Safety and Immunogenicity Study of a Bivalent Vaccine for Combined Prophylaxis of COVID-19 and Influenza in Non-Human Primates
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses, Cells and Proteins
2.1.1. Viruses
2.1.2. Cells
2.1.3. Proteins and Peptides
2.2. In Vitro Studies of the Experimental Vaccine Virus
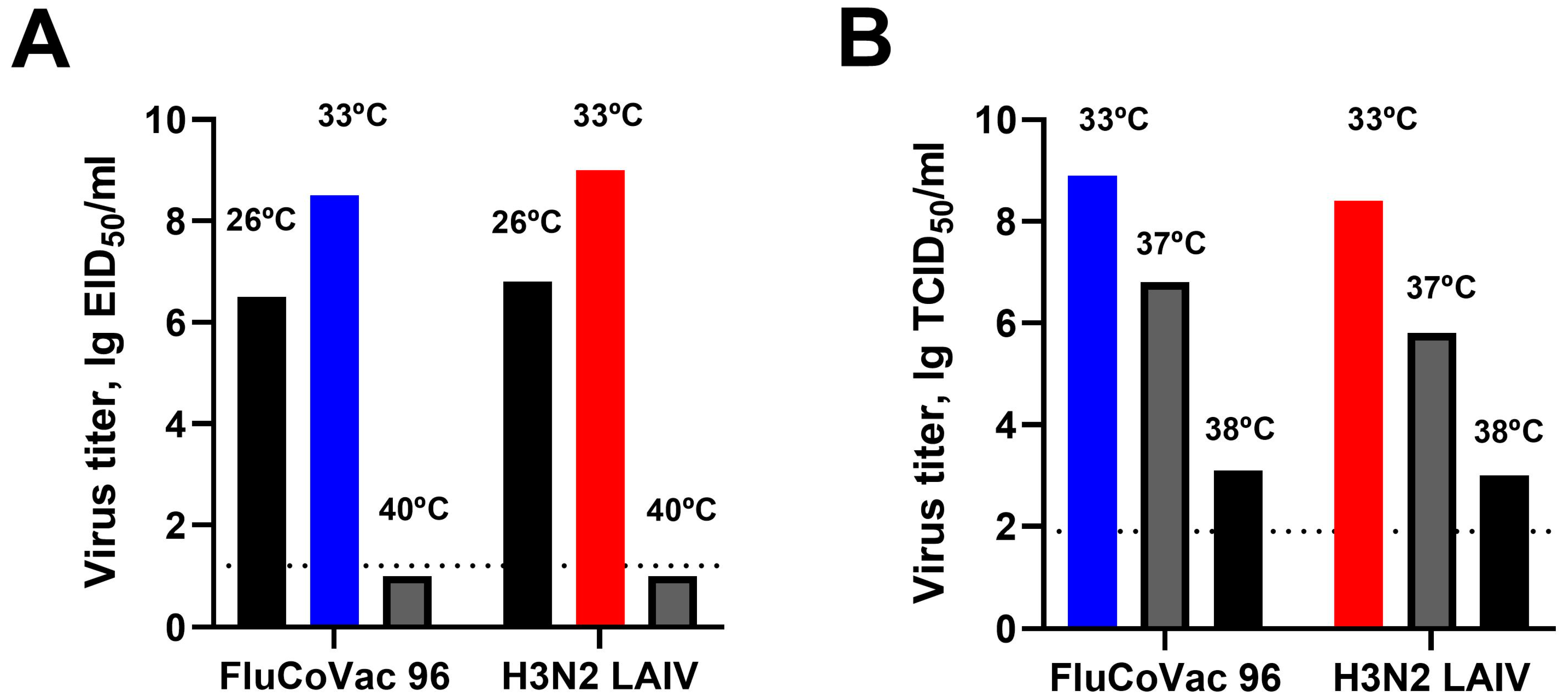
2.2.1. Replication in Eggs and MDCK Cells
2.2.2. Genetic Stability of Experimental Vaccine Virus
2.3. Experimental Manipulations on Rhesus Macaques
2.3.1. Immunization and Sample Collection
2.3.2. Clinical Assessment
2.3.3. Experimental Challenge with SARS-CoV-2
2.4. Assessment of Vaccine Virus Shedding
2.5. Assessment of Humoral Immune Responses
2.5.1. Hemagglutination Inhibition (HAI) Assay
2.5.2. ELISA
2.5.3. Microneutralization Assay
2.6. Assessment of Cell-Mediated Immune Responses
2.6.1. ELISPOT
2.6.2. Intracellular Cytokine Staining (ICS)
2.7. Histopathological Analysis
2.8. Statistical Analyses
3. Results
3.1. In Vitro Characterization of the Recombinant LAIV/CoV-2 Virus (FluCoVac-96)
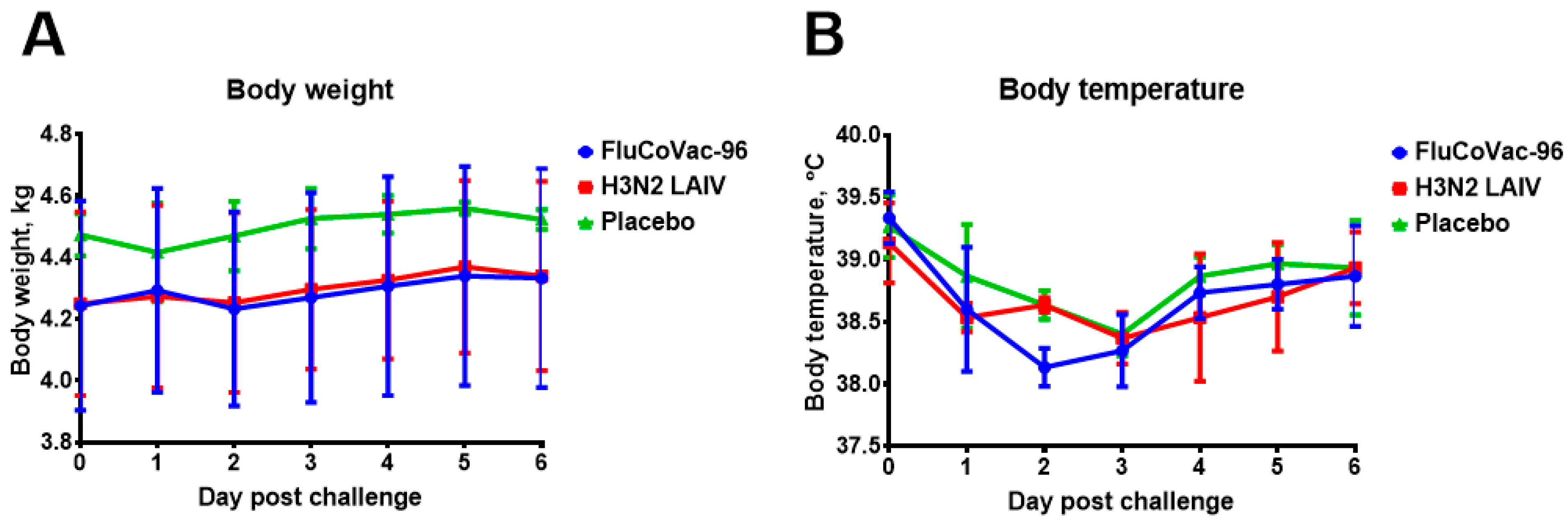
3.2. Safety of the FluCoVac-96 Strain in Rhesus Monkeys
3.2.1. Blood Parameters of Animals after Immunization
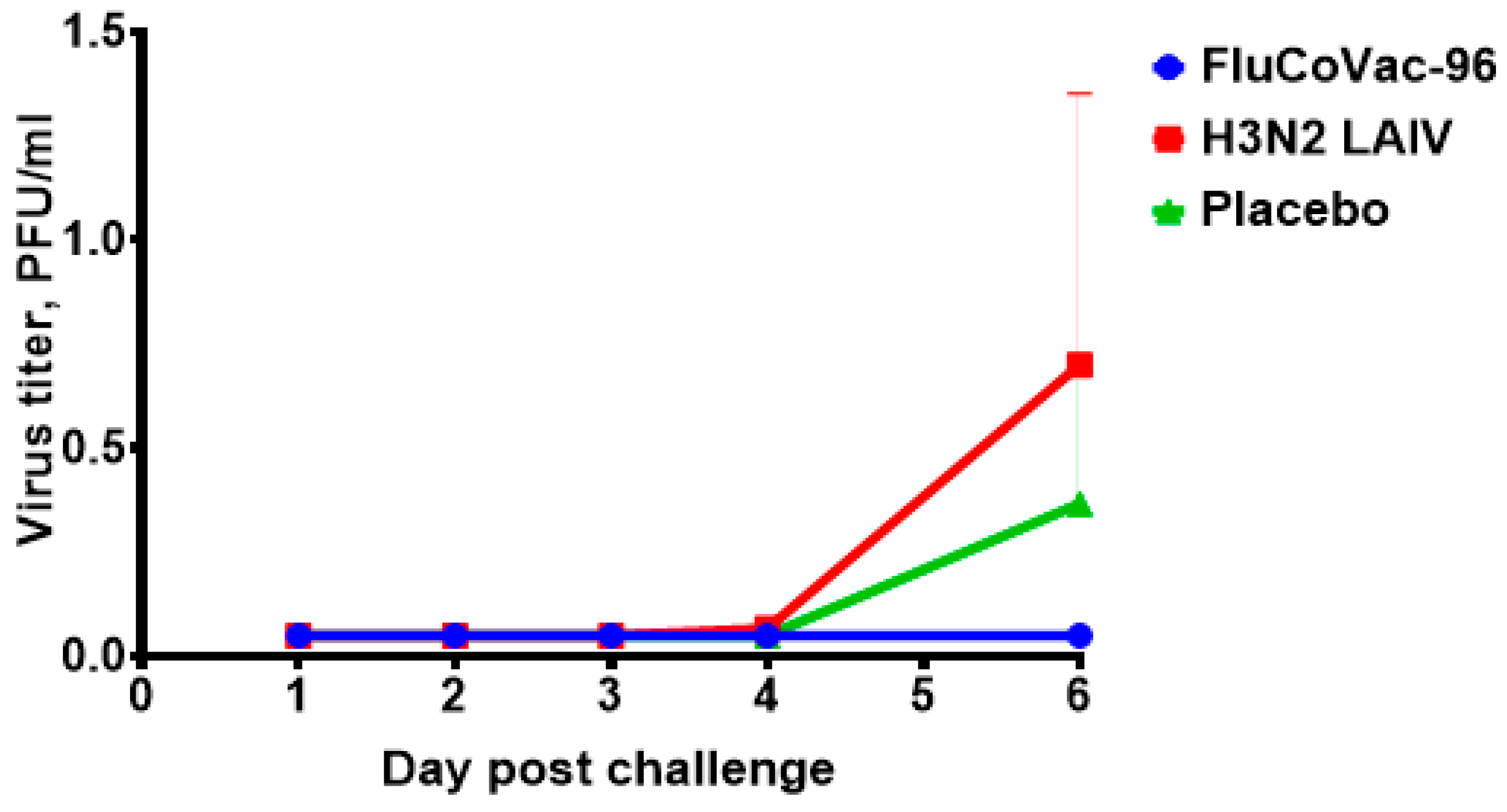
3.2.2. Replication of Vaccine Viruses in the Upper Respiratory Tract of Monkeys
3.3. Immunogenicity of the FluCoVac-96 Strain in Rhesus Monkeys
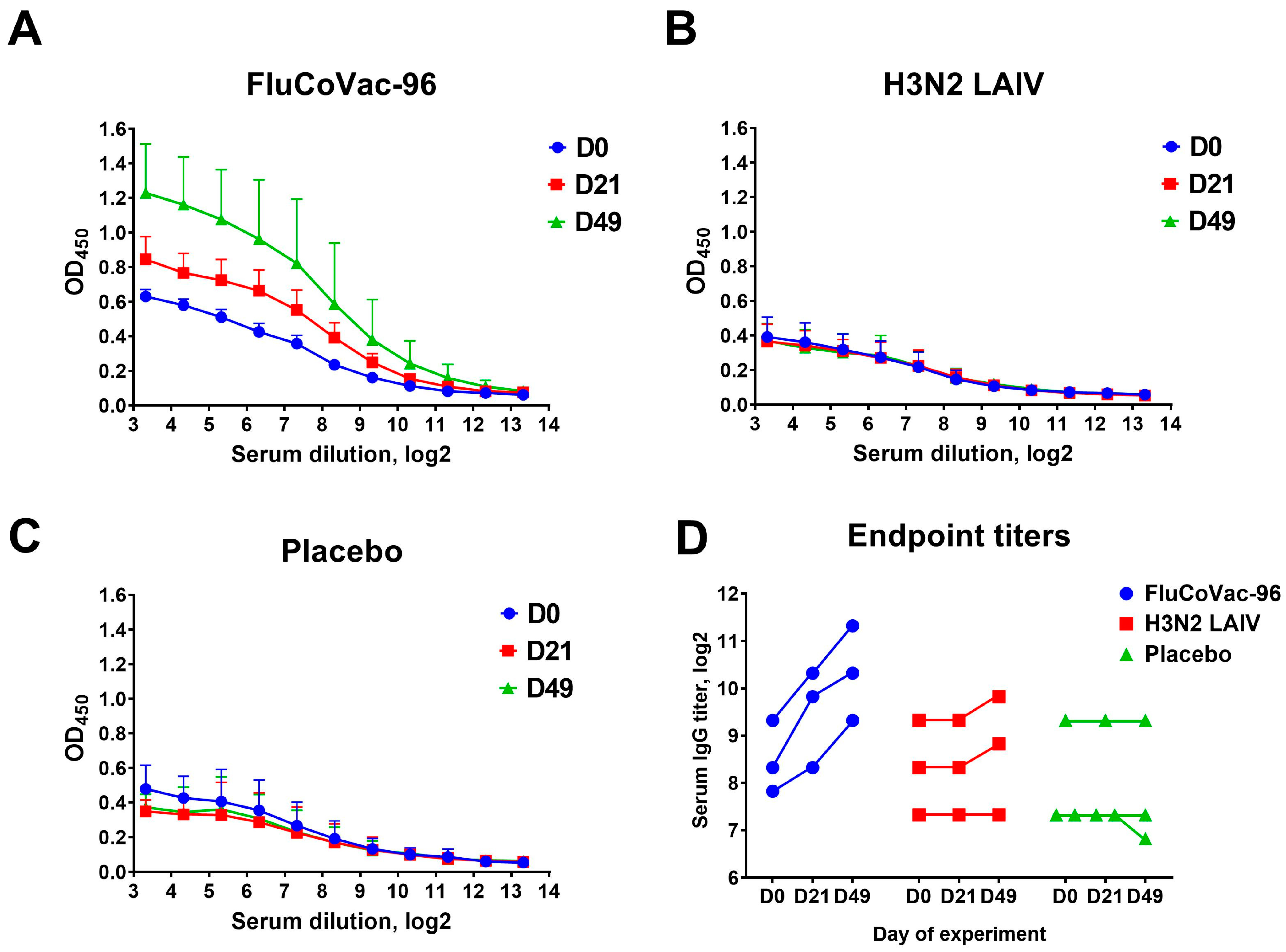
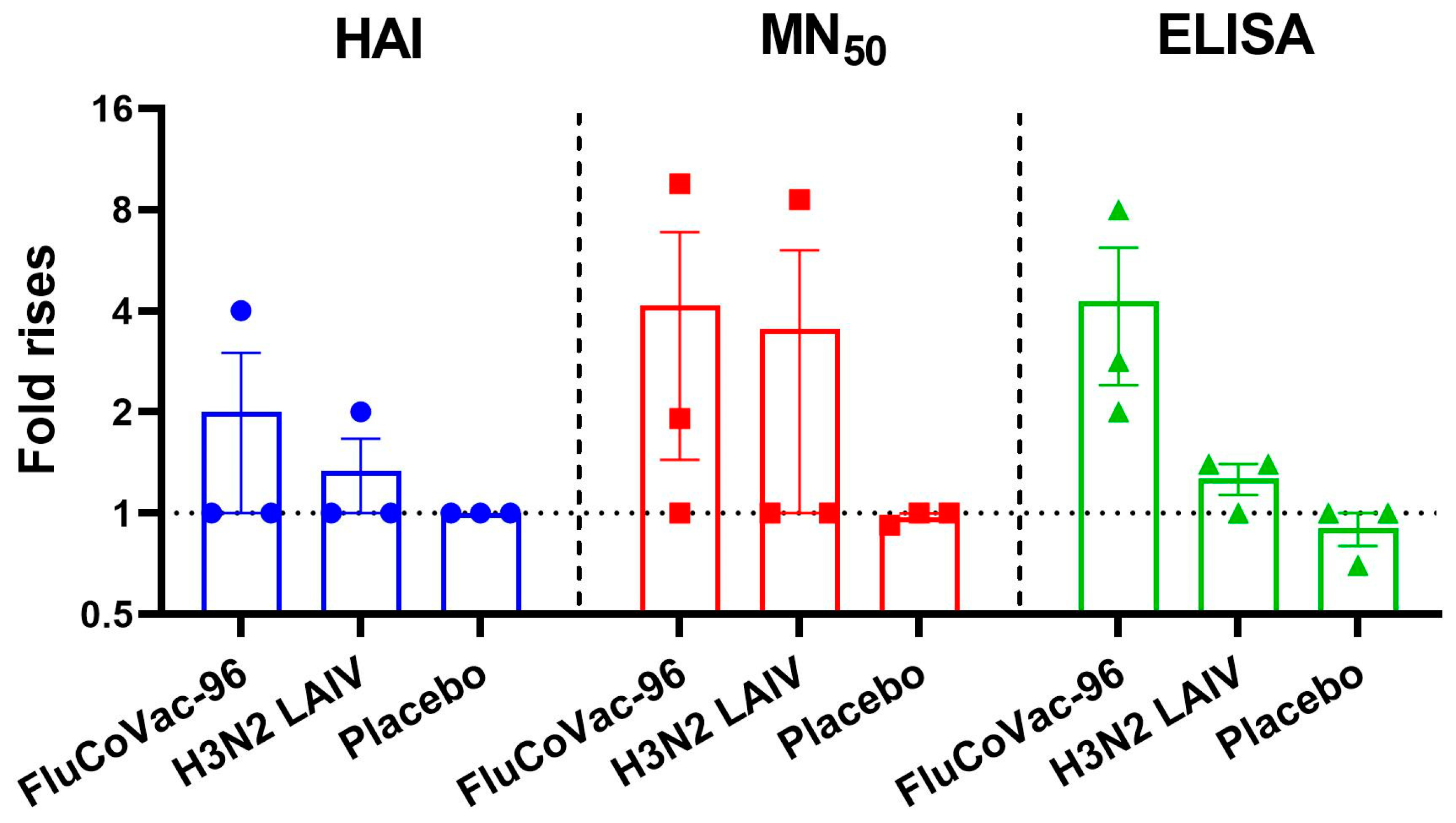
3.3.1. Serum Antibodies to Influenza Virus
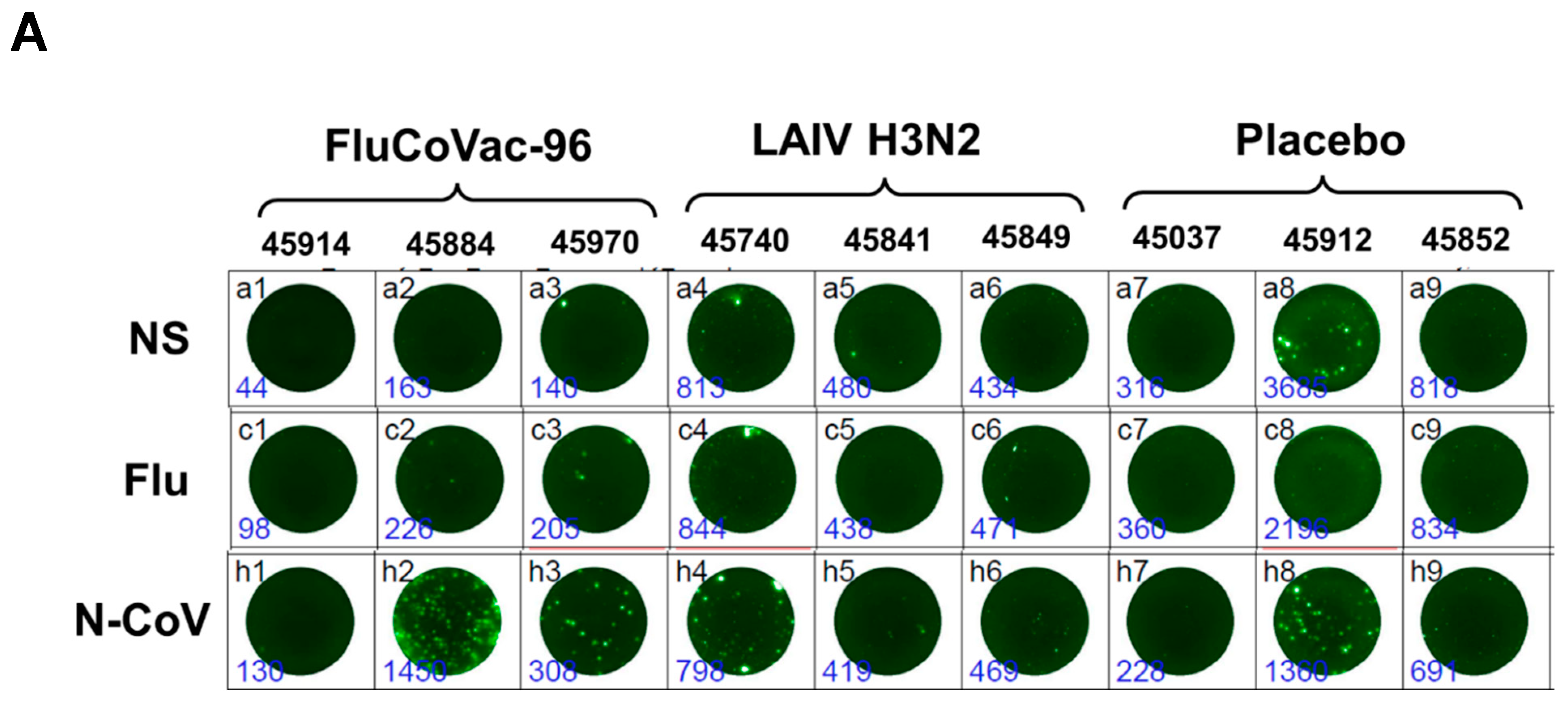
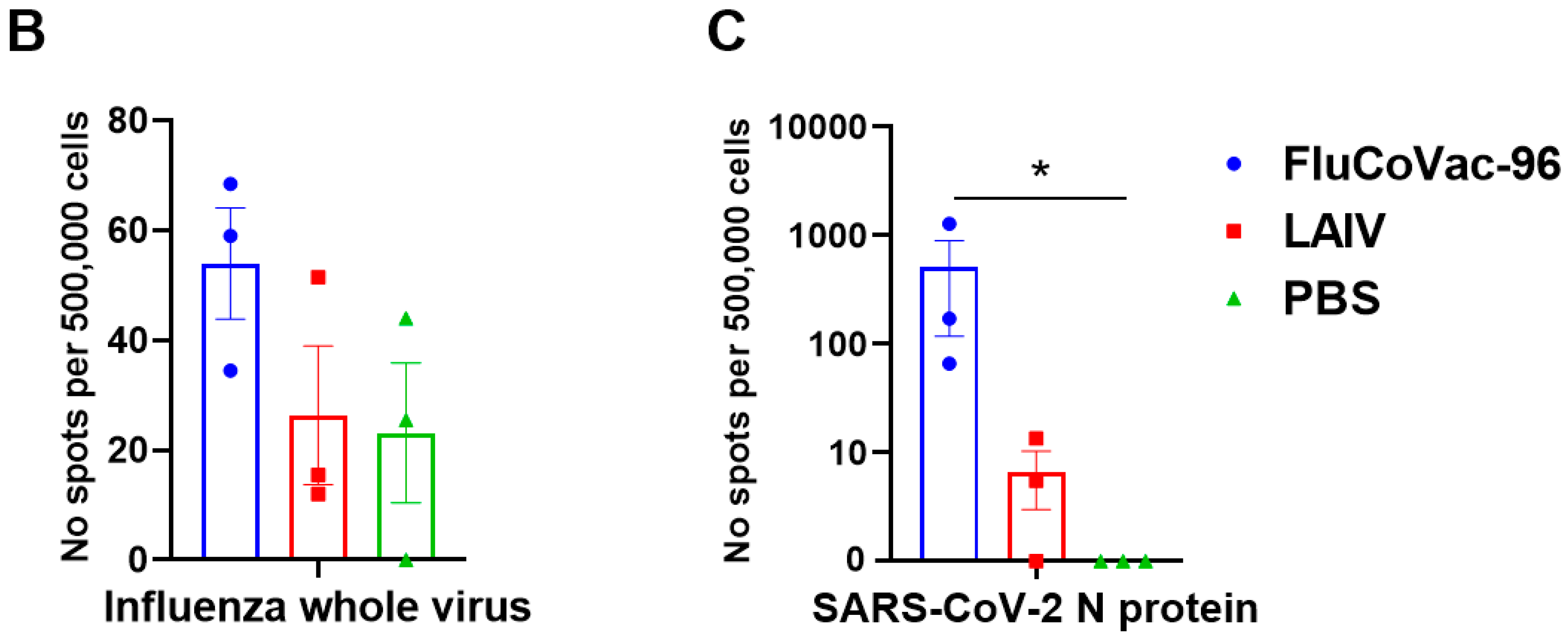
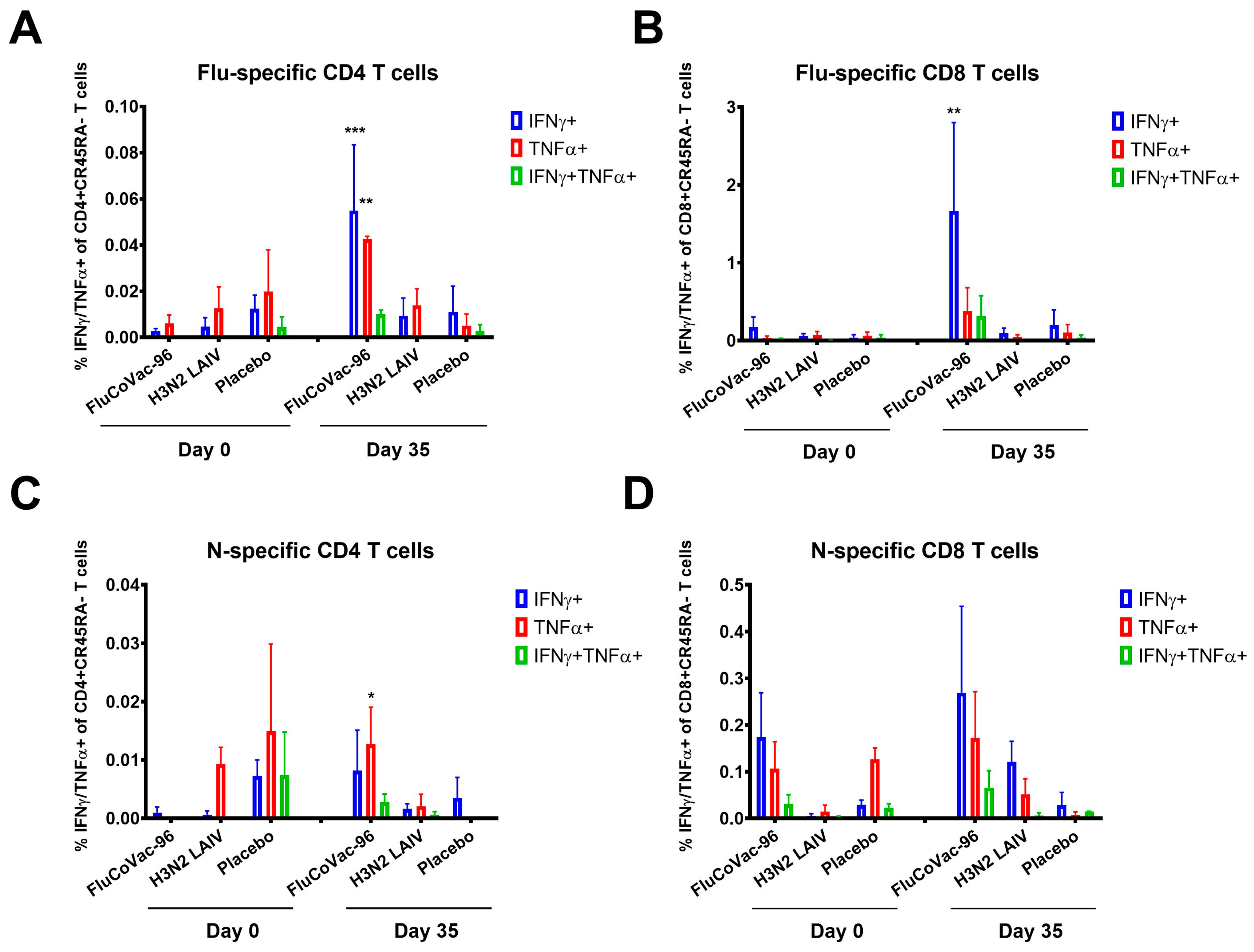
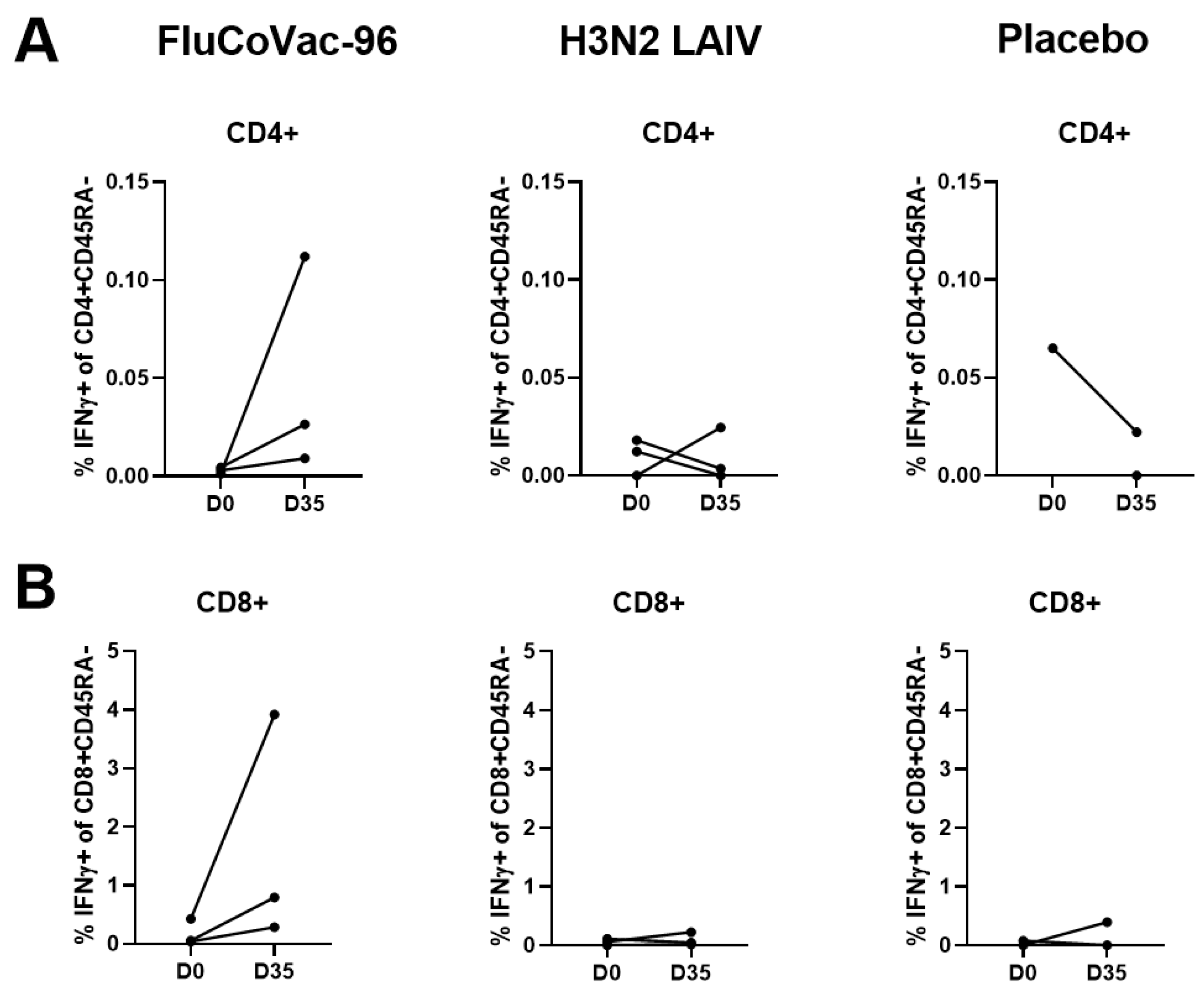
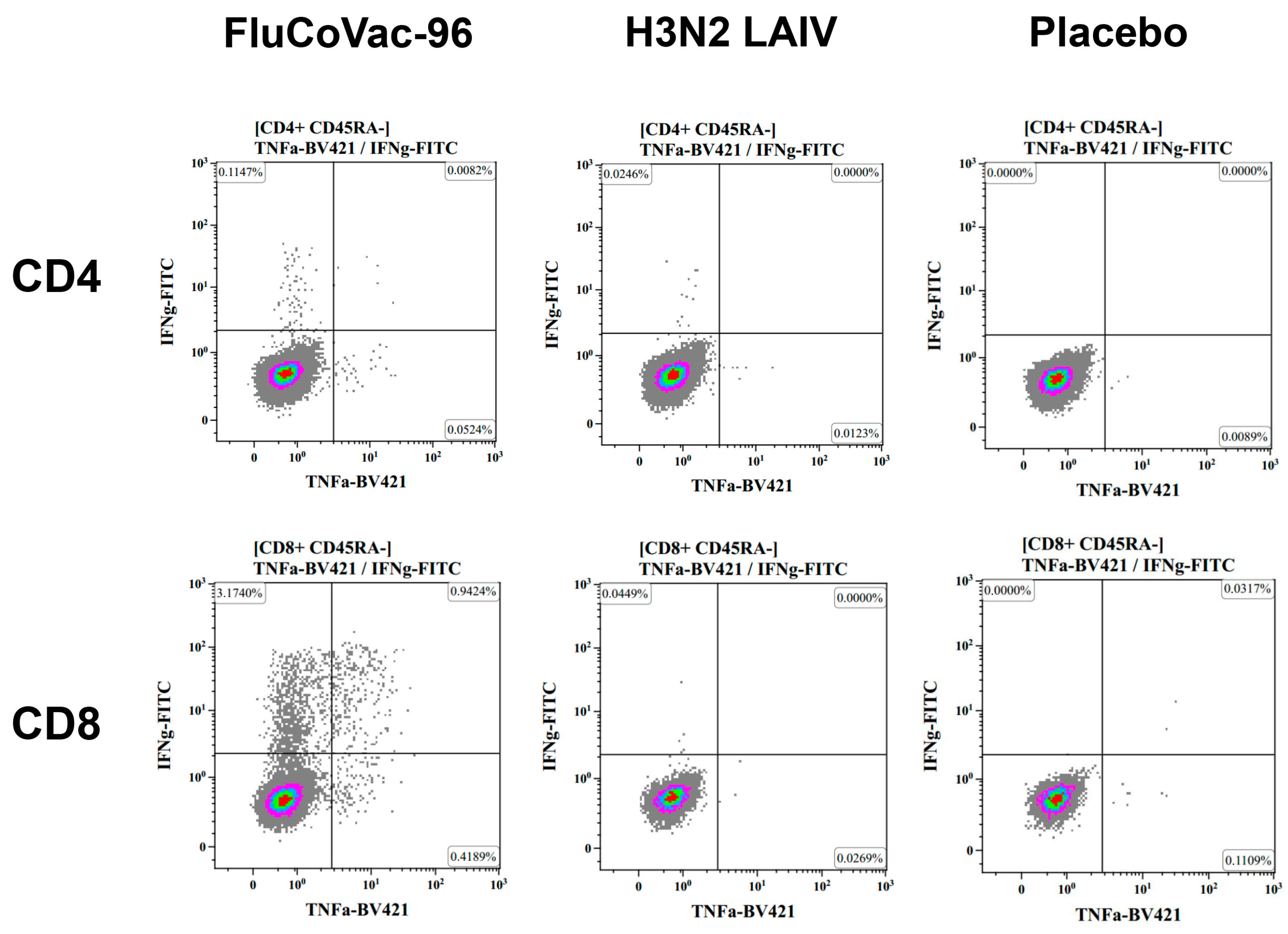
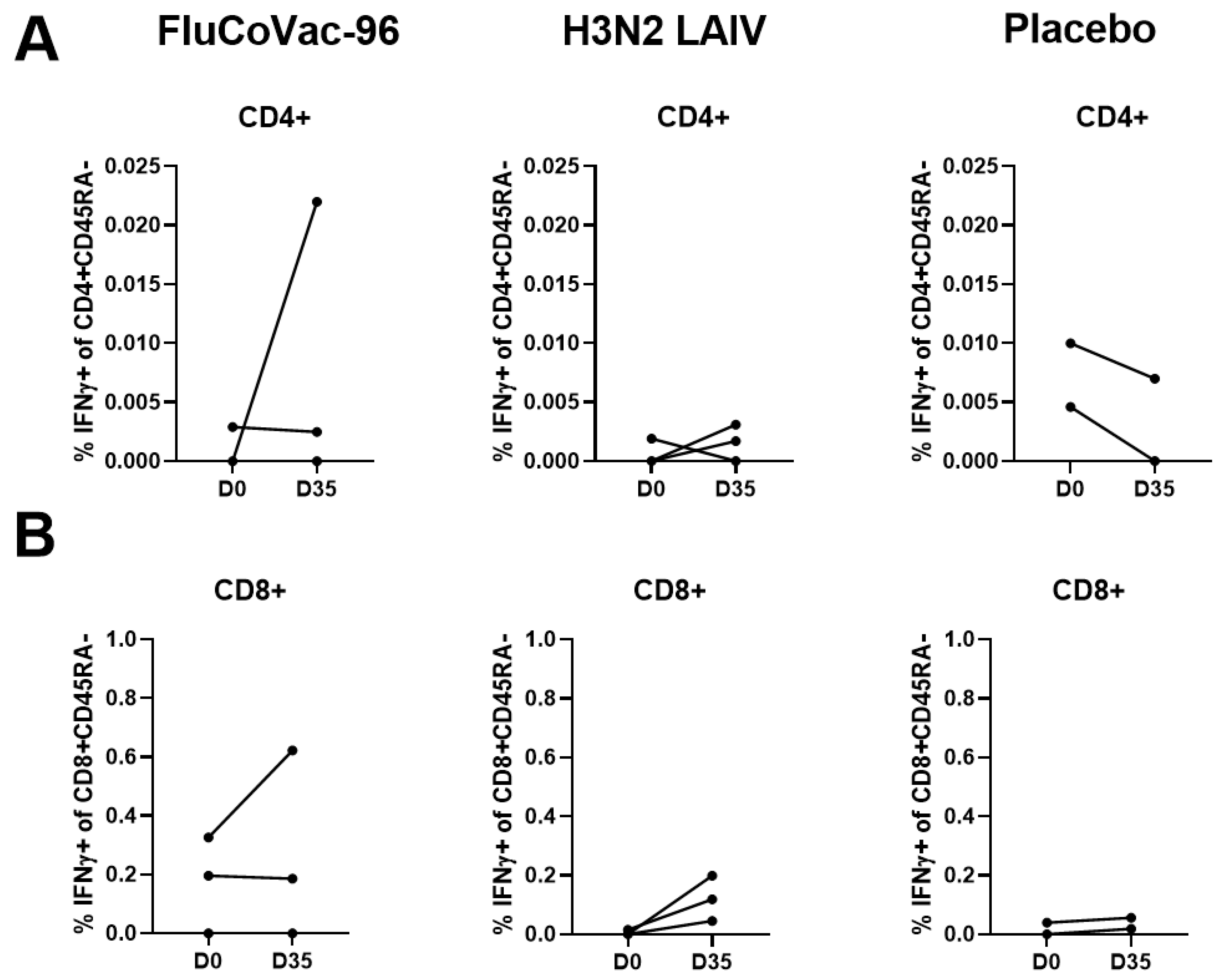
3.3.2. Cell-Mediated Immune Responses to SARS-CoV-2 and Influenza Virus
IFN-γ FluoroSpot Assay
Intracellular Cytokine Staining with Flow Cytometry
3.4. Protection against Challenge with SARS-CoV-2 Virus
Histopathology Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, A.T.; Linster, M.; Tan, C.W.; Le Bert, N.; Chia, W.N.; Kunasegaran, K.; Zhuang, Y.; Tham, C.Y.L.; Chia, A.; Smith, G.J.D.; et al. Early Induction of Functional SARS-CoV-2-Specific T Cells Associates with Rapid Viral Clearance and Mild Disease in COVID-19 Patients. Cell Rep. 2021, 34, 108728. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Strålin, K.; Gorin, J.