Abstract
The development of HIV prophylactic vaccines is facing an impasse, since all phase IIb/III clinical trials were halted in 2023 without demonstrating efficacy. Thus, the field is in need of developing novel immunogens and vaccination strategies that induce broadly neutralising antibodies together with potent Fc-dependent effector functions, as well as protective cross-reactive CD4+ and CD8+ T cell responses. Nucleic acid vaccines, particularly mRNA vaccines, have been one of the major groundbreaking advances in the current decade. Nucleic acid vaccines may help recalibrate the HIV vaccine field towards the use of delivery systems that allow the proper expression of immunogens as a sole antigen (i.e., membrane-bound trimeric envelope glycoproteins) or even to be displayed in a multiantigen platform that will be synthesised by the host. In this review, we will summarise how the multiple HIV vaccine strategies pursued in the last 40 years of HIV research have driven current vaccine development, which are the most relevant immunogens identified so far to induce balanced adaptive immune responses, and how they can benefit from the acceptance of nucleic acid vaccines in the market by reducing the limitations of previous delivery systems. The incorporation of nucleic acid vaccines into the current heterogeneous repertoire of vaccine platforms may represent an invaluable opportunity to reignite the fight against HIV.
1. Introduction
Vaccines are indisputably one of the most successful achievements in the biomedical field. Vaccination programmes have been pivotal to extending life expectancy in the last century [1,2]. Yet, infectious diseases represent a continuous threat to society. For instance, estimates indicate that there is a 2% chance of new pathogens emerging in any given year [3]. And while emerging pathogens can mould history, as recently demonstrated by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic or previously by the Human Immunodeficiency Virus (HIV), so can vaccines. And indeed, nucleic acid vaccines were game changers during the SARS-CoV-2 pandemic. Both the adaptation of classical vaccine platforms and the approval of novel technologies yielded several candidates that lowered the disease burden of the Coronavirus Disease 2019 (COVID-19) [4].
The SARS-CoV-2 pandemic experienced an important achievement with the approval of the first nucleic acid-based vaccines for human use, either as deoxyribnonucleic acid (DNA) vaccines [5], or messenger ribonucleic acid (mRNA) vaccines [6,7]. mRNA vaccines proved to be especially efficacious and competitive, and they are considered as one of the vaccine platforms that might achieve the highest growth in the near future, even beyond the infectious disease field [8,9]. However, research on nucleic acid vaccines predates COVID-19, and these types of vaccines were already being developed for HIV.
In contrast to the sharp success of SARS-CoV-2 vaccines, forty years of vaccine research against HIV have only yielded modest successes in efficacy trials (i.e., RV144) [10] or no success at all [11,12,13,14,15]. While it is true that SARS-CoV-2 vaccine development benefited from many years of scientific advances in both coronaviruses and other fields (e.g., gene delivery, mRNA engineering, and HIV vaccine research) [16,17,18,19,20], it is still remarkable that vaccines with such a high efficacy could move from bench to market within such a short time. The introduction of SARS-CoV-2 nucleic acid vaccines into the market, have shown that mRNA vaccine technology is a powerful tool for speeding up the development of novel vaccines in several areas including the HIV vaccine field.
In this review, we will provide a summary of the different HIV vaccine strategies that have been tested from the beginning of the acquired immunodeficiency syndrome (AIDS) pandemic, with a particular focus on the selected immunogens and vaccine platforms. In addition, we will discuss how HIV vaccine field can benefit from the different advantages of nucleic acid vaccines.
2. Which Immunogens Should We Use and Why?
HIV immunogens have been classically categorised into two main groups: those designed to elicit protective humoral responses, mainly neutralizing antibodies (NAbs), and those devised to induce potent cellular immune responses associated with the control of disease progression [21,22].
2.1. Elicitation of Protective Humoral Responses
HIV envelope glycoprotein (Env) is the only viral protein present on the viral surface, and it is the target of NAbs [23,24]. It is a highly glycosylated heterotrimeric protein generated by the oligomerisation of three gp160 molecules. Each gp160 monomer contains two subunits (gp120 and gp41) that are responsible for CD4 recognition and membrane fusion, respectively [25]. However, Env induces poorly protective CD4+ and CD8+ T cell responses [26].
Env accumulates the highest degree of genetic variability of HIV, which can be as high as 40% across HIV subtypes [27]. Despite this, Env has several conserved domains that play a major role in protein architecture and function (Figure 1), such as the CD4 binding site (CD4bs) in gp120, or the fusion peptide (FP) and the Membrane Proximal External Region (MPER) in the gp41 subunit [23,28,29,30]. These functional domains are known as HIV vulnerability sites since they are targeted by NAbs. However, owing to HIV’s exceptional mutational rate, HIV can evade the immune pressure exerted by these NAbs and escape their effect [31]. Furthermore, stemming from HIV’s enormous genetic diversity, NAb sensitivity varies among different HIV clades or subtypes.
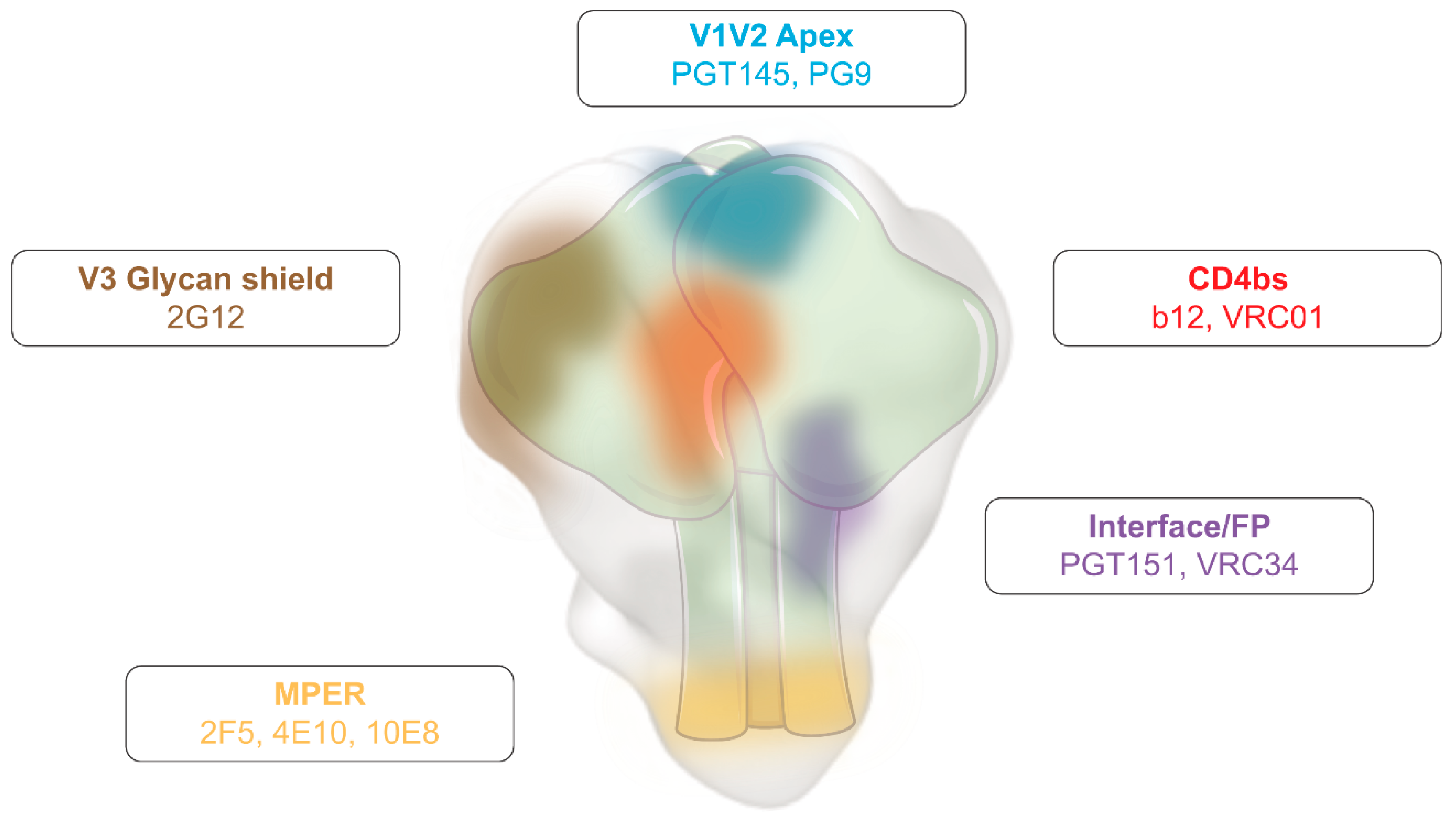
Figure 1.
Human immunodeficiency virus (HIV) Envelope glycoprotein (Env) vulnerability map. Schematic representation of Env, highlighting the main vulnerability domains and examples of broadly neutralising antibodies (bNAbs) that target these regions. Adapted from [28].
Despite the enormous antigenic variability of Env and the rapid virus/immune response adaptation, broadly neutralizing antibodies (bNAbs) that block multiple HIV subtypes showed in vivo antiviral activity in humans [32,33,34]. bNabs are only elicited in 1% of people living with HIV (PLWH) [35], and target several of Env’s vulnerability domains (e.g., CD4bs [b12 and VRC01], MPER [2F5, 10E8], among others) (Figure 1). They are the result of a long and complex antigen selection process, which explains part of their main features, such as the high degree of somatic hypermutation and the long time to elicitation after infection [36]. Still, their detection in humans is proof that they can be elicited by a proper stimulation [37].
bNAbs can cross-neutralise a broad range of HIV variants, and protect from HIV acquisition, particularly if a combination of bNAbs that target different vulnerability regions are co-administered [38,39,40]. Therefore, their elicitation by vaccination may be pivotal for the success of any prophylactic HIV vaccine [29].
Strong efforts have been taken for the design of Env-based immunogens that can elicit bNAbs. In an initial attempt, Env glycoproteins and subunit vaccines were tested in multiple forms (Gp160/gp140, gp120 [AIDSVAX B/B or B/E], or gp41) with limited success [14,15,41,42,43,44]. These strategies were unable to induce broadly protective responses, and the virus was able to evade them [31]. Despite the fact that these initial immunogens failed at eliciting NAbs, more advanced Env-based immunogens have been developed in the last few years. Some examples of these rationally designed HIV immunogens are: (i) eOD-GT8, that displays CD4bs antigens to prime germline precursors of known bNAbs [45]; (ii) linear epitope-based vaccines such as the fusion peptide (FP) or the MPER, designed to reduce the generation of non-neutralising antibodies [30,46]; or (iii) stable trimeric Env immunogens that maintain the prefusion native conformation of Env and are mainly targeted by Nabs, which may be SOSIP trimers [47,48,49], native flexible linked (NFL) trimers [50], or uncleaved prefusion optimised (UFO) trimers [51]. Although these immunogens can elicit cross-neutralising responses, they need the coadministration of adjuvants to increase their immunogenicity, particularly when they are assayed as recombinant proteins [e.g., alum phosphate [15,43,52], squalene-based adjuvants [53,54,55], or complex liposomes [56,57]].
In addition to neutralising humoral responses, antibody-dependent cellular responses (like antibody-dependent cellular cytotoxicity [ADCC] or antibody-dependent cell phagocytosis [ADCP]) can also play a pivotal role in providing certain degree of protection against HIV, as demonstrated by the RV144 vaccine Thai trial [58,59,60]. Importantly, these functions are mainly mediated by the constant region (Fc) of IgG1 and IgG3 immunoglobulins, whose production after antibody class-switch recombination is modulated by the cytokine milieu [61] Since vaccine properties and the adjuvant used may influence this milieu, both factors are also determinant to elicitation of optimal humoral responses.
2.2. Elicitation of Protective T Cell Responses
Conversely to Env, the structural polyprotein composed by the group-specific antigen (Gag) and polymerase (Pol) Gag-Pol, and the “negative factor” (Nef) accessory protein can strongly stimulate T cell responses [62]. Anti-Gag-Pol antibodies can be elicited during HIV infection or vaccination, but they are non-neutralising since these proteins are located inside the viral particle. That is why they their administration as subunit proteins is not useful as a vaccination strategy. Still, they remain as useful diagnostic tools [63].
Gag, Pol and Nef have several conserved domains that can be exploited to generate broad and protective T cell responses [64]. In fact, CD4+ T helper responses against Gag have been correlated with a slower progression to AIDS [65,66]. Additionally, some CD8+ T cell responses may play a major role in controlling HIV since a few HLA-B alleles have been associated with long-term non-progressor and elite controller patient profiles [67,68], highlighting their potential as vaccine immunogens.
