)|Learn Science at Scitable. Available online: https://www.nature.com/scitable/topicpage/basic-local-alignment-search-tool-blast-29096/ (accessed on 19 October 2023).
- Cai, Y.; Rodriguez, S.; Hebel, H. DNA vaccine manufacture: Scale and quality. Expert Rev. Vaccines 2009, 8, 1277–1291. [Google Scholar] [CrossRef]
- General Principles for the Development of Vaccines to Protect against Global Infectious Diseases; CDC, 21 CFR 10.115(g)(4)(i); Food and Drug Administration: Rockville, MD, USA, 2011.
- Ensuring the Safety of Vaccines in the United States|CDC. Available online: https://www.cdc.gov/vaccines/hcp/conversations/ensuring-safe-vaccines.html (accessed on 12 November 2023).
- Rabail, R.; Ahmed, W.; Ilyas, M.; Rajoka, M.S.; Hassoun, A.; Khalid, A.R.; Khan, M.R.; Aadil, R.M. The Side Effects and Adverse Clinical Cases Reported after COVID-19 Immunization. Vaccines 2022, 10, 488. [Google Scholar] [CrossRef]
- Couzin, J.; Kaiser, J. As Gelsinger Case Ends, Gene Therapy Suffers Another Blow. Science 2005, 307, 1028. [Google Scholar] [CrossRef]
- Rinde, M. The Death of Jesse Gelsinger, 20 Years Later. Science History Institute. Available online: https://sciencehistory.org/stories/magazine/the-death-of-jesse-gelsinger-20-years-later/ (accessed on 2 December 2023).
- Kaiser, J. Decades after a Tragic Failure, Gene Therapy Successfully Treats a Rare Liver Disease. Available online: https://www.science.org/content/article/decades-after-tragic-failure-gene-therapy-successfully-treats-rare-liver-disease (accessed on 2 December 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozak, M.; Hu, J. DNA Vaccines: Their Formulations, Engineering and Delivery. Vaccines 2024, 12, 71. https://doi.org/10.3390/vaccines12010071
Kozak M, Hu J. DNA Vaccines: Their Formulations, Engineering and Delivery. Vaccines. 2024; 12(1):71. https://doi.org/10.3390/vaccines12010071
Chicago/Turabian StyleKozak, Michael, and Jiafen Hu. 2024. "DNA Vaccines: Their Formulations, Engineering and Delivery" Vaccines 12, no. 1: 71. https://doi.org/10.3390/vaccines12010071
APA StyleKozak, M., & Hu, J. (2024). DNA Vaccines: Their Formulations, Engineering and Delivery. Vaccines, 12(1), 71. https://doi.org/10.3390/vaccines12010071