-B.; Olsson, A.; Llewellyn-Lacey, S.; Kamal, H.; Bogdanovic, G.; Muschiol, S.; et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 2020, 183, 158–168.e14. [Google Scholar] [CrossRef] [PubMed]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of Protection against SARS-CoV-2 in Rhesus Macaques. Nature 2021, 590, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Markov, N.S.; Ren, Z.; Senkow, K.J.; Grant, R.A.; Gao, C.A.; Malsin, E.S.; Sichizya, L.; Kihshen, H.; Helmin, K.A.; Jovisic, M.; et al. Distinctive evolution of alveolar T cell responses is associated with clinical outcomes in unvaccinated patients with SARS-CoV-2 pneumonia. Nat. Immunol. 2024, 25, 1607–1622. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Kuwahara, K.; Li, L.; Liu, Z.; Li, T.; Zhu, H.; Liu, J.; Xu, Y.; Xie, J.; et al. Immunodominant SARS Coronavirus Epitopes in Humans Elicited Both Enhancing and Neutralizing Effects on Infection in Non-Human Primates. ACS Infect. Dis. 2016, 2, 361–376. [Google Scholar] [CrossRef]
- Zayou, L.; Prakash, S.; Dhanushkodi, N.R.; Quadiri, A.; Ibraim, I.C.; Singer, M.; Salem, A.; Shaik, A.M.; Suzer, B.; Chilukuri, A.; et al. A Multi-Epitope/CXCL11 Prime/Pull Coronavirus Mucosal Vaccine Boosts the Frequency and the Function of Lung-Resident Memory CD4+ and CD8+ T Cells and Enhanced Protection against COVID-19-like Symptoms and Death Caused by SARS-CoV-2 Infection. J. Virol. 2023, 97, e01096-23. [Google Scholar] [CrossRef]
- Abdelaziz, M.O.; Raftery, M.J.; Weihs, J.; Bielawski, O.; Edel, R.; Köppke, J.; Vladimirova, D.; Adler, J.M.; Firsching, T.; Voß, A.; et al. Early Protective Effect of a (“Pan”) Coronavirus Vaccine (PanCoVac) in Roborovski Dwarf Hamsters after Single-Low Dose Intranasal Administration. Front. Immunol. 2023, 14, 1166765. [Google Scholar] [CrossRef]
- Perdiguero, B.; Marcos-Villar, L.; López-Bravo, M.; Sánchez-Cordón, P.J.; Zamora, C.; Valverde, J.R.; Sorzano, C.Ó.S.; Sin, L.; Álvarez, E.; Ramos, M.; et al. Immunogenicity and Efficacy of a Novel Multi-Patch SARS-CoV-2/COVID-19 Vaccine Candidate. Front. Immunol. 2023, 14, 1160065. [Google Scholar] [CrossRef]
- Arieta, C.M.; Xie, Y.J.; Rothenberg, D.A.; Diao, H.; Harjanto, D.; Meda, S.; Marquart, K.; Koenitzer, B.; Sciuto, T.E.; Lobo, A.; et al. The T-Cell-Directed Vaccine BNT162b4 Encoding Conserved Non-Spike Antigens Protects Animals from Severe SARS-CoV-2 Infection. Cell 2023, 186, 2392–2409.e21. [Google Scholar] [CrossRef]