Immunization studies have tested Gag-Pol and Nef immunogens as nucleic acid vaccines (as plasmid DNA or through viral vectors) [69,70,71]. The most used viral vectors include poxviruses [e.g., vaccinia viruses (MVA or NYVAC)] [72], canarypox viruses (e.g., ALVAC) [10], and adenoviral vectors (Ad5) [73]. Some examples of Gag-based immunogens administered as vaccines are: (i) mosaic proteins, that were bioinformatically designed to cover most HIV group M variants [74]; (ii) HIV.consv constructs, that encoded for the most conserved Gag elements [75]; or (iii) HTI immunogen, that contained conserved peptides that are targeted by effective cellular immune responses [76]. Nef has been included together with Gag and Pol in many of vaccine strategies (e.g., STEP, Phambili, HVTN505, PrEPVacc) [77]. These types of vaccines have been extensively evaluated for their capability to prime or boost cellular responses designed to kill infected cells, but they may also be relevant for prophylactic vaccine strategies [78].
Overall, considering the properties of each group of immunogens an optimal HIV vaccine strategy should induce a balanced adaptive immune response in which both protective humoral and cellular immune responses are elicited [21].
3. HIV Vaccine Efficacy Trials: Past Failures, Future Successes
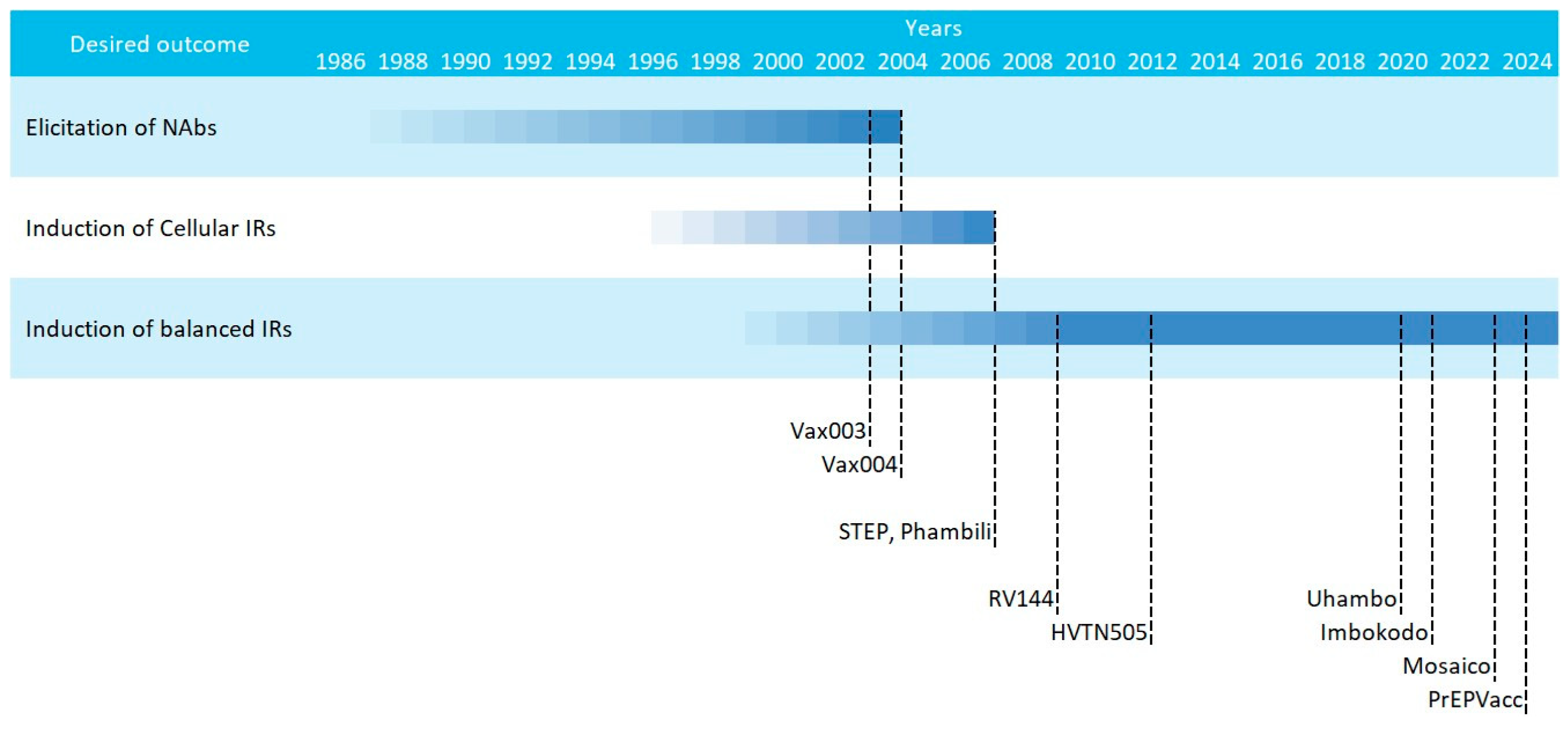
The current consensus that HIV vaccines should aim at inducing potent and balanced humoral and cellular responses was coined after the underperformance of multiple phase IIb/III HIV vaccine efficacy trials in the early 2000s. These attempts can be classified in three main waves, that followed different strategies [21,79]: (i) an early phase in which immunogens based on recombinant Env proteins were tested for the induction of humoral responses (Vax003 and Vax004); (ii) a second phase that saw the introduction of viral vectored vaccines that coded for HIV genes designed to induce cellular responses (STEP and Phambili); and (iii) a later phase in which vaccines were designed for inducing balanced humoral and cellular responses (RV144, Mosaico, PrEPVacc, etc.) (Figure 2).
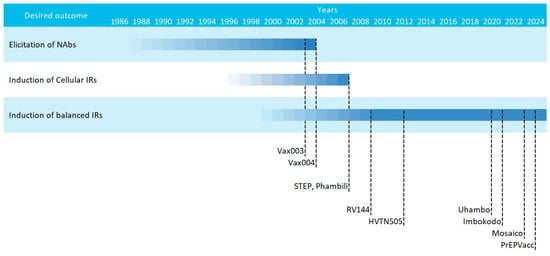
Figure 2.
Scheme of the multiple HIV vaccine clinical trials classified by strategy. Starting dates are gradual and ending dates represent the publication year.
Despite these different strategies, researchers agreed from the beginning that the main issue with HIV vaccines would be the enormous global diversity of HIV. HIV group M is the main group (represents 90% of HIV infections) and encompasses multiple subtypes (A, B, C, D, F, G, H, J, and K) and circulating recombinant forms (CRF) [80]. Intragroup variability within a subtype can be as high as 15%, while it can reach up to 40% genetic variability between different subtypes [27]. Hence, two strategies were foreseen: either vaccines were designed to induce a broad response covering all subtypes and variants, a feat we have not yet achieved, or vaccines should be tailored to the predominant subtype in a specific geographical region.
That was the situation with the two initial phase III trials (Vax003 and Vax004) that concluded in the year 2000, in which the gp120 subunit vaccine AIDSVAX was adapted to match predominant subtypes in Thailand (subtypes B/E) or in developed countries (subtype B) (clinicaltrials.gov: NCT00002441 and NCT00006327, respectively) [43]. These trials also differed on the target population; while Vax003 in Thailand targeted drug users, Vax004 in USA and the Netherlands was aimed at men who have sex with men (MSM) (Table 1). Still, although the antibody responses induced by these vaccines were robust, their cross-neutralising capacity was very low, and none of them showed overall efficacy, even if some groups showed non-significant trends of protection [81].

Table 1.
HIV vaccine efficacy trials performed up to date.
Once Vax003 and Vax004 ended, two phase IIb trials aimed at inducing potent T cell responses were being initiated and led by Merck (STEP study/HVTN502 and Phambili study/HVTN503) (Table 1). These trials rested on the fact that neutralising antibodies were not correlating with HIV disease progression, but cellular responses against Gag, Pol and Nef were [82]. Subtype B gag, pol, and nef were DNA encoded and delivered with adenoviral vectors (MRK Ad5). Despite testing the vaccine in two different settings with different prevalent subtypes (USA and Australia for STEP and South Africa for Phambili), vaccines were not adapted to the circulating South-African strain. In any case, both studies were halted in late 2007, not even a year after they started, since researchers found a higher incidence in the vaccinated group than the placebo one [70]. Further analyses indicated an association between higher HIV incidence and pre-existing anti-Ad5 antibodies, highlighting one of the main limitations of viral vector vaccines: the generation of anti-vector immune responses [11,83], a problem that can be bypassed with plasmidic DNA or mRNA vaccines.
Parallel to STEP and Phambili trials, a more successful phase III attempt was carried out. The RV144 (clinicaltrials.gov: NCT00223080) was designed to stimulate both arms of the adaptive immune system. The vaccination regimen included subtype B Gag-Pol and membrane-bound subtype AE Env immunogens delivered with a canarypox vector (vCP1521) named ALVAC-HIV and administered in four doses. The last two doses were co-administered with AIDSVAX B/E to boost humoral responses [44]. Despite some discussion on whether the foundation for this clinical trial were solid enough [84,85], the truth is that this is the only HIV vaccine efficacy trial to provide a positive but moderate degree of protection (31.2% protection of vaccinees over placebos) [10]. Deeper analyses highlighted the role of ADCC-mediating V1V2 humoral responses as correlates of protection [59], while anti-Env IgAs were associated with increased risk of HIV infection [58]. However, these results were considered too poor to merit further commercial development [86].
After the relative success of the RV144 Thai trial, all developed efficacy trials focused on the elicitation of balanced adaptive responses, using a similar protocol (HVTN702/Uhambo) or opening new avenues (HVTN505 and the HVTN705/Imbokodo-HVTN706/Mosaico trials). However, none of these trials achieved a similar success to the one reported with RV144.
In chronological order, HVTN505 started in 2009, and tested for the first time in a phase IIb trial a DNA vaccine (clinicaltrials.gov: NCT00865566). In this trial, vaccinees were primed with six plasmids encoding for subtype B gag, pol, nef, subtype A, B and C env and boosted with a mix of four Ad5 coding for subtype B gag-pol and subtype A, B and C env. Unfortunately, this trial was stopped due to futility [87], and only modest anti-Env CD8+ cytotoxic responses were enhanced in vaccinated uninfected volunteers [88].
The HVTN702/Uhambo phase IIb/III clinical trial (clinicaltrials.gov: NCT02968849) tried to reproduce the RV144 success in South Africa by adapting the vaccine components to a subtype C variant (Table 1) [89], but it was stopped due to futility [54].
Another HIV vaccine strategy that was tested in efficacy trials was the mosaic constructs [90,91]. Two efficacy trials were performed in parallel using the same strategy, but the booster was adapted to the target population: HVTN705/Imbokodo focused on women from sub-Saharan Africa, while HVTN706/Mosaico targeted MSM from America and Europe (Table 1). The vaccination regimen was similar to RV144 and included priming with four doses of four viral vectors (Ad26) that encoded four mosaic constructs: Mos1.Gag-pol, Mos2.Gag-pol, Mos1.Env, and Mos2S.Env. Boosting was performed with a soluble protein (subtype C gp140 trimer for Imbokodo and a mosaic gp120 for Mosaico) in parallel with the third and fourth doses (clinicaltrials.gov: NCT03060629 and NCT03964415). Imbokodo was stopped in summer 2021 for futility, since it showed a non-significant trend of protection among vaccinees (25.2%) [92]. Mosaico continued until early 2023, when it was also stopped for futility [13].
The most recent addition to this lengthy list of halted clinical trials is PrEPVacc (clinicaltrials.gov: NCT04066881). In this phase IIb study performed in Uganda, two vaccinee groups were compared to placebo control, but all volunteers received pre-exposure prophylaxis (PrEP) treatment [77]. The main vaccine component was a DNA vaccine (DNA-HIV-PT123) encoding for subtype C gag, env and pol-nef [53]. The two vaccinated arms received either AIDSVAX on weeks 0, 4, 24 and 48 or subtype C gp140 (weeks 0 and 4) and subtype C gp140 with the MVA vector on weeks 24 and 48 [55]. The vaccine arm of this trial was stopped by recommendation of the independent data monitoring committee in December 2023 due to the absence of vaccine efficacy observed in the interim analysis [93].
As of January 2024, there are no active candidates in efficacy trials, despite a few examples of mRNA vaccines in phase I trials [94,95]. Furthermore, considering the lack of success in the previous trials, reticence may arise among stakeholders to continue pursuing HIV vaccines without a solid lead candidate, as discussed 20 years ago when the RV144 was initialising [84,85]. However, the breakthrough of mRNA vaccine platforms may turn the tables in this sense and reignite the field after reaching this critical impasse. Considering the contribution of the HIV vaccine field to our understanding of protective mechanisms of SARS-CoV-2 vaccines, it seems appropriate that the same field should now seize the opportunities opened by these novel mRNA vaccines [96]. The main advantages provided by mRNA vaccines and their relevance in HIV vaccine development will be discussed in the following sections.
4. Nucleic Acid Vaccines and Their Delivery
Nucleic acid vaccines can be categorized into three groups: viral vectored vaccines, DNA vaccines, and mRNA vaccines. DNA- or RNA-encoded products are not novel in the biomedical field, since DNA or RNA viral vectors have been widely used in several applications ranging from gene therapy to vaccinology. For instance, viral vectors share many advantages with plasmid DNA or mRNA platforms, like a straightforward production protocol or a versatility to adapt the proteins they encode on a nucleic acid level, instead of having to produce, purify, and validate each product [97]. Furthermore, their protocols are well-optimised and standardised worldwide, facilitating technology exchanges and fast adaptation to new threats and challenges [98].
Nucleic acid-encoded vaccines are administered intramuscularly but instead of providing a direct stimulation to the immune system, they first need to be internalised by somatic cells that will synthesize the encoded antigens (Figure 3). They can encode soluble proteins that, once secreted, can stimulate the immune system in a similar fashion as subunit proteins, but additionally, during synthesis, they will be also processed by the proteosome and presented to CD8+ cytotoxic lymphocytes via major histocompatibility complex (MHC)-I. Alternatively, immunogens containing the transmembrane domain can be encoded and synthesized as membrane-anchored antigens, or multimeric presentations can be designed (as virus-like particles [VLPs]). In both cases aiming to better recruit B cell precursors and lower the affinity threshold [99]. As a case in point, Comirnaty (BNT162b2) and Spikevax (mRNA-1273) mRNA vaccines encode membrane-bound SARS-CoV-2 Spikes [100].
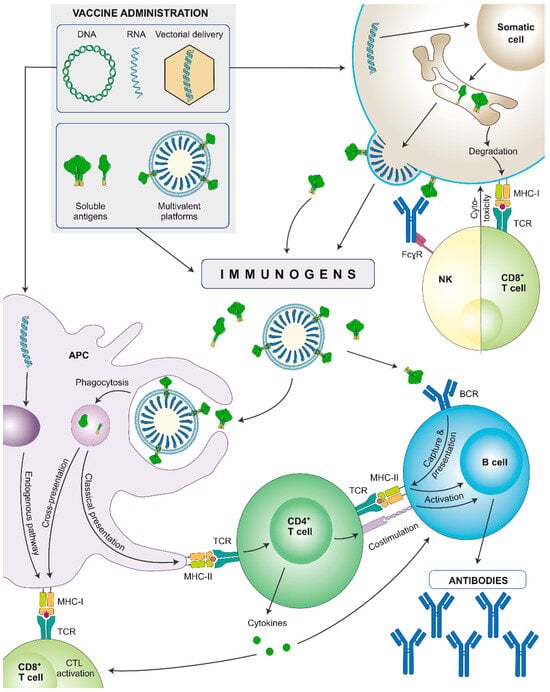
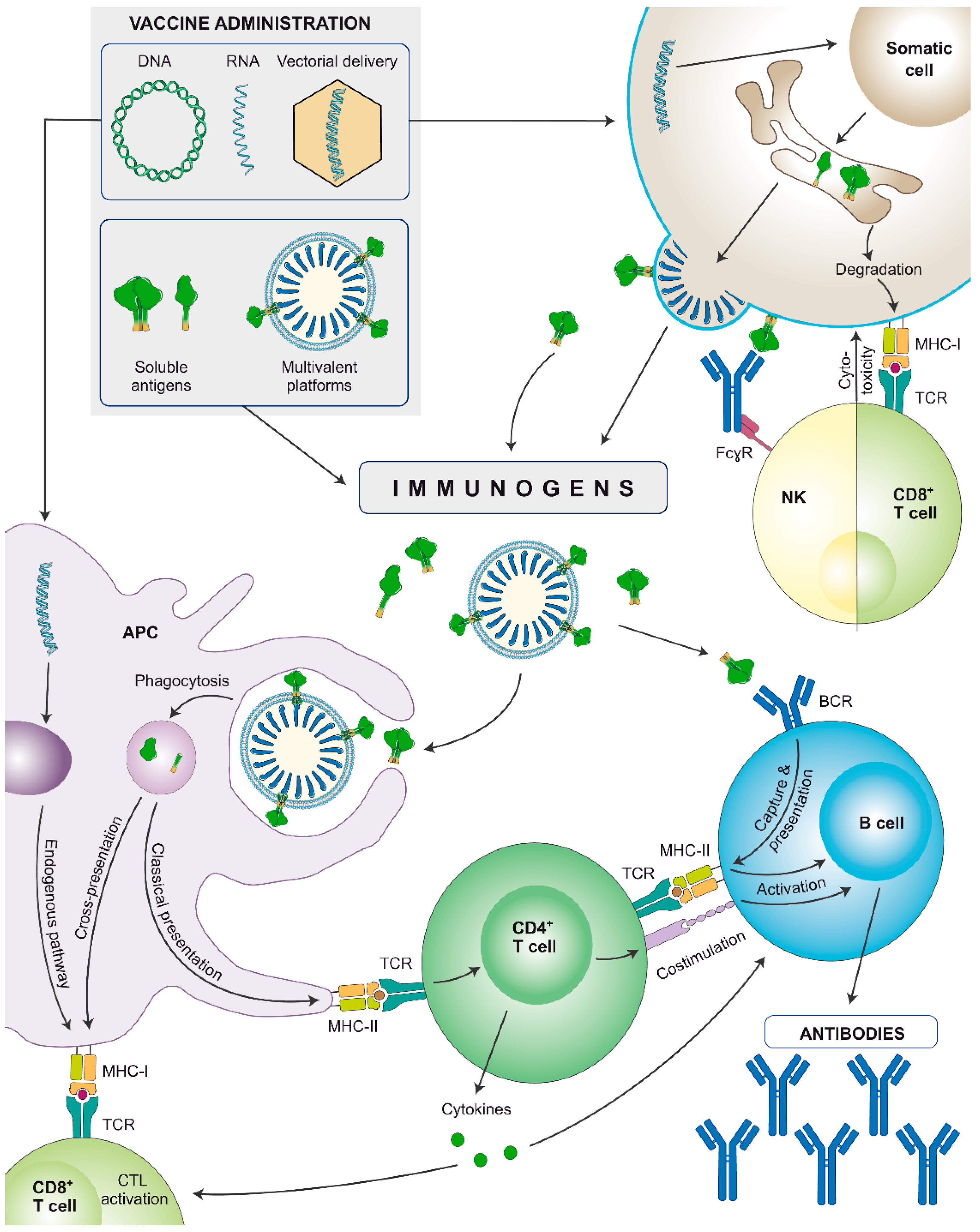
Figure 3.
Schematic representation of the immune stimulation pathways of nucleic acid-encoded or soluble recombinant protein-based vaccines (subunit proteins or multivalent platforms). Nucleic acids are internalised by somatic cells that synthesize the immunogens. They can be secreted or expressed on the cell surface, depending on the immunogen design. Secreted immunogens will be recognised by professional antigen-presenting cells (APCs: B cells, dendritic cells, and/or macrophages), triggering an adaptive immune response. Additionally, APCs also internalise DNA and/or mRNA and may be transfected by viral vectors to produce the antigen, that will be presented through major histocompatibility complex (MHC)-I molecules, stimulating cellular responses. Cells: innate APC (purple), somatic cell (brown), NK cell (yellow), CD8+ T lymphocyte (light green), CD4+ T lymphocyte (dark green), B lymphocyte (blue). Proteins: antibodies (blue), monomeric/trimeric Env (green).
Furthermore, DNA and/or mRNA can be internalised by antigen-presenting cells (APCs), and the encoded proteins will be synthesized, processed, and presented via MHC to activate cellular responses. Overall, both nucleic acid-encoded and subunit vaccines induce strong humoral responses, but the former will better stimulate the cellular arm, and hence, induce a more balanced immune response [101,102]. Still, adjuvants can further help by polarising and potentiating the immune response induced by nucleic acid vaccines.
4.1. Viral Vectored HIV Vaccines
There are several examples of viral vectored HIV vaccines tested in efficacy trials, as previously discussed, and viral vectors approved for use in humans (e.g., the canarypox vector ALVAC-HIV, vaccinia viruses [NYVAC, MVA, Tiantan], different adenoviruses [Ad5, Ad26, Ad35, chimpanzee adenovirus]) [72,103,104,105]. Their utility is indisputable, but there are some limitations that should be highlighted, such as pre-existing anti-vector responses that can interfere with the immunogenicity of the delivered antigen, as shown in the STEP study [11,106], or their elicitation upon vaccination. Considering that an HIV vaccine may require the administration of multiple doses [107], the issue with anti-vector responses is no minor concern, since it can reduce the effectivity of homologous vaccine regimens [108,109]. Furthermore, the potential of replication-competent vectors to reactivate is especially concerning in immunocompromised vaccinees, such as PLWH [110]. Even though there are many research lines with viral vectors trying to solve these matters (e.g., replication deficient vectors and combination of different serotypes and heterologous regimens), plasmid DNA and mRNA vaccines are an attractive alternative to bypass these limitations (Box 1) [111]. For example, SARS-CoV-2 mRNA vaccines have demonstrated efficacy in swiftly adjusting to emergent variants capable of evading prior immune responses. This characteristic of mRNA technology holds significant promise for HIV vaccine development, given the challenge posed by HIV’s capacity to circumvent immune defences, due to its exceptionally elevated mutational rate [112].
4.2. DNA and mRNA HIV Vaccines
DNA and mRNA vaccines have been extensively pursued in the last decades, both in preclinical and early phase clinical trials. However, no commercial nucleic acid vaccine was licensed until the SARS-CoV-2 pandemic hit. As of January 2024, there are two licensed mRNA [7,113] vaccines and one licensed DNA vaccine [114], all against SARS-CoV-2, and many more are being developed, even beyond the infectious disease field. Together with the advantages described for viral vectored vaccines (i.e., fast production and purification, versatility encoding proteins, and pseudotyping), which places them as great vaccine platforms against such a heterogeneous virus as HIV or in pandemic preparedness contexts against emerging viruses (Box 1), DNA and mRNA vaccines do not pose such a strong threat of inducing strong anti-vector responses compared to viral vectors [97,115]. This lower elicitation of anti-vector responses also allows for a longer persistence of the antigen and increases the immunogenicity of the platform. Still, DNA vaccines have sparked some concerns regarding their risk of inducing anti-DNA antibodies and potential integration of DNA into the host cell’s nucleus [116], which are slowly dissipating after their access to the market.
Box 1. Advantages and disadvantages of DNA and mRNA vaccines.
Advantages:
- -
- Versatility to encode various forms of antigens (no size limitation for encapsulation):
- ∘
- Subunit antigens
- ∘
- Membrane-bound antigens
- ∘
- Multivalent platforms
- -
- Unique production and purification strategies for all immunogens. No need to optimise downstream purification protocols since the host will be the bioreactor.
- -
- Fast sequence modification: easy to adapt to new threats (emerging pathogens or variants), which accelerates screening speed in preclinical trials.
- -
- Less adverse effects than viral vector vaccines (capillary leak syndrome, vector reactivation, anti-vector responses)
Disadvantages:
- -
- Global production and purification procedures have yet to be easily accessible in developing countries.
- -
- Poor thermostability: requires ultra-freezers for adequate conservation.
- -
- Elevated costs due to intellectual property protection compared to conventional vaccine platforms (viral vectors, inactivated pathogens, subunit proteins).
- -
- Heterogeneous protein production. This limitation is especially relevant for mRNA delivery of proteins in which the therapeutic window is narrower.
4.2.1. Nucleic Acid Vaccines Encoding Viral Particles
DNA vaccines have been tested in clinical trials against HIV [55], SARS-CoV-2 [114], and other diseases too [117]. Regarding HIV, two DNA vaccine products have been tested in efficacy trials (Table 1): in the HVTN505 and in PrEPVacc, as aforementioned. Despite the negative results yielded by these clinical trials, their safety and tolerability have been extensively demonstrated, which can facilitate future attempts with similar platforms. Furthermore, there are a series of DNA vaccine platforms proving their safety profile in phase I/II trials (Table 2). These vaccine candidates in early clinical trials are designed both for the induction of cellular responses against Gag-Pol-Nef proteins (p24CE, HIV.consv, MAG-DNA, or GEO-D03, among others) or humoral responses against Env (DNA-C, DNA Nat-B/ConS, Pennvax, etc.). In these cases, DNA vaccines can also encode cytokines that will act as adjuvants, like IL-12 or GM-CSF [118,119,120].

Table 2.
DNA and mRNA HIV vaccines tested in the last 10 years.
DNA vaccines are often administered using plasmid DNA vectors that contain the gene of interest, a promoter, and a polyadenylation site. One commercially available vector is pVAX1, which follows FDA recommendations for DNA vaccines [121]. The main challenge that nucleic acid administration faces is their limited incorporation into host cells, requiring the use of physical or chemical delivery systems [122]. These systems, such as liposomes or in vivo electroporation, can enhance the transfection efficiency into host cells and improve the desired immune response against the immunogen by mediating an adjuvant effect [123,124].
RNA vaccines gained a significant momentum during the SARS-CoV-2 pandemic due to their successful development and efficacy [125]. This success stemmed from many years of research trying to reduce the immunogenicity of synthetic mRNA, which led to diminishing the rate of rejection and the fast degradation of delivered mRNA [126], and improving their delivery [19]. Before 2020, some attempts at developing HIV RNA vaccines were slowly moving towards clinical trials [18,127], but they were facing some reluctance caused by their novelty [16,128]. However, the success of mRNA vaccines for COVID-19 paved the way for more RNA-based vaccines to enter clinical trials [129]. Still, it is important to remember that regardless of the benefits of the platform used (DNA, mRNA, or viral vectors), if the immunogens that they encode do not elicit potent and protective immune responses, the strategy will still be futile.