- Boulton, S.; Poutou, J.; Gill, R.; Alluqmani, N.; He, X.; Singaravelu, R.; Crupi, M.J.F.; Petryk, J.; Austin, B.; Angka, L.; et al. A T Cell-Targeted Multi-Antigen Vaccine Generates Robust Cellular and Humoral Immunity against SARS-CoV-2 Infection. Mol. Ther. Methods Clin. Dev. 2023, 31, 101110. [Google Scholar] [CrossRef]
- Wang, C.Y.; Kuo, B.-S.; Lee, Y.-H.; Ho, Y.-H.; Pan, Y.-H.; Yang, Y.-T.; Chang, H.-C.; Fu, L.-F.; Peng, W.-J. UB-612 Pan-SARS-CoV-2 T Cell Immunity-Promoting Vaccine Protects against COVID-19 Moderate-Severe Disease. iScience 2024, 27, 108887. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, C.Y.; Kuo, H.-K.; Peng, W.-J.; Huang, J.-H.; Kuo, B.-S.; Lin, F.; Liu, Y.-J.; Liu, Z.; Wu, H.-T.; et al. A Novel RBD-Protein/Peptide Vaccine Elicits Broadly Neutralizing Antibodies and Protects Mice and Macaques against SARS-CoV-2. Emerg. Microbes Infect. 2022, 11, 2724–2734. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Hwang, K.-P.; Kuo, H.-K.; Peng, W.-J.; Shen, Y.-H.; Kuo, B.-S.; Huang, J.-H.; Liu, H.; Ho, Y.-H.; Lin, F.; et al. A Multitope SARS-CoV-2 Vaccine Provides Long-Lasting B Cell and T Cell Immunity against Delta and Omicron Variants. J. Clin. Investig. 2022, 132, e157707. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, J.S.; Tandler, C.; Marconato, M.; Nelde, A.; Habibzada, T.; Rittig, S.M.; Tegeler, C.M.; Maringer, Y.; Jaeger, S.U.; Denk, M.; et al. Phase I/II Trial of a Peptide-Based COVID-19 T-Cell Activator in Patients with B-Cell Deficiency. Nat. Commun. 2023, 14, 5032. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, P.; Yuan, L.; Zhang, L.; Zhang, L.; Zhao, H.; Chen, C.; Wang, X.; Han, J.; Chen, Y.; et al. A Live Attenuated Virus-Based Intranasal COVID-19 Vaccine Provides Rapid, Prolonged, and Broad Protection against SARS-CoV-2. Sci. Bull. 2022, 67, 1372–1387. [Google Scholar] [CrossRef]
- Loes, A.N.; Gentles, L.E.; Greaney, A.J.; Crawford, K.H.D.; Bloom, J.D. Attenuated Influenza Virions Expressing the SARS-CoV-2 Receptor-Binding Domain Induce Neutralizing Antibodies in Mice. Viruses 2020, 12, 987. [Google Scholar] [CrossRef]
- Koonpaew, S.; Kaewborisuth, C.; Srisutthisamphan, K.; Wanitchang, A.; Thaweerattanasinp, T.; Saenboonrueng, J.; Poonsuk, S.; Jengarn, J.; Viriyakitkosol, R.; Kramyu, J.; et al. A Single-Cycle Influenza A Virus-Based SARS-CoV-2 Vaccine Elicits Potent Immune Responses in a Mouse Model. Vaccines 2021, 9, 850. [Google Scholar] [CrossRef]
- Chaparian, R.R.; Harding, A.T.; Hamele, C.E.; Riebe, K.; Karlsson, A.; Sempowski, G.D.; Heaton, N.S.; Heaton, B.E. A Virion-Based Combination Vaccine Protects against Influenza and SARS-CoV-2 Disease in Mice. J. Virol. 2022, 96, e00689-22. [Google Scholar] [CrossRef]