In this sense, the high versatility and robustness of production processes of mRNA vaccine platforms can also prove very useful to accelerate vaccine testing. Since their safety profile is more than demonstrated, smaller, more directed experimental medicine vaccine trials (EMVT) can be performed to answer more directed scientific questions to screen for specific immunogens. This new discovery methods could accelerate vaccine science by testing multiple immunogens in smaller clinical trials, rather than betting on individual products to advance in conventional clinical trials [130].
There are currently three mRNA HIV vaccine candidates in phase I clinical trials. These vaccines integrate both the use of mRNA delivery with novel immunogens like the eOD-GT8 oligomer designed to prime CD4bs germline neutralising antibodies (clinicaltrials.gov: NCT05414786 and NCT05001373) or native Env trimers to induce potent neutralising responses (clinicaltrials.gov: NCT05217641). If these trials report successful results regarding their safety and tolerability profiles, and some insights on the immunological profiles they induce, we can expect to see them in combination with Gag-Pol and Nef immunogens to trigger a balanced adaptive immunity in phase II and III clinical trials soon.
The main challenges that mRNA faces in this context of HIV vaccine development are mainly logistical (Box 1), since the poor thermostable profile and current elevated costs hinder their introduction in developing countries. This stands especially true for Sub-Saharan African countries, where an effective prophylactic vaccine would be most useful to limit transmission. Improvement of the thermostability profile of mRNA [131,132], building better production and storage facilities on site [133], or the development of DNA vaccines as an alternative to mRNA whenever needed, could bypass this limitation.
4.2.2. Nucleic Acid Vaccines Encoding Virus-like Particles
Another strategy to boost the immunogenicity of subunit vaccines is to express the immunogen on multivalent platforms. Most multivalent platforms use nanotechnology-based strategies such as liposomes, or polymers, but there are also biological approaches that mimic the virus morphology, like virus-like particles (VLPs). These latter strategies can also benefit from nucleic acid vaccine platforms.
VLPs are as heterogeneous as viruses themselves, and most of them can be used to deliver relevant antigens [134]. However, in a context of an HIV vaccine, it makes sense to develop HIV-based VLPs that mimic the structure of the virus and deliver HIV humoral and cellular immunogens in their native conformation, since size, stability and proper antigen display are key properties that determine the immunogenicity of multivalent platforms [135,136].
HIV VLPs are produced by the expression of Gag, HIV’s main structural protein. Similar to HIV virion production, Gag buds on the membrane and VLPs protrude and are excreted, resulting in non-infectious, non-replicative immature VLPs that recapitulate the viral structure [137]. These HIV Gag VLPs can and have been used as immunogens to elicit potent cellular responses by vaccination [138,139], and even tested in humans [140,141,142]. Furthermore, membrane-bound immunogens can be passively incorporated on the VLP surface during budding, resulting in multivalent antigen carriers that express the immunogen in a native-like platform and can trigger both humoral and cellular responses [143].
Unfortunately, immunogens are usually incorporated at the surface of HIV Gag VLPs at low densities mirroring Env incorporation at the surface of HIV virions [144], which hinders their ability to induce potent immune responses by reducing antibodies’ avidity. That is why many groups have explored strategies to increase the antigen density at the surface of VLPs by modifying the transmembrane and/or intracellular domains [145,146,147,148], by introducing multimerization tags [149] or by fusing the immunogen with Gag [150,151].
A relevant feature of HIV Gag VLPs is that they can be produced in a wide array of in vitro platforms depending on the desired properties [137], but they can also be encoded and produced in vitro upon administration of nucleic acid vaccines [120]. After vaccination with a VLP-encoding nucleic acid vaccine, cells will uptake the DNA or mRNA and start secreting VLPs with immunogens on their surface that can be processed by APCs (Figure 3). Once Env epitopes bind their cognate B cell receptor (BCR), B cells will phagocytose the VLP and present epitopes from that same immunogen via MHC-II. Furthermore, they can also display antigens from proteins present within the particle (like Gag), and increase the chances of getting validated by a wider repertoire of CD4+ T helper cells, a phenomenon known as intrastructural help [152,153].
There are multiple examples of HIV VLP vaccine candidates formulated as nucleic acid vaccines or administered in heterologous regimens tested in preclinical models (namely mice, macaques, rabbits, or guinea pigs) and early clinical trials [120,148,154,155,156]. Table 3 provides a comprehensive list of some relevant examples of vaccine candidates formulated as nucleic acid encoded VLPs or administered in heterologous regimens and the main immunological results derived from their administration.

Table 3.
Immunogenicity studies of Gag-based VLPs as nucleic acid vaccine candidates for HIV.
4.3. Summary and Future Directions
In conclusion, expanding the heterogeneous armamentarium of vaccines and platforms available for developing preventive vaccination strategies against HIV is crucial to achieve success. As described by many researchers, a successful HIV-1 vaccine will not only depend on developing immunogens and platforms that elicit strong immune responses but also on understanding the synergistic effects of different platforms, immunogens, and adjuvants.
In this sense, we strongly believe that immunisation strategies with new antigens based on native Env trimers (i.e., SOSIP, NFL, or UFO) displayed as membrane-anchored antigens on cells or, more relevantly, on the surface of multivalent platforms like VLPs, could elicit broadly neutralising humoral responses. Furthermore, VLPs can also contain relevant Gag-, Pol- and/or Nef-based immunogens that can trigger protective cellular responses, achieving a balanced immune response. These multivalent platforms can further benefit from nucleic acid vaccines (mainly DNA and mRNA delivery systems), which facilitates and accelerates upstream and downstream manufacturing and the adaptation of vaccines against highly diverse viruses like HIV or influenza viruses, or against emerging viruses or viral variants of concern, that can pose a threat for humans.
Considering that both nucleic acid delivery systems, as well as VLPs have proven safe in clinical trials, we deem feasible that they could be successfully used in combination as vaccine platforms to display key immunogens in a future attempt to develop a prophylactic HIV vaccine, either alone or in combination with other immunogens in heterologous prime/boost systems. These strategies could provide a fresh push to pursue a new wave of clinical trials for the prevention of HIV infections, and even beyond this field.
Author Contributions
All authors supervised the scientific content of the manuscript. F.T.-F. drafted the manuscript and figures. F.T.-F., J.C. and J.B. edited the manuscript and discussed the contents. All authors have read and agreed to the published version of the manuscript.
Funding
Research in J.C. and J.B. laboratories is funded by “Ministerio de Ciencia, Innovación y Universidades” grant number PID2022-139831OB-I00 (to J.C.) and “Instituto de Salud Carlos III” and the “European Social Fund”, grant numbers PI20/00093 and PI/23/01269 (to J.B.). This work was supported by the CERCA Program (2021 SGR 00452; Generalitat de Catalunya).
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest. J.B. and J.C. are inventors of the patent WO/2018/020324, developed at the IrsiCaixa AIDS Research Institute. Outside this work BC, JB and JC are founders and shareholders of AlbaJuna Therapeutics, S. L.
References
- Rappuoli, R.; Pizza, M.; Del Giudice, G.; De Gregorio, E. Vaccines, New Opportunities for a New Society. Proc. Natl. Acad. Sci. USA 2014, 111, 12288–12293. [Google Scholar] [CrossRef]
- Vanderslott, S.; Dattani, S.; Spooner, F.; Roser, M. Vaccination. Available online: https://ourworldindata.org/vaccination (accessed on 27 January 2024).
- Marani, M.; Katul, G.G.; Pan, W.K.; Parolari, A.J. Intensity and Frequency of Extreme Novel Epidemics. Proc. Natl. Acad. Sci. USA 2021, 118, e2105482118. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Fernández, M.; Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; González-Acedo, A.; Ramos-Torrecillas, J.; Illescas-Montes, R. The Current Status of COVID-19 Vaccines. A Scoping Review. Drug Discov. Today 2022, 27, 103336. [Google Scholar] [CrossRef]
- Khobragade, A.; Bhate, S.; Ramaiah, V.; Deshpande, S.; Giri, K.; Phophle, H.; Supe, P.; Godara, I.; Revanna, R.; Nagarkar, R.; et al. Efficacy, Safety, and Immunogenicity of the DNA SARS-CoV-2 Vaccine (ZyCoV-D): The Interim Efficacy Results of a Phase 3, Randomised, Double-Blind, Placebo-Controlled Study in India. Lancet 2022, 399, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, Y.; Tong, X.; Kang, J.; Avendaño, M.J.; Serrano, E.F.; García-Salum, T.; Pardo-Roa, C.; Riquelme, A.; Medina, R.A.; Alter, G. Preserved Omicron Spike Specific Antibody Binding and Fc-Recognition across COVID-19 Vaccine Platforms. medRxiv 2021. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. New Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Krienke, C.; Kolb, L.; Diken, E.; Streuber, M.; Kirchhoff, S.; Bukur, T.; Akilli-Öztürk, Ö.; Kranz, L.M.; Berger, H.; Petschenka, J.; et al. A Noninflammatory MRNA Vaccine for Treatment of Experimental Autoimmune Encephalomyelitis. Science 2021, 371, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA Delivery to Dendritic Cells Exploits Antiviral Defence for Cancer Immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; de Souza, M.; Adams, E.; et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. New Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar] [CrossRef]
- Gray, G.E.; Allen, M.; Moodie, Z.; Churchyard, G.; Bekker, L.-G.; Nchabeleng, M.; Mlisana, K.; Metch, B.; de Bruyn, G.; Latka, M.H.; et al. Safety and Efficacy of the HVTN 503/Phambili Study of a Clade-B-Based HIV-1 Vaccine in South Africa: A Double-Blind, Randomised, Placebo-Controlled Test-of-Concept Phase 2b Study. Lancet Infect. Dis. 2011, 11, 507–515. [Google Scholar] [CrossRef]