- Moser, M.J.; Hill-Batorski, L.; Bowen, R.A.; Matejka, S.M.; Marshall, D.; Kawaoka, Y.; Neumann, G.; Bilsel, P. Intranasal Single-Replication Influenza Vector Induces Cross-Reactive Serum and Mucosal Antibodies against SARS-CoV-2 Variants. Vaccines 2023, 11, 1063. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, L.; Li, Y.; Liu, Q.; Deng, L.; Lu, Y.; Zhang, X.; Li, S.; Ge, J.; Bu, Z.; et al. An Influenza Virus Vector Candidate Vaccine Stably Expressing SARS-CoV-2 Receptor-Binding Domain Produces High and Long-Lasting Neutralizing Antibodies in Mice. Vet. Microbiol. 2022, 271, 109491. [Google Scholar] [CrossRef]
- Isakova-Sivak, I.; Stepanova, E.; Matyushenko, V.; Niskanen, S.; Mezhenskaya, D.; Bazhenova, E.; Krutikova, E.; Kotomina, T.; Prokopenko, P.; Neterebskii, B.; et al. Development of a T Cell-Based COVID-19 Vaccine Using a Live Attenuated Influenza Vaccine Viral Vector. Vaccines 2022, 10, 1142. [Google Scholar] [CrossRef] [PubMed]
- Boonnak, K.; Paskel, M.; Matsuoka, Y.; Vogel, L.; Subbarao, K. Evaluation of Replication, Immunogenicity and Protective Efficacy of a Live Attenuated Cold-Adapted Pandemic H1N1 Influenza Virus Vaccine in Non-Human Primates. Vaccine 2012, 30, 5603–5610. [Google Scholar] [CrossRef] [PubMed]
- Skinner, J.A.; Zurawski, S.M.; Sugimoto, C.; Vinet-Oliphant, H.; Vinod, P.; Xue, Y.; Russell-Lodrigue, K.; Albrecht, R.A.; García-Sastre, A.; Salazar, A.M.; et al. Immunologic Characterization of a Rhesus Macaque H1N1 Challenge Model for Candidate Influenza Virus Vaccine Assessment. Clin. Vaccine Immunol. 2014, 21, 1668–1680. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Tiwary, M.; Wu, H.L.; Tailor, N.; Vendramelli, R.; Audet, J.; Warner, B.M.; Tierney, K.; Albietz, A.; Truong, T.; et al. Pandemic 1918 Influenza Virus Does Not Cause Lethal Infection in Rhesus or Cynomolgus Macaques. J. Virol. 2022, 96, e00728-22. [Google Scholar] [CrossRef]
- Berendt, R.F.; McDonough, W.E.; Walker, J.S. Persistence of Diplococcus Pneumoniae after Influenza Virus Infection in Macaca mulatta. Infect. Immun. 1974, 10, 369–374. [Google Scholar] [CrossRef]
- Berendt, R.F. Simian Model for the Evaluation of Immunity to Influenza. Infect. Immun. 1974, 9, 101–105. [Google Scholar] [CrossRef]
- Short, S.J.; Lubach, G.R.; Karasin, A.I.; Olsen, C.W.; Styner, M.; Knickmeyer, R.C.; Gilmore, J.H.; Coe, C.L. Maternal Influenza Infection During Pregnancy Impacts Postnatal Brain Development in the Rhesus Monkey. Biol. Psychiatry 2010, 67, 965–973. [Google Scholar] [CrossRef]
- McCarthy, K.R.; Von Holle, T.A.; Sutherland, L.L.; Oguin, T.H.; Sempowski, G.D.; Harrison, S.C.; Moody, M.A. Differential Immune Imprinting by Influenza Virus Vaccination and Infection in Nonhuman Primates. Proc. Natl. Acad. Sci. USA 2021, 118, e2026752118. [Google Scholar] [CrossRef]
- Shinya, K.; Gao, Y.; Cilloniz, C.; Suzuki, Y.; Fujie, M.; Deng, G.; Zhu, Q.; Fan, S.; Makino, A.; Muramoto, Y.; et al. Integrated Clinical, Pathologic, Virologic, and Transcriptomic Analysis of H5N1 Influenza Virus-Induced Viral Pneumonia in the Rhesus Macaque. J. Virol. 2012, 86, 6055–6066. [Google Scholar] [CrossRef]
- Davis, A.S.; Taubenberger, J.K.; Bray, M. The Use of Nonhuman Primates in Research on Seasonal, Pandemic and Avian Influenza, 1893–2014. Antivir. Res. 2015, 117, 75–98. [Google Scholar] [CrossRef]
- Zheng, H.; Li, H.; Guo, L.; Liang, Y.; Li, J.; Wang, X.; Hu, Y.; Wang, L.; Liao, Y.; Yang, F.; et al. Virulence and Pathogenesis of SARS-CoV-2 Infection in Rhesus Macaques: A Nonhuman Primate Model of COVID-19 Progression. PLoS Pathog. 2020, 16, e1008949. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhao, Y.; Yu, W.; Yang, Y.; Gao, J.; Wang, J.; Kuang, D.; Yang, M.; Yang, J.; Ma, C.; et al. Comparison of Nonhuman Primates Identified the Suitable Model for COVID-19. Signal Transduct. Target. Ther. 2020, 5, 157. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an Inactivated Vaccine Candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Qi, F.; Xu, Y.; Li, F.; Liu, P.; Liu, J.; Bao, L.; Deng, W.; Gao, H.; Xiang, Z.; et al. Age-related Rhesus Macaque Models of COVID-19. Anim. Models Exp. Med. 2020, 3, 93–97. [Google Scholar] [CrossRef]
- Witt, A.N.; Green, R.D.; Winterborn, A.N. A Meta-Analysis of Rhesus Macaques (Macaca mulatta), Cynomolgus Macaques (Macaca fascicularis), African Green Monkeys (Chlorocebus aethiops), and Ferrets (Mustela putorius furo) as Large Animal Models for COVID-19. Comp. Med. 2021, 71, 433–441. [Google Scholar] [CrossRef]
- Yu, J.; Tostanoski, L.H.; Peter, L.; Mercado, N.B.; McMahan, K.; Mahrokhian, S.H.; Nkolola, J.P.; Liu, J.; Li, Z.; Chandrashekar, A.; et al. DNA Vaccine Protection against SARS-CoV-2 in Rhesus Macaques. Science 2020, 369, 806–811. [Google Scholar] [CrossRef]