- Moodie, Z.; Dintwe, O.; Sawant, S.; Grove, D.; Huang, Y.; Janes, H.; Heptinstall, J.; Omar, F.L.; Cohen, K.; De Rosa, S.C.; et al. Analysis of the HIV Vaccine Trials Network 702 Phase 2b-3 HIV-1 Vaccine Trial in South Africa Assessing RV144 Antibody and T-Cell Correlates of HIV-1 Acquisition Risk. J. Infect. Dis. 2022, 226, 246–257. [Google Scholar] [CrossRef]
- Crabtree Ramírez, B.; González Hernández, L.A.; Cabrera, C.; del Río, C.; González Rodríguez, A.; Sierra Madero, J. Mexican Perspective on the Mosaico HIV Vaccine Trial. Lancet HIV 2023, 10, e426–e427. [Google Scholar] [CrossRef]
- Flynn, N.M.; Forthal, D.N.; Harro, C.D.; Judson, F.N.; Mayer, K.H.; Para, M.F. Placebo-Controlled Phase 3 Trial of a Recombinant Glycoprotein 120 Vaccine to Prevent HIV-1 Infection. J. Infect. Dis. 2005, 191, 654–665. [Google Scholar] [CrossRef]
- Pitisuttithum, P.; Gilbert, P.; Gurwith, M.; Heyward, W.; Martin, M.; van Griensven, F.; Hu, D.; Tappero, J.W.; Choopanya, K. Randomized, Double-Blind, Placebo-Controlled Efficacy Trial of a Bivalent Recombinant Glycoprotein 120 HIV-1 Vaccine among Injection Drug Users in Bangkok, Thailand. J. Infect. Dis. 2006, 194, 1661–1671. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. MRNA Vaccines—A New Era in Vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Sanders, R.W.; Moore, J.P. Virus Vaccines: Proteins Prefer Prolines. Cell Host Microbe 2021, 29, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Esteban, I.; Pastor-Quiñones, C.; Usero, L.; Plana, M.; García, F.; Leal, L. In the Era of MRNA Vaccines, Is There Any Hope for HIV Functional Cure? Viruses 2021, 13, 501. [Google Scholar] [CrossRef] [PubMed]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. MRNA Vaccine Delivery Using Lipid Nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.-W.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.F.; Wiehe, K.; Borrow, P.; Saunders, K.O.; Korber, B.; Wagh, K.; McMichael, A.J.; Kelsoe, G.; Hahn, B.H.; Alt, F.; et al. Author Correction: Strategies for HIV-1 Vaccines That Induce Broadly Neutralizing Antibodies. Nat. Rev. Immunol. 2023, 23, 265. [Google Scholar] [CrossRef] [PubMed]
- Mothe, B.; Brander, C. HIV T-Cell Vaccines. Adv. Exp. Med. Biol. 2018, 1075, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, B. Molecular Mechanism of HIV-1 Entry. Trends Microbiol. 2019, 27, 878–891. [Google Scholar] [CrossRef]
- Burton, D.R.; Hangartner, L. Broadly Neutralizing Antibodies to HIV and Their Role in Vaccine Design. Annu. Rev. Immunol. 2016, 34, 635–659. [Google Scholar] [CrossRef]
- Kesavardhana, S.; Varadarajan, R. Stabilizing the Native Trimer of HIV-1 Env by Destabilizing the Heterodimeric Interface of the Gp41 Postfusion Six-Helix Bundle. J. Virol. 2014, 88, 9590–9604. [Google Scholar] [CrossRef]
- Masemola, A.; Mashishi, T.; Khoury, G.; Mohube, P.; Mokgotho, P.; Vardas, E.; Colvin, M.; Zijenah, L.; Katzenstein, D.; Musonda, R.; et al. Hierarchical Targeting of Subtype C Human Immunodeficiency Virus Type 1 Proteins by CD8+ T Cells: Correlation with Viral Load. J. Virol. 2004, 78, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Hemelaar, J. The Origin and Diversity of the HIV-1 Pandemic. Trends Mol. Med. 2012, 18, 182–192. [Google Scholar] [CrossRef] [PubMed]
- West, A.P.J.; Scharf, L.; Scheid, J.F.; Klein, F.; Bjorkman, P.J.; Nussenzweig, M.C. Structural Insights on the Role of Antibodies in HIV-1 Vaccine and Therapy. Cell 2014, 156, 633–648. [Google Scholar] [CrossRef]
- Sadanand, S.; Suscovich, T.J.; Alter, G. Broadly Neutralizing Antibodies Against HIV: New Insights to Inform Vaccine Design. Annu. Rev. Med. 2016, 67, 185–200. [Google Scholar] [CrossRef]
- Molinos-Albert, L.M.; Clotet, B.; Blanco, J.; Carrillo, J. Immunologic Insights on the Membrane Proximal External Region: A Major Human Immunodeficiency Virus Type-1 Vaccine Target. Front. Immunol. 2017, 8, 1154. [Google Scholar] [CrossRef]
- Bonsignori, M.; Liao, H.-X.; Gao, F.; Williams, W.B.; Alam, S.M.; Montefiori, D.C.; Haynes, B.F. Antibody-Virus Co-Evolution in HIV Infection: Paths for HIV Vaccine Development. Immunol. Rev. 2017, 275, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Gaebler, C.; Nogueira, L.; Stoffel, E.; Oliveira, T.Y.; Breton, G.; Millard, K.G.; Turroja, M.; Butler, A.; Ramos, V.; Seaman, M.S.; et al. Prolonged Viral Suppression with Anti-HIV-1 Antibody Therapy. Nature 2022, 606, 368–374. [Google Scholar] [CrossRef]
- Niessl, J.; Baxter, A.E.; Mendoza, P.; Jankovic, M.; Cohen, Y.Z.; Butler, A.L.; Lu, C.L.; Dubé, M.; Shimeliovich, I.; Gruell, H.; et al. Combination Anti-HIV-1 Antibody Therapy Is Associated with Increased Virus-Specific T Cell Immunity. Nat. Med. 2020, 26, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, P.; Gruell, H.; Nogueira, L.; Pai, J.A.; Butler, A.L.; Millard, K.; Lehmann, C.; Suárez, I.; Oliveira, T.Y.; Lorenzi, J.C.C.; et al. Combination Therapy with Anti-HIV-1 Antibodies Maintains Viral Suppression. Nature 2018, 561, 479. [Google Scholar] [CrossRef] [PubMed]
- Simek, M.D.; Rida, W.; Priddy, F.H.; Pung, P.; Carrow, E.; Laufer, D.S.; Lehrman, J.K.; Boaz, M.; Tarragona-Fiol, T.; Miiro, G.; et al. Human Immunodeficiency Virus Type 1 Elite Neutralizers: Individuals with Broad and Potent Neutralizing Activity Identified by Using a High-Throughput Neutralization Assay Together with an Analytical Selection Algorithm. J. Virol. 2009, 83, 7337–7348. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, L. Broadly Neutralizing Antibodies and Vaccine Design against HIV-1 Infection. Front. Med. 2020, 14, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Subbaraman, H.; Schanz, M.; Trkola, A. Broadly Neutralizing Antibodies: What Is Needed to Move from a Rare Event in HIV-1 Infection to Vaccine Efficacy? Retrovirology 2018, 15, 52. [Google Scholar] [CrossRef] [PubMed]
- Julg, B.; Liu, P.-T.; Wagh, K.; Fischer, W.M.; Abbink, P.; Mercado, N.B.; Whitney, J.B.; Nkolola, J.P.; McMahan, K.; Tartaglia, L.J.; et al. Protection against a Mixed SHIV Challenge by a Broadly Neutralizing Antibody Cocktail. Sci. Transl. Med. 2017, 9, eaao4235. [Google Scholar] [CrossRef] [PubMed]
- Julg, B.; Barouch, D.H. Neutralizing Antibodies for HIV-1 Prevention. Curr. Opin. HIV AIDS 2019, 14, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Corey, L.; Gilbert, P.B.; Juraska, M.; Montefiori, D.C.; Morris, L.; Karuna, S.T.; Edupuganti, S.; Mgodi, N.M.; deCamp, A.C.; Rudnicki, E.; et al. Two Randomized Trials of Neutralizing Antibodies to Prevent HIV-1 Acquisition. New Engl. J. Med. 2021, 384, 1003–1014. [Google Scholar] [CrossRef]
- Rusche, J.R.; Lynn, D.L.; Robert-Guroff, M.; Langlois, A.J.; Lyerly, H.K.; Carson, H.; Krohn, K.; Ranki, A.; Gallo, R.C.; Bolognesi, D.P. Humoral Immune Response to the Entire Human Immunodeficiency Virus Envelope Glycoprotein Made in Insect Cells. Proc. Natl. Acad. Sci. USA 1987, 84, 6924–6928. [Google Scholar] [CrossRef]
- Steimer, K.S.; Scandella, C.J.; Skiles, P.V.; Haigwood, N.L. Neutralization of Divergent HIV-1 Isolates by Conformation-Dependent Human Antibodies to Gp120. Science 1991, 254, 105–108. [Google Scholar] [CrossRef]
- Billich, A. AIDSVAX. VaxGen. Curr. Opin. Investig. Drugs 2001, 2, 1203–1208. [Google Scholar]
- Rerks-Ngarm, S.; Brown, A.E.; Khamboonruang, C.; Thongcharoen, P.; Kunasol, P. HIV/AIDS Preventive Vaccine ‘Prime-Boost’ Phase III Trial: Foundations and Initial Lessons Learned from Thailand. AIDS 2006, 20, 1471–1479. [Google Scholar]
- Jardine, J.G.; Kulp, D.W.; Havenar-Daughton, C.; Sarkar, A.; Briney, B.; Sok, D.; Sesterhenn, F.; Ereño-Orbea, J.; Kalyuzhniy, O.; Deresa, I.; et al. HIV-1 Broadly Neutralizing Antibody Precursor B Cells Revealed by Germline-Targeting Immunogen. Science 2016, 351, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Caillat, C.; Guilligay, D.; Sulbaran, G.; Weissenhorn, W. Neutralizing Antibodies Targeting HIV-1 Gp41. Viruses 2020, 12, 1210. [Google Scholar] [CrossRef]
- Sanders, R.W.; Vesanen, M.; Schuelke, N.; Master, A.; Schiffner, L.; Kalyanaraman, R.; Paluch, M.; Berkhout, B.; Maddon, P.J.; Olson, W.C.; et al. Stabilization of the Soluble, Cleaved, Trimeric Form of the Envelope Glycoprotein Complex of Human Immunodeficiency Virus Type 1. J. Virol. 2002, 76, 8875–8889. [Google Scholar] [CrossRef] [PubMed]
- Pauthner, M.G.; Nkolola, J.P.; Havenar-Daughton, C.; Murrell, B.; Reiss, S.M.; Bastidas, R.; Prévost, J.; Nedellec, R.; von Bredow, B.; Abbink, P.; et al. Vaccine-Induced Protection from Homologous Tier 2 SHIV Challenge in Nonhuman Primates Depends on Serum-Neutralizing Antibody Titers. Immunity 2019, 50, 241–252.e6. [Google Scholar] [CrossRef]
- Sliepen, K.; Han, B.W.; Bontjer, I.; Mooij, P.; Garces, F.; Behrens, A.-J.; Rantalainen, K.; Kumar, S.; Sarkar, A.; Brouwer, P.J.M.; et al. Structure and Immunogenicity of a Stabilized HIV-1 Envelope Trimer Based on a Group-M Consensus Sequence. Nat. Commun. 2019, 10, 2355. [Google Scholar] [CrossRef] [PubMed]
- Dubrovskaya, V.; Tran, K.; Ozorowski, G.; Guenaga, J.; Wilson, R.; Bale, S.; Cottrell, C.A.; Turner, H.L.; Seabright, G.; O’Dell, S.; et al. Vaccination with Glycan-Modified HIV NFL Envelope Trimer-Liposomes Elicits Broadly Neutralizing Antibodies to Multiple Sites of Vulnerability. Immunity 2019, 51, 915–929.e7. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Paynter, J.; Antanasijevic, A.; Allen, J.D.; Eldad, M.; Lee, Y.Z.; Copps, J.; Newby, M.L.; He, L.; Chavez, D.; et al. Single-Component Multilayered Self-Assembling Protein Nanoparticles Presenting Glycan-Trimmed Uncleaved Prefusion Optimized Envelope Trimmers as HIV-1 Vaccine Candidates. Nat. Commun. 2023, 14, 1985. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Excler, J.-L.; Michael, N.L. Lessons from the RV144 Thai Phase III HIV-1 Vaccine Trial and the Search for Correlates of Protection. Annu. Rev. Med. 2015, 66, 423–437. [Google Scholar] [CrossRef]
- Moodie, Z.; Walsh, S.R.; Laher, F.; Maganga, L.; Herce, M.E.; Naidoo, S.; Hosseinipour, M.C.; Innes, C.; Bekker, L.-G.; Grunenberg, N.; et al. Antibody and Cellular Responses to HIV Vaccine Regimens with DNA Plasmid as Compared with ALVAC Priming: An Analysis of Two Randomized Controlled Trials. PLoS Med. 2020, 17, e1003117. [Google Scholar] [CrossRef]
- Gray, G.E.; Bekker, L.-G.; Laher, F.; Malahleha, M.; Allen, M.; Moodie, Z.; Grunenberg, N.; Huang, Y.; Grove, D.; Prigmore, B.; et al. Vaccine Efficacy of ALVAC-HIV and Bivalent Subtype C Gp120-MF59 in Adults. New Engl. J. Med. 2021, 384, 1089–1100. [Google Scholar] [CrossRef]