- Vogel, A.B.; Kanevsky, I.; Che, Y.; Swanson, K.A.; Muik, A.; Vormehr, M.; Kranz, L.M.; Walzer, K.C.; Hein, S.; Güler, A.; et al. BNT162b Vaccines Protect Rhesus Macaques from SARS-CoV-2. Nature 2021, 592, 283–289. [Google Scholar] [CrossRef]
- Mercado, N.B.; Zahn, R.; Wegmann, F.; Loos, C.; Chandrashekar, A.; Yu, J.; Liu, J.; Peter, L.; McMahan, K.; Tostanoski, L.H.; et al. Single-Shot Ad26 Vaccine Protects against SARS-CoV-2 in Rhesus Macaques. Nature 2020, 586, 583. [Google Scholar] [CrossRef]
- Tseng, C.-T.; Sbrana, E.; Iwata-Yoshikawa, N.; Newman, P.C.; Garron, T.; Atmar, R.L.; Peters, C.J.; Couch, R.B. Immunization with SARS Coronavirus Vaccines Leads to Pulmonary Immunopathology on Challenge with the SARS Virus. PLoS ONE 2012, 7, e35421. [Google Scholar] [CrossRef]
- Bigay, J.; Le Grand, R.; Martinon, F.; Maisonnasse, P. Vaccine-Associated Enhanced Disease in Humans and Animal Models: Lessons and Challenges for Vaccine Development. Front. Microbiol. 2022, 13, 932408. [Google Scholar] [CrossRef]
- Ebenig, A.; Muraleedharan, S.; Kazmierski, J.; Todt, D.; Auste, A.; Anzaghe, M.; Gömer, A.; Postmus, D.; Gogesch, P.; Niles, M.; et al. Vaccine-Associated Enhanced Respiratory Pathology in COVID-19 Hamsters after TH2-Biased Immunization. Cell Rep. 2022, 40, 111214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Magazine, N.; McGee, M.C.; Carossino, M.; Veggiani, G.; Kousoulas, K.G.; August, A.; Huang, W. Th2 and Th17-Associated Immunopathology Following SARS-CoV-2 Breakthrough Infection in Spike-Vaccinated ACE2-Humanized Mice. J. Med. Virol. 2024, 96, e29408. [Google Scholar] [CrossRef] [PubMed]
- Matyushenko, V.; Isakova-Sivak, I.; Kudryavtsev, I.; Goshina, A.; Chistyakova, A.; Stepanova, E.; Prokopenko, P.; Sychev, I.; Rudenko, L. Detection of IFNγ-Secreting CD4+ and CD8+ Memory T Cells in COVID-19 Convalescents after Stimulation of Peripheral Blood Mononuclear Cells with Live SARS-CoV-2. Viruses 2021, 13, 1490. [Google Scholar] [CrossRef] [PubMed]
- Rak, A.; Gorbunov, N.; Kostevich, V.; Sokolov, A.; Prokopenko, P.; Rudenko, L.; Isakova-Sivak, I. Assessment of Immunogenic and Antigenic Properties of Recombinant Nucleocapsid Proteins of Five SARS-CoV-2 Variants in a Mouse Model. Viruses 2023, 15, 230. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty per Cent Endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Directive 2010/63/EU of the European Parliament and of the Council of September 22, 2010, on the Protection of Animals Used for Scientific Purposes. 2010. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0063 (accessed on 24 September 2024).
- Shu, B.; Wu, K.-H.; Emery, S.; Villanueva, J.; Johnson, R.; Guthrie, E.; Berman, L.; Warnes, C.; Barnes, N.; Klimov, A.; et al. Design and Performance of the CDC Real-Time Reverse Transcriptase PCR Swine Flu Panel for Detection of 2009 A (H1N1) Pandemic Influenza Virus. J. Clin. Microbiol. 2011, 49, 2614–2619. [Google Scholar] [CrossRef]
- Matyushenko, V.; Isakova-Sivak, I.; Smolonogina, T.; Dubrovina, I.; Tretiak, T.; Rudenko, L. Genotyping Assay for Differentiation of Wild-Type and Vaccine Viruses in Subjects Immunized with Live Attenuated Influenza Vaccine. PLoS ONE 2017, 12, e0180497. [Google Scholar] [CrossRef]
- World Health Organization. WHO Manual on Animal Influenza Diagnosis and Surveillance; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Reactivity Database. Available online: https://www.nhpreagents.org/ReactivityDatabase (accessed on 10 June 2024).
- Magalhaes, I.; Vudattu, N.K.; Ahmed, R.K.; Kühlmann-Berenzon, S.; Ngo, Y.; Sizemore, D.R.; Wehlin, L.; Weichold, F.; Andersson, J.; Skeiky, Y.A.; et al. High Content Cellular Immune Profiling Reveals Differences between Rhesus Monkeys and Men. Immunology 2010, 131, 128–140. [Google Scholar] [CrossRef]
- Bjornson-Hooper, Z.B.; Fragiadakis, G.K.; Spitzer, M.H.; Chen, H.; Madhireddy, D.; Hu, K.; Lundsten, K.; McIlwain, D.R.; Nolan, G.P. A Comprehensive Atlas of Immunological Differences between Humans, Mice, and Non-Human Primates. Front. Immunol. 2022, 13, 867015. [Google Scholar] [CrossRef]
- Pitcher, C.J.; Hagen, S.I.; Walker, J.M.; Lum, R.; Mitchell, B.L.; Maino, V.C.; Axthelm, M.K.; Picker, L.J. Development and Homeostasis of T Cell Memory in Rhesus Macaque. J. Immunol. 2002, 168, 29–43. [Google Scholar] [CrossRef]
- WHO. Recommendations to Assure the Quality, Safety and Efficacy of Influenza Vaccines (Human, Live Attenuated) for Intranasal Administration, Annex 4, TRS No 977. Available online: https://www.who.int/publications/m/item/influenza-attenuated-intranasal-administration-annex-4-trs-no-977 (accessed on 5 September 2024).
- Kiseleva, I.; Su, Q.; Toner, T.J.; Szymkowiak, C.; Kwan, W.-S.; Rudenko, L.; Shaw, A.R.; Youil, R. Cell-Based Assay for the Determination of Temperature Sensitive and Cold Adapted Phenotypes of Influenza Viruses. J. Virol. Methods 2004, 116, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Isakova-Sivak, I.; Chen, L.-M.; Matsuoka, Y.; Voeten, J.T.M.; Kiseleva, I.; Heldens, J.G.M.; van den Bosch, H.; Klimov, A.; Rudenko, L.; Cox, N.J.; et al. Genetic Bases of the Temperature-Sensitive Phenotype of a Master Donor Virus Used in Live Attenuated Influenza Vaccines: A/Leningrad/134/17/57 (H2N2). Virology 2011, 412, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Serum Institute of India Pvt. Ltd. Nasovac-S Influenza Vaccine, Live, Attenuated (Human) [Package Insert]; Serum Institute of India Pvt. Ltd.: Pune, India, February 2021; Available online: https://extranet.who.int/prequal/sites/default/files/vwa_vaccine/pq_297_Nasovac-S_1dose_SII_PI_NH-2021.pdf (accessed on 24 September 2024).
- Grohskopf, L.A. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023–24 Influenza Season. MMWR Recomm. Rep. 2023, 72, 1–25. [Google Scholar] [CrossRef]
- Isakova-Sivak, I.; Matyushenko, V.; Stepanova, E.; Matushkina, A.; Kotomina, T.; Mezhenskaya, D.; Prokopenko, P.; Kudryavtsev, I.; Kopeykin, P.; Sivak, K.; et al. Recombinant Live Attenuated Influenza Vaccine Viruses Carrying Conserved T-Cell Epitopes of Human Adenoviruses Induce Functional Cytotoxic T-Cell Responses and Protect Mice against Both Infections. Vaccines 2020, 8, 196. [Google Scholar] [CrossRef]
- Kotomina, T.; Korenkov, D.; Matyushenko, V.; Prokopenko, P.; Rudenko, L.; Isakova-Sivak, I. Live Attenuated Influenza Vaccine Viral Vector Induces Functional Cytotoxic T-Cell Immune Response against Foreign CD8+ T-Cell Epitopes Inserted into NA and NS1 Genes Using the 2A Self-Cleavage Site. Hum. Vaccines Immunother. 2018, 14, 2964–2970. [Google Scholar] [CrossRef]
- Matyushenko, V.; Kotomina, T.; Kudryavtsev, I.; Mezhenskaya, D.; Prokopenko, P.; Matushkina, A.; Sivak, K.; Muzhikyan, A.; Rudenko, L.; Isakova-Sivak, I. Conserved T-Cell Epitopes of Respiratory Syncytial Virus (RSV) Delivered by Recombinant Live Attenuated Influenza Vaccine Viruses Efficiently Induce RSV-Specific Lung-Localized Memory T Cells and Augment Influenza-Specific Resident Memory T-Cell Responses. Antivir. Res. 2020, 182, 104864. [Google Scholar] [CrossRef]
- Mooij, P.; Balla-Jhagjhoorsingh, S.S.; Beenhakker, N.; van Haaften, P.; Baak, I.; Nieuwenhuis, I.G.; Heidari, S.; Wolf, H.; Frachette, M.-J.; Bieler, K.; et al. Comparison of Human and Rhesus Macaque T-Cell Responses Elicited by Boosting with NYVAC Encoding Human Immunodeficiency Virus Type 1 Clade C Immunogens. J. Virol. 2009, 83, 5881–5889. [Google Scholar] [CrossRef]
- Vaccari, M.; Franchini, G. Memory T Cells in Rhesus Macaques. Adv. Exp. Med. Biol. 2010, 684, 126–144. [Google Scholar] [CrossRef]
- Burel, J.G.; Apte, S.H.; Groves, P.L.; McCarthy, J.S.; Doolan, D.L. Polyfunctional and IFN-γ Monofunctional Human CD4+ T Cell Populations Are Molecularly Distinct. JCI Insight 2017, 2, e87499. [Google Scholar] [CrossRef]
- Imai, N.; Tawara, I.; Yamane, M.; Muraoka, D.; Shiku, H.; Ikeda, H. CD4+ T Cells Support Polyfunctionality of Cytotoxic CD8+ T Cells with Memory Potential in Immunological Control of Tumor. Cancer Sci. 2020, 111, 1958–1968. [Google Scholar] [CrossRef]
- Jacob-Dolan, C.; Yu, J.; McMahan, K.; Giffin, V.; Chandrashekar, A.; Martinot, A.J.; Anioke, T.; Powers, O.C.; Hall, K.; Hope, D.; et al. Immunogenicity and Protective Efficacy of GBP510/AS03 Vaccine against SARS-CoV-2 Delta Challenge in Rhesus Macaques. NPJ Vaccines 2023, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Hu, X.; Zhou, Y.; Rao, J.; Zhang, X.; Peng, Y.; Zhao, J.; Yao, Y.; Liu, K.; Liang, M.; et al. Infection and Pathogenesis of the Delta Variant of SARS-CoV-2 in Rhesus Macaque. Virol. Sin. 2022, 37, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Gagne, M.; Corbett, K.S.; Flynn, B.J.; Foulds, K.E.; Wagner, D.A.; Andrew, S.F.; Todd, J.-P.M.; Honeycutt, C.C.; McCormick, L.; Nurmukhambetova, S.T.; et al. Protection from SARS-CoV-2 Delta One Year after mRNA-1273 Vaccination in Rhesus Macaques Coincides with Anamnestic Antibody Response in the Lung. Cell 2022, 185, 113–130.e15. [Google Scholar] [CrossRef] [PubMed]
- Salguero, F.J.; White, A.D.; Slack, G.S.; Fotheringham, S.A.; Bewley, K.R.; Gooch, K.E.; Longet, S.; Humphries, H.E.; Watson, R.J.; Hunter, L.; et al. Comparison of Rhesus and Cynomolgus Macaques as an Infection Model for COVID-19. Nat. Commun. 2021, 12, 1260. [Google Scholar] [CrossRef]
- Routhu, N.K.; Gangadhara, S.; Lai, L.; Davis Gardner, M.E.; Floyd, K.; Shiferaw, A.; Bartsch, Y.C.; Fischinger, S.; Khoury, G.; Rahman, S.A.; et al. A Modified Vaccinia Ankara Vaccine Expressing Spike and Nucleocapsid Protects Rhesus Macaques against SARS-CoV-2 Delta Infection. Sci. Immunol. 2022, 7, eabo0226. [Google Scholar] [CrossRef]