- Hosseinipour, M.C.; Innes, C.; Naidoo, S.; Mann, P.; Hutter, J.; Ramjee, G.; Sebe, M.; Maganga, L.; Herce, M.E.; deCamp, A.C.; et al. Phase 1 Human Immunodeficiency Virus (HIV) Vaccine Trial to Evaluate the Safety and Immunogenicity of HIV Subtype C DNA and MF59-Adjuvanted Subtype C Envelope Protein. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 72, 50–60. [Google Scholar] [CrossRef]
- Nielsen, C.M.; Ogbe, A.; Pedroza-Pacheco, I.; Doeleman, S.E.; Chen, Y.; Silk, S.E.; Barrett, J.R.; Elias, S.C.; Miura, K.; Diouf, A.; et al. Protein/AS01B Vaccination Elicits Stronger, More Th2-Skewed Antigen-Specific Human T Follicular Helper Cell Responses than Heterologous Viral Vectors. Cell Rep. Med. 2021, 2, 100207. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, M.; Wang, T. Liposomes Used as a Vaccine Adjuvant-Delivery System: From Basics to Clinical Immunization. J. Control. Release 2019, 303, 130–150. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.F.; Gilbert, P.B.; McElrath, M.J.; Zolla-Pazner, S.; Tomaras, G.D.; Alam, S.M.; Evans, D.T.; Montefiori, D.C.; Karnasuta, C.; Sutthent, R.; et al. Immune-Correlates Analysis of an HIV-1 Vaccine Efficacy Trial. New Engl. J. Med. 2012, 366, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Wren, L.; Kent, S.J. HIV Vaccine Efficacy Trial: Glimmers of Hope and the Potential Role of Antibody-Dependent Cellular Cytotoxicity. Hum. Vaccin. 2011, 7, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Corey, L.; Gilbert, P.B.; Tomaras, G.D.; Haynes, B.F.; Pantaleo, G.; Fauci, A.S. Immune Correlates of Vaccine Protection against HIV-1 Acquisition: A Review. Sci. Transl. Med. 2015, 7, 310rv7. [Google Scholar] [CrossRef]
- Kracker, S.; Radbruch, A. Immunoglobulin Class Switching: In Vitro Induction and Analysis. Methods Mol. Biol. 2004, 271, 149–159. [Google Scholar] [CrossRef]
- McMichael, A.J.; Rowland-Jones, S.L. Cellular Immune Responses to HIV. Nature 2001, 410, 980–987. [Google Scholar] [CrossRef]
- Gray, E.R.; Bain, R.; Varsaneux, O.; Peeling, R.W.; Stevens, M.M.; McKendry, R.A. P24 Revisited: A Landscape Review of Antigen Detection for Early HIV Diagnosis. AIDS 2018, 32, 2089–2102. [Google Scholar] [CrossRef]
- Coplan, P.M.; Gupta, S.B.; Dubey, S.A.; Pitisuttithum, P.; Nikas, A.; Mbewe, B.; Vardas, E.; Schechter, M.; Kallas, E.G.; Freed, D.C.; et al. Cross-Reactivity of Anti-HIV-1 T Cell Immune Responses among the Major HIV-1 Clades in HIV-1-Positive Individuals from 4 Continents. J. Infect. Dis. 2005, 191, 1427–1434. [Google Scholar] [CrossRef]
- Laher, F.; Ranasinghe, S.; Porichis, F.; Mewalal, N.; Pretorius, K.; Ismail, N.; Buus, S.; Stryhn, A.; Carrington, M.; Walker, B.D.; et al. HIV Controllers Exhibit Enhanced Frequencies of Major Histocompatibility Complex Class II Tetramer(+) Gag-Specific CD4(+) T Cells in Chronic Clade C HIV-1 Infection. J. Virol. 2017, 91, e02477-16. [Google Scholar] [CrossRef]
- Pernas, M.; Tarancón-Diez, L.; Rodríguez-Gallego, E.; Gómez, J.; Prado, J.G.; Casado, C.; Dominguez-Molina, B.; Olivares, I.; Coiras, M.; León, A.; et al. Factors Leading to the Loss of Natural Elite Control of HIV-1 Infection. J. Virol. 2018, 92, e01805-17. [Google Scholar] [CrossRef]
- Payne, R.P.; Kløverpris, H.; Sacha, J.B.; Brumme, Z.; Brumme, C.; Buus, S.; Sims, S.; Hickling, S.; Riddell, L.; Chen, F.; et al. Efficacious Early Antiviral Activity of HIV Gag- and Pol-Specific HLA-B 2705-Restricted CD8+ T Cells. J. Virol. 2010, 84, 10543–10557. [Google Scholar] [CrossRef] [PubMed]
- Sundaramurthi, J.C.; Ashokkumar, M.; Swaminathan, S.; Hanna, L.E. HLA Based Selection of Epitopes Offers a Potential Window of Opportunity for Vaccine Design against HIV. Vaccine 2017, 35, 5568–5575. [Google Scholar] [PubMed]
- Rouphael, N.G.; Morgan, C.; Li, S.S.; Jensen, R.; Sanchez, B.; Karuna, S.; Swann, E.; Sobieszczyk, M.E.; Frank, I.; Wilson, G.J.; et al. DNA Priming and Gp120 Boosting Induces HIV-Specific Antibodies in a Randomized Clinical Trial. J. Clin. Investig. 2019, 129, 4769–4785. [Google Scholar] [CrossRef] [PubMed]
- Sekaly, R.-P. The Failed HIV Merck Vaccine Study: A Step Back or a Launching Point for Future Vaccine Development? J. Exp. Med. 2008, 205, 7–12. [Google Scholar] [CrossRef]
- Msafiri, F.; Joachim, A.; Held, K.; Nadai, Y.; Chissumba, R.M.; Geldmacher, C.; Aboud, S.; Stöhr, W.; Viegas, E.; Kroidl, A.; et al. Frequent Anti-V1V2 Responses Induced by HIV-DNA Followed by HIV-MVA with or without CN54rgp140/GLA-AF in Healthy African Volunteers. Microorganisms 2020, 8, 1722. [Google Scholar] [CrossRef] [PubMed]
- Perdiguero, B.; Pérez, P.; Marcos-Villar, L.; Albericio, G.; Astorgano, D.; Álvarez, E.; Sin, L.; Gómez, C.E.; García-Arriaza, J.; Esteban, M. Highly Attenuated Poxvirus-Based Vaccines Against Emerging Viral Diseases. J. Mol. Biol. 2023, 435, 168173. [Google Scholar] [CrossRef]
- Kajon, A.E.; Weinberg, J.B.; Spindler, K.R. Adenoviruses. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-801238-3. [Google Scholar]
- Barouch, D.H.; O’Brien, K.L.; Simmons, N.L.; King, S.L.; Abbink, P.; Maxfield, L.F.; Sun, Y.-H.; La Porte, A.; Riggs, A.M.; Lynch, D.M.; et al. Mosaic HIV-1 Vaccines Expand the Breadth and Depth of Cellular Immune Responses in Rhesus Monkeys. Nat. Med. 2010, 16, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Ondondo, B.; Murakoshi, H.; Clutton, G.; Abdul-Jawad, S.; Wee, E.G.-T.; Gatanaga, H.; Oka, S.; McMichael, A.J.; Takiguchi, M.; Korber, B.; et al. Novel Conserved-Region T-Cell Mosaic Vaccine With High Global HIV-1 Coverage Is Recognized by Protective Responses in Untreated Infection. Mol. Ther. 2016, 24, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Mothe, B.; Hu, X.; Llano, A.; Rosati, M.; Olvera, A.; Kulkarni, V.; Valentin, A.; Alicea, C.; Pilkington, G.R.; Sardesai, N.Y.; et al. A Human Immune Data-Informed Vaccine Concept Elicits Strong and Broad T-Cell Specificities Associated with HIV-1 Control in Mice and Macaques. J. Transl. Med. 2015, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Laher, F.; Bekker, L.-G.; Garrett, N.; Lazarus, E.M.; Gray, G.E. Review of Preventative HIV Vaccine Clinical Trials in South Africa. Arch. Virol. 2020, 165, 2439–2452. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Crotty, S. HIV Vaccinology: 2021 Update. Semin. Immunol. 2021, 51, 101470. [Google Scholar] [CrossRef]
- Esparza, J. A Brief History of the Global Effort to Develop a Preventive HIV Vaccine. Vaccine 2013, 31, 3502–3518. [Google Scholar] [CrossRef]
- Rhee, S.-Y.; Shafer, R.W. Geographically-Stratified HIV-1 Group M Pol Subtype and Circulating Recombinant Form Sequences. Sci. Data 2018, 5, 180148. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Peterson, M.L.; Follmann, D.; Hudgens, M.G.; Francis, D.P.; Gurwith, M.; Heyward, W.L.; Jobes, D.V.; Popovic, V.; Self, S.G.; et al. Correlation between Immunologic Responses to a Recombinant Glycoprotein 120 Vaccine and Incidence of HIV-1 Infection in a Phase 3 HIV-1 Preventive Vaccine Trial. J. Infect. Dis. 2005, 191, 666–677. [Google Scholar] [CrossRef]
- Harrer, T.; Harrer, E.; Kalams, S.A.; Elbeik, T.; Staprans, S.I.; Feinberg, M.B.; Cao, Y.; Ho, D.D.; Yilma, T.; Caliendo, A.M.; et al. Strong Cytotoxic T Cell and Weak Neutralizing Antibody Responses in a Subset of Persons with Stable Nonprogressing HIV Type 1 Infection. AIDS Res. Hum. Retroviruses 1996, 12, 585–592. [Google Scholar] [CrossRef]
- Buchbinder, S.P.; Mehrotra, D.V.; Duerr, A.; Fitzgerald, D.W.; Mogg, R.; Li, D.; Gilbert, P.B.; Lama, J.R.; Marmor, M.; Del Rio, C.; et al. Efficacy Assessment of a Cell-Mediated Immunity HIV-1 Vaccine (the Step Study): A Double-Blind, Randomised, Placebo-Controlled, Test-of-Concept Trial. Lancet 2008, 372, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.R.; Desrosiers, R.C.; Doms, R.W.; Feinberg, M.B.; Gallo, R.C.; Hahn, B.; Hoxie, J.A.; Hunter, E.; Korber, B.; Landay, A.; et al. A Sound Rationale Needed for Phase III HIV-1 Vaccine Trials. Science 2004, 303, 316. [Google Scholar] [CrossRef] [PubMed]
- Belshe, R.; Franchini, G.; Girard, M.P.; Gotch, F.; Kaleebu, P.; Marthas, M.L.; McChesney, M.B.; McCullough, R.; Mhalu, F.; Salmon-Ceron, D.; et al. Support for the RV144 HIV Vaccine Trial. Science 2004, 305, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Russell, N.D.; Marovich, M.A. Pox-Protein Public Private Partnership Program and Upcoming HIV Vaccine Efficacy Trials. Curr. Opin. HIV AIDS 2016, 11, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.M.; Sobieszczyk, M.E.; Janes, H.; Karuna, S.T.; Mulligan, M.J.; Grove, D.; Koblin, B.A.; Buchbinder, S.P.; Keefer, M.C.; Tomaras, G.D.; et al. Efficacy Trial of a DNA/RAd5 HIV-1 Preventive Vaccine. New Engl. J. Med. 2013, 369, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Janes, H.E.; Cohen, K.W.; Frahm, N.; De Rosa, S.C.; Sanchez, B.; Hural, J.; Magaret, C.A.; Karuna, S.; Bentley, C.; Gottardo, R.; et al. Higher T-Cell Responses Induced by DNA/RAd5 HIV-1 Preventive Vaccine Are Associated with Lower HIV-1 Infection Risk in an Efficacy Trial. J. Infect. Dis. 2017, 215, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Bekker, L.-G.; Moodie, Z.; Grunenberg, N.; Laher, F.; Tomaras, G.D.; Cohen, K.W.; Allen, M.; Malahleha, M.; Mngadi, K.; Daniels, B.; et al. Subtype C ALVAC-HIV and Bivalent Subtype C Gp120/MF59 HIV-1 Vaccine in Low-Risk, HIV-Uninfected, South African Adults: A Phase 1/2 Trial. Lancet HIV 2018, 5, e366–e378. [Google Scholar] [CrossRef]
- Baden, L.R.; Stieh, D.J.; Sarnecki, M.; Walsh, S.R.; Tomaras, G.D.; Kublin, J.G.; McElrath, M.J.; Alter, G.; Ferrari, G.; Montefiori, D.; et al. Safety and Immunogenicity of Two Heterologous HIV Vaccine Regimens in Healthy, HIV-Uninfected Adults (TRAVERSE): A Randomised, Parallel-Group, Placebo-Controlled, Double-Blind, Phase 1/2a Study. Lancet HIV 2020, 7, e688–e698. [Google Scholar] [CrossRef]
- Barouch, D.H.; Tomaka, F.L.; Wegmann, F.; Stieh, D.J.; Alter, G.; Robb, M.L.; Michael, N.L.; Peter, L.; Nkolola, J.P.; Borducchi, E.N.; et al. Evaluation of a Mosaic HIV-1 Vaccine in a Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 1/2a Clinical Trial (APPROACH) and in Rhesus Monkeys (NHP 13-19). Lancet 2018, 392, 232–243. [Google Scholar] [CrossRef]
- Gray, G. Phase 2b efficacy trial of mosaic HIV-1 vaccine regimen in African women (Imbokodo). In Proceedings of the Conference on Retroviruses and Opportunistic Infections (CROI), Virtual, 12–16 February 2022. [Google Scholar]
- IAVI. IAVI Statement on PrEPVacc Trial. Available online: https://www.iavi.org/features/iavi-statement-on-prepvacc-trial/ (accessed on 28 January 2024).
- Abbasi, J. First MRNA HIV Vaccine Clinical Trial Launches. JAMA 2022, 327, 909. [Google Scholar] [CrossRef]
- Fortner, A.; Bucur, O. MRNA-Based Vaccine Technology for HIV. Discoveries 2022, 10, e150. [Google Scholar] [CrossRef]
- Shepherd, B.O.; Chang, D.; Vasan, S.; Ake, J.; Modjarrad, K. HIV and SARS-CoV-2: Tracing a Path of Vaccine Research and Development. Curr. HIV/AIDS Rep. 2022, 19, 86–93. [Google Scholar] [CrossRef]
- Hobernik, D.; Bros, M. DNA Vaccines-How Far From Clinical Use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef] [PubMed]
- Ertl, H.C.J. Viral Vectors as Vaccine Carriers. Curr. Opin. Virol. 2016, 21, 1–8. [Google Scholar] [CrossRef]
- Melzi, E.; Willis, J.R.; Ma, K.M.; Lin, Y.C.; Kratochvil, S.; Berndsen, Z.T.; Landais, E.A.; Kalyuzhniy, O.; Nair, U.; Warner, J.; et al. Membrane-Bound MRNA Immunogens Lower the Threshold to Activate HIV Env V2 Apex-Directed Broadly Neutralizing B Cell Precursors in Humanized Mice. Immunity 2022, 55, 2168–2186.e6. [Google Scholar] [CrossRef]
- Cari, L.; Naghavi Alhosseini, M.; Mencacci, A.; Migliorati, G.; Nocentini, G. Differences in the Expression Levels of SARS-CoV-2 Spike Protein in Cells Treated with MRNA-Based COVID-19 Vaccines: A Study on Vaccines from the Real World. Vaccines 2023, 11, 879. [Google Scholar] [CrossRef]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and Challenges in the Delivery of MRNA-Based Vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, H.; Meng, L.; Li, F.; Yu, C. Comparison of Immune Responses Elicited by SARS-CoV-2 MRNA and Recombinant Protein Vaccine Candidates. Front. Immunol. 2022, 13, 906457. [Google Scholar] [CrossRef] [PubMed]
- Barry, M. Single-Cycle Adenovirus Vectors in the Current Vaccine Landscape. Expert Rev. Vaccines 2018, 17, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Krüger, N.; Schulz, S.; Cossmann, A.; Rocha, C.; Kempf, A.; Nehlmeier, I.; Graichen, L.; Moldenhauer, A.-S.; Winkler, M.S.; et al. The Omicron Variant Is Highly Resistant against Antibody-Mediated Neutralization: Implications for Control of the COVID-19 Pandemic. Cell 2022, 185, 447–456.e11. [Google Scholar] [CrossRef] [PubMed]
- Nkolola, J.P.; Barouch, D.H. Prophylactic HIV-1 Vaccine Trials: Past, Present, and Future. Lancet HIV 2023, 11, E117–E124. [Google Scholar] [CrossRef] [PubMed]
- Sasso, E.; D’Alise, A.M.; Zambrano, N.; Scarselli, E.; Folgori, A.; Nicosia, A. New Viral Vectors for Infectious Diseases and Cancer. Semin. Immunol. 2020, 50, 101430. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.R. Advancing an HIV Vaccine; Advancing Vaccinology. Nat. Rev. Immunol. 2019, 19, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous COVID-19 Booster Vaccinations. New Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Olszevicki, S.; Gaiano, A.; Salazar, M.; Regairaz, L.; Varela Baino, A.N.; Bartel, E.; Varela, T.; González Martínez, V.V.; Pesci, S.; et al. Protection of Homologous and Heterologous Boosters after Primary Schemes of RAd26-RAd5, ChAdOx1 NCoV-19 and BBIBP-CorV during the Omicron Outbreak in Adults of 50 Years and Older in Argentina: A Test-Negative Case–Control Study. Lancet Reg. Health 2023, 27, 1000607. [Google Scholar] [CrossRef] [PubMed]
- Parks, C.L. Replication-Competent Viral Vectors for Vaccine Delivery. In Human Vaccines: Emerging Technologies in Design and Development; Elsevier: Amsterdam, The Netherlands, 2017; pp. 25–63. [Google Scholar] [CrossRef]
- Gu, J.; Xu, Z.; Liu, Q.; Tang, S.; Zhang, W.; Xie, S.; Chen, X.; Chen, J.; Yong, K.T.; Yang, C.; et al. Building a Better Silver Bullet: Current Status and Perspectives of Non-Viral Vectors for MRNA Vaccines. Adv. Healthc. Mater. 2024, 13, 2302409. [Google Scholar] [CrossRef]
- Scheaffer, S.M.; Lee, D.; Whitener, B.; Ying, B.; Wu, K.; Liang, C.Y.; Jani, H.; Martin, P.; Amato, N.J.; Avena, L.E.; et al. Bivalent SARS-CoV-2 MRNA Vaccines Increase Breadth of Neutralization and Protect against the BA.5 Omicron Variant in Mice. Nat. Med. 2022, 29, 247–257. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. New Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Momin, T.; Kansagra, K.; Patel, H.; Sharma, S.; Sharma, B.; Patel, J.; Mittal, R.; Sanmukhani, J.; Maithal, K.; Dey, A.; et al. Safety and Immunogenicity of a DNA SARS-CoV-2 Vaccine (ZyCoV-D): Results of an Open-Label, Non-Randomized Phase I Part of Phase I/II Clinical Study by Intradermal Route in Healthy Subjects in India. eClinicalMedicine 2021, 38, 101020. [Google Scholar] [CrossRef]
- Hanke, T. New Vector and Vaccine Platforms: MRNA, DNA, Viral Vectors. Curr. Opin. HIV AIDS 2022, 17, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Langer, B.; Renner, M.; Scherer, J.; Schüle, S.; Cichutek, K. Safety Assessment of Biolistic DNA Vaccination. Methods Mol. Biol. 2013, 940, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Arun Kumar, S.; Jhan, Y.Y.; Bishop, C.J. Engineering DNA Vaccines against Infectious Diseases. Acta Biomater. 2018, 80, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Eusébio, D.; Neves, A.R.; Costa, D.; Biswas, S.; Alves, G.; Cui, Z.; Sousa, Â. Methods to Improve the Immunogenicity of Plasmid DNA Vaccines. Drug Discov. Today 2021, 26, 2575–2592. [Google Scholar] [CrossRef] [PubMed]
- Elizaga, M.L.; Li, S.S.; Kochar, N.K.; Wilson, G.J.; Allen, M.A.; Tieu, H.V.N.; Frank, I.; Sobieszczyk, M.E.; Cohen, K.W.; Sanchez, B.; et al. Safety and Tolerability of HIV-1 Multiantigen PDNA Vaccine given with IL-12 Plasmid DNA via Electroporation, Boosted with a Recombinant Vesicular Stomatitis Virus HIV Gag Vaccine in Healthy Volunteers in a Randomized, Controlled Clinical Trial. PLoS ONE 2018, 13, e0202753. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, S.P.; Grunenberg, N.A.; Sanchez, B.J.; Seaton, K.E.; Ferrari, G.; Moody, M.A.; Frahm, N.; Montefiori, D.C.; Hay, C.M.; Goepfert, P.A.; et al. Immunogenicity of a Novel Clade B HIV-1 Vaccine Combination: Results of Phase 1 Randomized Placebo Controlled Trial of an HIV-1 GM-CSF-Expressing DNA Prime with a Modified Vaccinia Ankara Vaccine Boost in Healthy HIV-1 Uninfected Adults. PLoS ONE 2017, 12, e0179597. [Google Scholar] [CrossRef] [PubMed]
- Klinman, D.M.; Klaschik, S.; Tross, D.; Shirota, H.; Steinhagen, F. FDA Guidance on Prophylactic DNA Vaccines: Analysis and Recommendations. Vaccine 2010, 28, 2801–2805. [Google Scholar] [CrossRef] [PubMed]
- Jorritsma, S.H.T.; Gowans, E.J.; Grubor-Bauk, B.; Wijesundara, D.K. Delivery Methods to Increase Cellular Uptake and Immunogenicity of DNA Vaccines. Vaccine 2016, 34, 5488–5494. [Google Scholar] [CrossRef]
- Qin, F.; Xia, F.; Chen, H.; Cui, B.; Feng, Y.; Zhang, P.; Chen, J.; Luo, M. A Guide to Nucleic Acid Vaccines in the Prevention and Treatment of Infectious Diseases and Cancers: From Basic Principles to Current Applications. Front. Cell Dev. Biol. 2021, 9, 633776. [Google Scholar] [CrossRef]
- Satkauskas, S.; Bureau, M.F.; Puc, M.; Mahfoudi, A.; Scherman, D.; Miklavcic, D.; Mir, L.M. Mechanisms of In Vivo DNA Electrotransfer: Respective Contributions of Cell Electropermeabilization and DNA Electrophoresis. Mol. Ther. 2002, 5, 133–140. [Google Scholar] [CrossRef]
- Pilkington, E.H.; Suys, E.J.A.; Trevaskis, N.L.; Wheatley, A.K.; Zukancic, D.; Algarni, A.; Al-Wassiti, H.; Davis, T.P.; Pouton, C.W.; Kent, S.J.; et al. From Influenza to COVID-19: Lipid Nanoparticle MRNA Vaccines at the Frontiers of Infectious Diseases. Acta Biomater. 2021, 131, 16–40. [Google Scholar] [CrossRef]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of Pseudouridine Into MRNA Yields Superior Nonimmunogenic Vector With Increased Translational Capacity and Biological Stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Leal, L.; Guardo, A.C.; Morón-López, S.; Salgado, M.; Mothe, B.; Heirman, C.; Pannus, P.; Vanham, G.; van den Ham, H.J.; Gruters, R.; et al. Phase I Clinical Trial of an Intranodally Administered MRNA-Based Therapeutic Vaccine against HIV-1 Infection. AIDS 2018, 32, 2533–2545. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.D.; Moody, M.A.; Thompson, A.B. Innovations in HIV-1 Vaccine Design. Clin. Ther. 2020, 42, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. How COVID Unlocked the Power of RNA Vaccines. Nature 2021, 589, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Prudden, H.; Tatoud, R.; Slack, C.; Shattock, R.; Anklesaria, P.; Bekker, L.G.; Buchbinder, S. Experimental Medicine for HIV Vaccine Research and Development. Vaccines 2023, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, D.J.A.; Anchordoquy, T.J.; Volkin, D.B.; Jiskoot, W.; Mastrobattista, E. Addressing the Cold Reality of MRNA Vaccine Stability. J. Pharm. Sci. 2021, 110, 997–1001. [Google Scholar] [CrossRef]
- Uddin, M.N.; Roni, M.A. Challenges of Storage and Stability of MRNA-Based COVID-19 Vaccines. Vaccines 2021, 9, 1033. [Google Scholar] [CrossRef]
- Saied, A.R.A. Building Africa’s First MRNA Vaccine Facility. Lancet 2023, 402, 287–288. [Google Scholar] [CrossRef]
- Fuenmayor, J.; Gòdia, F.; Cervera, L. Production of Virus-like Particles for Vaccines. N. Biotechnol. 2017, 39, 174–180. [Google Scholar] [CrossRef]
- Thalhauser, S.; Peterhoff, D.; Wagner, R.; Breunig, M. Critical Design Criteria for Engineering a Nanoparticulate HIV-1 Vaccine. J. Control. Release 2020, 317, 322–335. [Google Scholar] [CrossRef]
- Tokatlian, T.; Kulp, D.W.; Mutafyan, A.A.; Jones, C.A.; Menis, S.; Georgeson, E.; Kubitz, M.; Zhang, M.H.; Melo, M.B.; Silva, M.; et al. Enhancing Humoral Responses Against HIV Envelope Trimers via Nanoparticle Delivery with Stabilized Synthetic Liposomes. Sci. Rep. 2018, 8, 16527. [Google Scholar] [CrossRef]
- Cervera, L.; Gòdia, F.; Tarrés-Freixas, F.; Aguilar-Gurrieri, C.; Carrillo, J.; Blanco, J.; Gutiérrez-Granados, S. Production of HIV-1-Based Virus-like Particles for Vaccination: Achievements and Limits. Appl. Microbiol. Biotechnol. 2019, 103, 7367–7384. [Google Scholar] [CrossRef] [PubMed]
- Paliard, X.; Liu, Y.; Wagner, R.; Wolf, H.; Baenziger, J.; Walker, C.M. Priming of Strong, Broad, and Long-Lived HIV Type 1 P55gag-Specific CD8+ Cytotoxic T Cells after Administration of a Virus-Like Particle Vaccine in Rhesus Macaques. AIDS Res. Hum. Retroviruses 2000, 16, 273–282. [Google Scholar] [CrossRef]
- Deml, L.; Speth, C.; Dierich, M.P.; Wolf, H.; Wagner, R. Recombinant HIV-1 Pr55gag Virus-like Particles: Potent Stimulators of Innate and Acquired Immune Responses. Mol. Immunol. 2005, 42, 259–277. [Google Scholar] [CrossRef]
- Veenstra, J.; Williams, I.G.; Colebunders, R.; Dorrell, L.; Tchamouroff, S.E.; Patou, G.; Lange, J.M.A.; Weller, I.V.D.; Goeman, J.; Uthayakumar, S.; et al. Immunization with Recombinant P17/P24:Ty Virus-like Particles in Human Immunodeficiency Virus-Infected Persons. J. Infect. Dis. 1996, 174, 862–866. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peters, B.S.; Cheingsong-Popov, R.; Callow, D.; Foxall, R.; Patou, G.; Hodgkin, K.; Weber, J.N. A Pilot Phase II Study of the Safety and Immunogenicity of HIV P17/P24:VLP (P24-VLP) in Asymptomatic HIV Seropositive Subjects. J. Infect. 1997, 35, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, A.D.; Roggensack, M.; Jaramillo, A.B.; Smith, D.E.; Walker, A.; Gow, I.; McMurchie, M.; Harris, J.; Patou, G.; Cooper, D.A. Safety and Immunogenicity of a Candidate Therapeutic Vaccine, P24 Virus-like Particle, Combined with Zidovudine, in Asymptomatic Subjects. Community HIV Research Network Investigators. AIDS 1998, 12, 175–182. [Google Scholar] [CrossRef]
- Chen, C.W.; Saubi, N.; Joseph-Munné, J. Design Concepts of Virus-Like Particle-Based HIV-1 Vaccines. Front. Immunol. 2020, 11, 573157. [Google Scholar] [CrossRef]
- Klein, J.S.; Bjorkman, P.J. Few and Far between: How HIV May Be Evading Antibody Avidity. PLoS Pathog. 2010, 6, e1000908. [Google Scholar] [CrossRef]
- Deml, L.; Kratochwil, G.; Osterrieder, N.; Knüchel, R.; Wolf, H.; Wagner, R. Increased Incorporation of Chimeric Human Immunodeficiency Virus Type 1 Gp120 Proteins into Pr55gag Virus-Like Particles by an Epstein-Barr Virus Gp220/350-Derived Transmembrane Domain. Virology 1997, 235, 10–25. [Google Scholar] [CrossRef]
- Wang, B.-Z.; Liu, W.; Kang, S.-M.; Alam, M.; Huang, C.; Ye, L.; Sun, Y.; Li, Y.; Kothe, D.L.; Pushko, P.; et al. Incorporation of High Levels of Chimeric Human Immunodeficiency Virus Envelope Glycoproteins into Virus-like Particles. J. Virol. 2007, 81, 10869–10878. [Google Scholar] [CrossRef] [PubMed]
- Stano, A.; Leaman, D.P.; Kim, A.S.; Zhang, L.; Autin, L.; Ingale, J.; Gift, S.K.; Truong, J.; Wyatt, R.T.; Olson, A.J.; et al. Dense Array of Spikes on HIV-1 Virion Particles. J. Virol. 2017, 91, e00415-17. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.; van Diepen, M.; Galant, S.; Kruse, E.; Margolin, E.; Ximba, P.; Hermanus, T.; Moore, P.; Douglass, N.; Williamson, A.-L.; et al. Immunogenicity of HIV-1 Vaccines Expressing Chimeric Envelope Glycoproteins on the Surface of Pr55 Gag Virus-Like Particles. Vaccines 2020, 8, 54. [Google Scholar] [CrossRef]
- Escolano, A.; Gristick, H.B.; Abernathy, M.E.; Merkenschlager, J.; Gautam, R.; Oliveira, T.Y.; Pai, J.; West, A.P.J.; Barnes, C.O.; Cohen, A.A.; et al. Immunization Expands B Cells Specific to HIV-1 V3 Glycan in Mice and Macaques. Nature 2019, 570, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Tarrés-Freixas, F.; Aguilar-Gurrieri, C.; Rodríguez de la Concepción, M.L.; Urrea, V.; Trinité, B.; Ortiz, R.; Pradenas, E.; Blanco, P.; Marfil, S.; Molinos-Albert, L.M.; et al. An Engineered HIV-1 Gag-Based VLP Displaying High Antigen Density Induces Strong Antibody-Dependent Functional Immune Responses. NPJ Vaccines 2023, 8, 51. [Google Scholar] [CrossRef]
- Ortiz, R.; Barajas, A.; Pons-Grífols, A.; Trinité, B.; Tarrés-Freixas, F.; Rovirosa, C.; Urrea, V.; Barreiro, A.; Gonzalez-Tendero, A.; Cardona, M.; et al. Exploring FeLV-Gag-Based VLPs as a New Vaccine Platform-Analysis of Production and Immunogenicity. Int. J. Mol. Sci. 2023, 24, 9025. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, H.; Nabi, G.; McKinstry, W.J.; Khoo, K.K.; Mak, J.; Salazar, A.M.; Tenbusch, M.; Temchura, V.; Überla, K. Intrastructural Help: Harnessing T Helper Cells Induced by Licensed Vaccines for Improvement of HIV Env Antibody Responses to Virus-Like Particle Vaccines. J. Virol. 2018, 92, e00141-18. [Google Scholar] [CrossRef] [PubMed]
- Klessing, S.; Temchura, V.; Tannig, P.; Peter, A.S.; Christensen, D.; Lang, R.; Überla, K. CD4(+) T Cells Induced by Tuberculosis Subunit Vaccine H1 Can Improve the HIV-1 Env Humoral Response by Intrastructural Help. Vaccines 2020, 8, 604. [Google Scholar] [CrossRef]
- Smith, J.M.; Rao Amara, R.; Campbell, D.; Xu, Y.; Patel, M.; Sharma, S.; Butera, S.T.; Ellenberger, D.L.; Yi, H.; Chennareddi, L.; et al. DNA/MVA Vaccine for HIV Type 1: Effects of Codon-Optimization and the Expression of Aggregates or Virus-Like Particles on the Immunogenicity of the DNA Prime. AIDS Res. Hum. Retroviruses 2004, 20, 1335–1347. [Google Scholar] [CrossRef]
- Ellenberger, D.; Wyatt, L.; Li, B.; Buge, S.; Lanier, N.; Rodriguez, I.V.; Sariol, C.A.; Martinez, M.; Monsour, M.; Vogt, J.; et al. Comparative Immunogenicity in Rhesus Monkeys of Multi-Protein HIV-1 (CRF02_AG) DNA/MVA Vaccines Expressing Mature and Immature VLPs. Virology 2005, 340, 21–32. [Google Scholar] [CrossRef][Green Version]
- Perdiguero, B.; Sánchez-Corzo, C.; Sorzano, C.O.S.; Saiz, L.; Mediavilla, P.; Esteban, M.; Gómez, C.E. A Novel MVA-Based HIV Vaccine Candidate (MVA-Gp145-GPN) Co-Expressing Clade C Membrane-Bound Trimeric Gp145 Env and Gag-Induced Virus-Like Particles (VLPs) Triggered Broad and Multifunctional HIV-1-Specific T Cell and Antibody Responses. Viruses 2019, 11, 160. [Google Scholar] [CrossRef]
- Montefiori, D.C.; Safrit, J.T.; Lydy, S.L.; Barry, A.P.; Miroslawa, B.; Vo, H.T.; Klein, M.; Tartaglia, J.; Robinson, H.L.; Rovinski, B. Induction of Neutralizing Antibodies and Gag-Specific Cellular Immune Responses to an R5 Primary Isolate of Human Immunodeficiency Virus Type 1 in Rhesus Macaques. J. Virol. 2001, 75, 5879–5890. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Radaelli, A.; Zanotto, C.; Perletti, G.; Elli, V.; Vicenzi, E.; Poli, G.; De Giuli Morghen, C. Comparative Analysis of Immune Responses and Cytokine Profiles Elicited in Rabbits by the Combined Use of Recombinant Fowlpox Viruses, Plasmids and Virus-like Particles in Prime-Boost Vaccination Protocols against SHIV. Vaccine 2003, 21, 2052–2064. [Google Scholar] [CrossRef]
- Radaelli, A.; Bonduelle, O.; Beggio, P.; Mahe, B.; Pozzi, E.; Elli, V.; Paganini, M.; Zanotto, C.; Morghen, C.D.G.; Combadière, B. Prime-Boost Immunization with DNA, Recombinant Fowlpox Virus and VLPSHIV Elicit Both Neutralizing Antibodies and IFNγ-Producing T Cells against the HIV-Envelope Protein in Mice That Control Env-Bearing Tumour Cells. Vaccine 2007, 25, 2128–2138. [Google Scholar] [CrossRef] [PubMed]
- Buonaguro, L.; Devito, C.; Tornesello, M.L.; Schröder, U.; Wahren, B.; Hinkula, J.; Buonaguro, F.M. DNA-VLP Prime-Boost Intra-Nasal Immunization Induces Cellular and Humoral Anti-HIV-1 Systemic and Mucosal Immunity with Cross-Clade Neutralizing Activity. Vaccine 2007, 25, 5968–5977. [Google Scholar] [CrossRef] [PubMed]
- Lifei, Y.; Yufeng, S.; Xiaomin, L.; Xiaoxing, H.; Jingjing, L.; Heng, D.; Ping, Z.; Paul, Z. HIV-1 Virus-Like Particles Produced by Stably Transfected Drosophila S2 Cells: A Desirable Vaccine Component. J. Virol. 2012, 86, 7662–7676. [Google Scholar] [CrossRef]
- Kamdem Toukam, D.; Tenbusch, M.; Stang, A.; Temchura, V.; Storcksdieck genannt Bonsmann, M.; Grewe, B.; Koch, S.; Meyerhans, A.; Nchinda, G.; Kaptue, L.; et al. Targeting Antibody Responses to the Membrane Proximal External Region of the Envelope Glycoprotein of Human Immunodeficiency Virus. PLoS ONE 2012, 7, e38068. [Google Scholar] [CrossRef] [PubMed]
- Nabi, G.; Genannt Bonsmann, M.S.; Tenbusch, M.; Gardt, O.; Barouch, D.H.; Temchura, V.; Überla, K. GagPol-Specific CD4+T-Cells Increase the Antibody Response to Env by Intrastructural Help. Retrovirology 2013, 10, 117. [Google Scholar] [CrossRef]
- Ross, T.M.; Pereira, L.E.; Luckay, A.; McNicholl, J.M.; García-Lerma, J.G.; Heneine, W.; Eugene, H.S.; Pierce-Paul, B.R.; Zhang, J.; Hendry, R.M.; et al. A Polyvalent Clade B Virus-Like Particle HIV Vaccine Combined with Partially Protective Oral Preexposure Prophylaxis Prevents Simian–Human Immunodeficiency Virus Infection in Macaques and Primes for Virus-Amplified Immunity. AIDS Res. Hum. Retroviruses 2014, 30, 1072–1081. [Google Scholar] [CrossRef]
- Benen, T.D.; Tonks, P.; Kliche, A.; Kapzan, R.; Heeney, J.L.; Wagner, R. Development and Immunological Assessment of VLP-Based Immunogens Exposing the Membrane-Proximal Region of the HIV-1 Gp41 Protein. J. Biomed. Sci. 2014, 21, 79. [Google Scholar] [CrossRef]
- Vzorov, A.N.; Wang, L.; Chen, J.; Wang, B.-Z.; Compans, R.W. Effects of Modification of the HIV-1 Env Cytoplasmic Tail on Immunogenicity of VLP Vaccines. Virology 2016, 489, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Gangadhara, S.; Victor, B.; Shen, X.; Chen, X.; Nabi, R.; Kasturi, S.P.; Sabula, M.J.; Labranche, C.C.; Reddy, P.B.J.; et al. Virus-Like Particles Displaying Trimeric Simian Immunodeficiency Virus (SIV) Envelope Gp160 Enhance the Breadth of DNA/Modified Vaccinia Virus Ankara SIV Vaccine-Induced Antibody Responses in Rhesus Macaques. J. Virol. 2016, 90, 8842–8854. [Google Scholar] [CrossRef] [PubMed]
- Storcksdieck genannt Bonsmann, M.; Niezold, T.; Hannaman, D.; Überla, K.; Tenbusch, M. The Improved Antibody Response against HIV-1 after a Vaccination Based on Intrastructural Help Is Complemented by Functional CD8+ T Cell Responses. Vaccine 2016, 34, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhu, Q.; Huang, X.; Yang, L.; Song, Y.; Zhu, P.; Zhou, P. In Vivo Electroporation in DNA-VLP Prime-Boost Preferentially Enhances HIV-1 Envelope-Specific IgG2a, Neutralizing Antibody and CD8 T Cell Responses. Vaccine 2017, 35, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Pavez, C.; Ferreira, C.B.; Merino-Mansilla, A.; Fabra-Garcia, A.; Casadella, M.; Noguera-Julian, M.; Paredes, R.; Olvera, A.; Haro, I.; Brander, C.; et al. Guiding the Humoral Response against HIV-1 toward a MPER Adjacent Region by Immunization with a VLP-Formulated Antibody-Selected Envelope Variant. PLoS ONE 2018, 13, e0208345. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Dienger-Stambaugh, K.; Chen, X.; Wei, H.; Phan, S.; Beavis, A.C.; Singh, K.; Adhikary, N.R.D.; Tiwari, P.; Villinger, F.; et al. Parainfluenza Virus 5 Priming Followed by SIV/HIV Virus-Like-Particle Boosting Induces Potent and Durable Immune Responses in Nonhuman Primates. Front. Immunol. 2021, 12, 162. [Google Scholar] [CrossRef]
- Zhang, P.; Narayanan, E.; Liu, Q.; Tsybovsky, Y.; Boswell, K.; Ding, S.; Hu, Z.; Follmann, D.; Lin, Y.; Miao, H.; et al. A Multiclade Env–Gag VLP MRNA Vaccine Elicits Tier-2 HIV-1-Neutralizing Antibodies and Reduces the Risk of Heterologous SHIV Infection in Macaques. Nat. Med. 2021, 27, 2234–2245. [Google Scholar] [CrossRef]
- Beltran-Pavez, C.; Bontjer, I.; Gonzalez, N.; Pernas, M.; Merino-Mansilla, A.; Olvera, A.; Miro, J.M.; Brander, C.; Alcami, J.; Sanders, R.W.; et al. Potent Induction of Envelope-Specific Antibody Responses by Virus-Like Particle Immunogens Based on HIV-1 Envelopes from Patients with Early Broadly Neutralizing Responses. J. Virol. 2022, 96, e0134321. [Google Scholar] [CrossRef]
- Vazquez, T.; Torrieri-Damard, L.; Pitoiset, F.; Levacher, B.; Vigneron, J.; Mayr, L.; Brimaud, F.; Bonnet, B.; Moog, C.; Klatzmann, D.; et al. Particulate Antigens Administrated by Intranasal and Intravaginal Routes in a Prime-Boost Strategy Improve HIV-Specific TFH Generation, High-Quality Antibodies and Long-Lasting Mucosal Immunity. Eur. J. Pharm. Biopharm. 2023, 191, 124–138. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).