- He, X.; Chandrashekar, A.; Zahn, R.; Wegmann, F.; Yu, J.; Mercado, N.B.; McMahan, K.; Martinot, A.J.; Piedra-Mora, C.; Beecy, S.; et al. Low-Dose Ad26.COV2.S Protection against SARS-CoV-2 Challenge in Rhesus Macaques. Cell 2021, 184, 3467–3473.e11. [Google Scholar] [CrossRef]
- Sui, Y.; Li, J.; Zhang, R.; Prabhu, S.K.; Andersen, H.; Venzon, D.; Cook, A.; Brown, R.; Teow, E.; Velasco, J.; et al. Protection against SARS-CoV-2 Infection by a Mucosal Vaccine in Rhesus Macaques. JCI Insight 2021, 6, e148494. [Google Scholar] [CrossRef]
- van Doremalen, N.; Lambe, T.; Spencer, A.; Belij-Rammerstorfer, S.; Purushotham, J.N.; Port, J.R.; Avanzato, V.A.; Bushmaker, T.; Flaxman, A.; Ulaszewska, M.; et al. ChAdOx1 nCoV-19 Vaccine Prevents SARS-CoV-2 Pneumonia in Rhesus Macaques. Nature 2020, 586, 578–582. [Google Scholar] [CrossRef]
- Yadav, P.D.; Ella, R.; Kumar, S.; Patil, D.R.; Mohandas, S.; Shete, A.M.; Vadrevu, K.M.; Bhati, G.; Sapkal, G.; Kaushal, H.; et al. Immunogenicity and Protective Efficacy of Inactivated SARS-CoV-2 Vaccine Candidate, BBV152 in Rhesus Macaques. Nat. Commun. 2021, 12, 1386. [Google Scholar] [CrossRef]
- Prokopenko, P.; Matyushenko, V.; Rak, A.; Stepanova, E.; Chistyakova, A.; Goshina, A.; Kudryavtsev, I.; Rudenko, L.; Isakova-Sivak, I. Truncation of NS1 Protein Enhances T Cell-Mediated Cross-Protection of a Live Attenuated Influenza Vaccine Virus Expressing Wild-Type Nucleoprotein. Vaccines 2023, 11, 501. [Google Scholar] [CrossRef]
- Kingstad-Bakke, B.; Cleven, T.; Bussan, H.; Yount, B.L.; Uraki, R.; Iwatsuki-Horimoto, K.; Koga, M.; Yamamoto, S.; Yotsuyanagi, H.; Park, H.; et al. Airway Surveillance and Lung Viral Control by Memory T Cells Induced by COVID-19 mRNA Vaccine. JCI Insight 2023, 8, e172510. [Google Scholar] [CrossRef] [PubMed]
- Blair, R.V.; Vaccari, M.; Doyle-Meyers, L.A.; Roy, C.J.; Russell-Lodrigue, K.; Fahlberg, M.; Monjure, C.J.; Beddingfield, B.; Plante, K.S.; Plante, J.A.; et al. Acute Respiratory Distress in Aged, SARS-CoV-2–Infected African Green Monkeys but Not Rhesus Macaques. Am. J. Pathol. 2021, 191, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Yao, Y.-F.; Yang, X.-L.; Zhou, Y.-W.; Gao, G.; Peng, Y.; Yang, L.; Hu, X.; Xiong, J.; Jiang, R.-D.; et al. Infection with Novel Coronavirus (SARS-CoV-2) Causes Pneumonia in Rhesus Macaques. Cell Res. 2020, 30, 670–677. [Google Scholar] [CrossRef] [PubMed]
- van Doremalen, N.; Singh, M.; Saturday, T.A.; Yinda, C.K.; Perez-Perez, L.; Bohler, W.F.; Weishampel, Z.A.; Lewis, M.; Schulz, J.E.; Williamson, B.N.; et al. SARS-CoV-2 Omicron BA.1 and BA.2 Are Attenuated in Rhesus Macaques as Compared to Delta. Sci. Adv. 2022, 8, eade1860. [Google Scholar] [CrossRef]
- Wan, Y.; Shang, J.; Sun, S.; Tai, W.; Chen, J.; Geng, Q.; He, L.; Chen, Y.; Wu, J.; Shi, Z.; et al. Molecular Mechanism for Antibody-Dependent Enhancement of Coronavirus Entry. J. Virol. 2020, 94, e02015-19. [Google Scholar] [CrossRef]
- Bournazos, S.; Gupta, A.; Ravetch, J.V. The Role of IgG Fc Receptors in Antibody-Dependent Enhancement. Nat. Rev. Immunol. 2020, 20, 633–643. [Google Scholar] [CrossRef]
- Kudryavtsev, I.; Rubinstein, A.; Golovkin, A.; Kalinina, O.; Vasilyev, K.; Rudenko, L.; Isakova-Sivak, I. Dysregulated Immune Responses in SARS-CoV-2-Infected Patients: A Comprehensive Overview. Viruses 2022, 14, 1082. [Google Scholar] [CrossRef]
- Wong, L.-Y.R.; Perlman, S. Immune Dysregulation and Immunopathology Induced by SARS-CoV-2 and Related Coronaviruses—Are We Our Own Worst Enemy? Nat. Rev. Immunol. 2022, 22, 47–56. [Google Scholar] [CrossRef]
- Luo, F.; Liao, F.-L.; Wang, H.; Tang, H.-B.; Yang, Z.-Q.; Hou, W. Evaluation of Antibody-Dependent Enhancement of SARS-CoV Infection in Rhesus Macaques Immunized with an Inactivated SARS-CoV Vaccine. Virol. Sin. 2018, 33, 201–204. [Google Scholar] [CrossRef]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Steel, K.J.A.; Hemmings, O.; O’Byrne, A.; Kouphou, N.; Galao, R.P.; et al. Longitudinal Observation and Decline of Neutralizing Antibody Responses in the Three Months Following SARS-CoV-2 Infection in Humans. Nat. Microbiol. 2020, 5, 1598–1607. [Google Scholar] [CrossRef]
- Cohen, K.W.; Linderman, S.L.; Moodie, Z.; Czartoski, J.; Lai, L.; Mantus, G.; Norwood, C.; Nyhoff, L.E.; Edara, V.V.; Floyd, K.; et al. Longitudinal Analysis Shows Durable and Broad Immune Memory after SARS-CoV-2 Infection with Persisting Antibody Responses and Memory B and T Cells. Cell Rep. Med. 2021, 2, 100354. [Google Scholar] [CrossRef] [PubMed]
- Grant, E.J.; Quiñones-Parra, S.M.; Clemens, E.B.; Kedzierska, K. Human Influenza Viruses and CD8+ T Cell Responses. Curr. Opin. Virol. 2016, 16, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Bao, L.; Liu, J.; Xiao, C.; Liu, J.; Xue, J.; Lv, Q.; Qi, F.; Gao, H.; Yu, P.; et al. Primary Exposure to SARS-CoV-2 Protects against Reinfection in Rhesus Macaques. Science 2020, 369, eabc5343. [Google Scholar] [CrossRef]
- Chandrashekar, A.; Liu, J.; Martinot, A.J.; McMahan, K.; Mercado, N.B.; Peter, L.; Tostanoski, L.H.; Yu, J.; Maliga, Z.; Nekorchuk, M.; et al. SARS-CoV-2 Infection Protects against Rechallenge in Rhesus Macaques. Science 2020, 369, 812–817. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanova, E.; Isakova-Sivak, I.; Matyushenko, V.; Mezhenskaya, D.; Kudryavtsev, I.; Kostromitina, A.; Chistiakova, A.; Rak, A.; Bazhenova, E.; Prokopenko, P.; et al. Safety and Immunogenicity Study of a Bivalent Vaccine for Combined Prophylaxis of COVID-19 and Influenza in Non-Human Primates. Vaccines 2024, 12, 1099. https://doi.org/10.3390/vaccines12101099
Stepanova E, Isakova-Sivak I, Matyushenko V, Mezhenskaya D, Kudryavtsev I, Kostromitina A, Chistiakova A, Rak A, Bazhenova E, Prokopenko P, et al. Safety and Immunogenicity Study of a Bivalent Vaccine for Combined Prophylaxis of COVID-19 and Influenza in Non-Human Primates. Vaccines. 2024; 12(10):1099. https://doi.org/10.3390/vaccines12101099
Chicago/Turabian StyleStepanova, Ekaterina, Irina Isakova-Sivak, Victoria Matyushenko, Daria Mezhenskaya, Igor Kudryavtsev, Arina Kostromitina, Anna Chistiakova, Alexandra Rak, Ekaterina Bazhenova, Polina Prokopenko, and et al. 2024. "Safety and Immunogenicity Study of a Bivalent Vaccine for Combined Prophylaxis of COVID-19 and Influenza in Non-Human Primates" Vaccines 12, no. 10: 1099. https://doi.org/10.3390/vaccines12101099
APA StyleStepanova, E., Isakova-Sivak, I., Matyushenko, V., Mezhenskaya, D., Kudryavtsev, I., Kostromitina, A., Chistiakova, A., Rak, A., Bazhenova, E., Prokopenko, P., Kotomina, T., Donina, S., Novitskaya, V., Sivak, K., Karal-Ogly, D., & Rudenko, L. (2024). Safety and Immunogenicity Study of a Bivalent Vaccine for Combined Prophylaxis of COVID-19 and Influenza in Non-Human Primates. Vaccines, 12(10), 1099. https://doi.org/10.3390/vaccines12101099




