Neutralizing Antibodies against 10 SARS-CoV-2 Variants at Two Years Post-COVISHIELD Vaccination with Special Reference to Omicron Subvariants and Booster Administration
Abstract
1. Introduction
2. Methodology
2.1. Vaccine
2.2. Study Population and Samples
2.3. Ethical Approval
2.4. MSD Assay
2.5. Statistical Analysis
3. Results
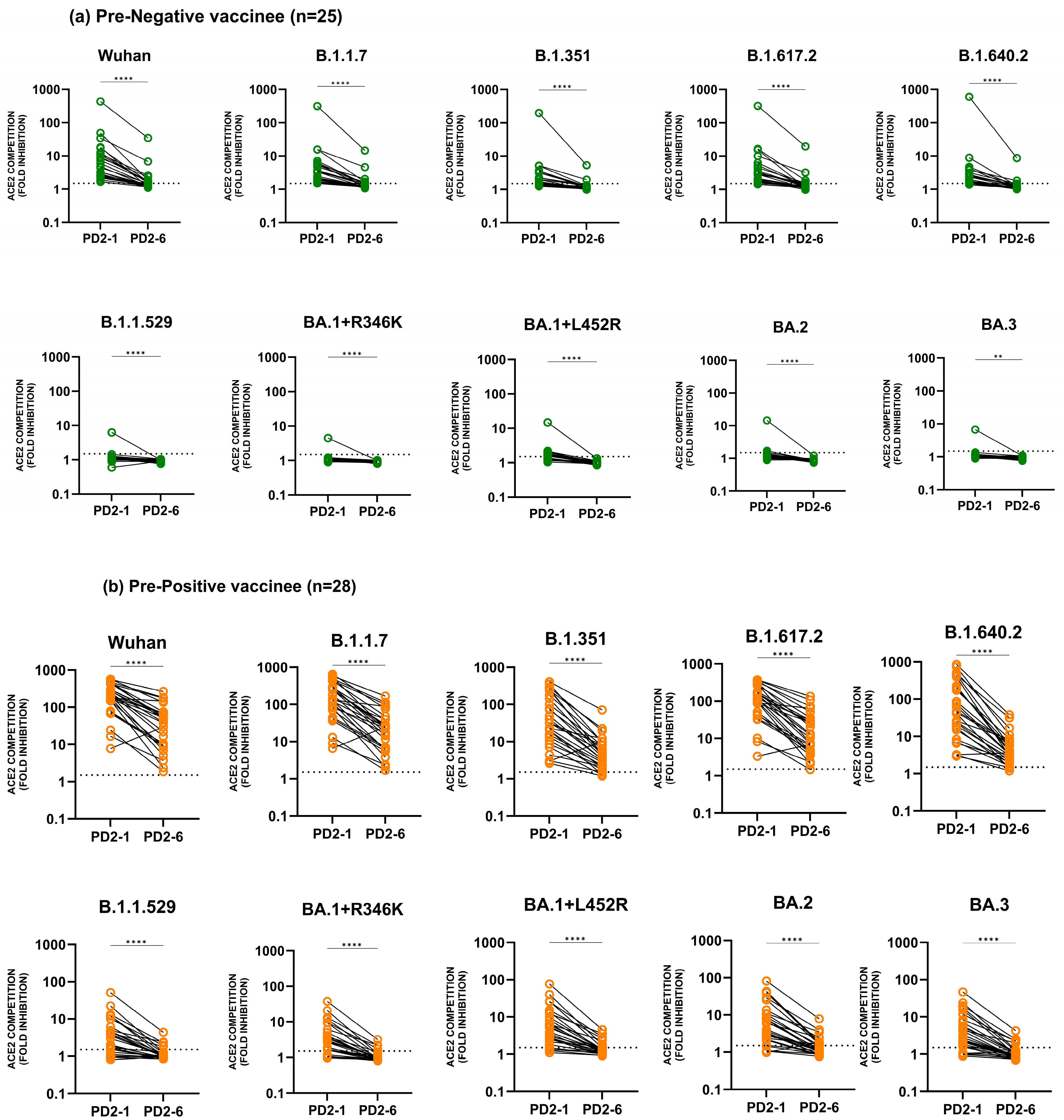
3.1. Neutralizing Antibody Response against Different Variants until Six Months Post-Vaccination
3.1.1. Seropositivity
3.1.2. Anti-Variant Neutralizing Antibody Levels
3.2. Administration of a Booster Dose and Follow-Up until Two Years Post-Vaccination
3.2.1. Seropositivity Post-Booster and at 2 Years Post-Vaccination
3.2.2. Neutralizing Antibody Levels Post-Booster and at Two Years Post-Vaccination
3.3. Comparison of Anti-Variant Antibody Levels among Pre-Negatives and Pre-Positives
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fontanet, A.; Autran, B.; Lina, B.; Kieny, M.P.; Karim, S.S.A.; Sridhar, D. SARS-CoV-2 Variants and Ending the COVID-19 Pandemic. Lancet 2021, 397, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Arankalle, V.; Kulkarni-Munje, A.; Kulkarni, R.; Palkar, S.; Patil, R.; Oswal, J.; Lalwani, S.; Mishra, A.C. Immunogenicity of Two COVID-19 Vaccines Used in India: An Observational Cohort Study in Health Care Workers from a Tertiary Care Hospital. Front. Immunol. 2022, 13, 928501. [Google Scholar] [CrossRef]
- Patil, R.; Palkar, S.; Mishra, A.; Patil, R.; Arankalle, V. Variable Neutralizing Antibody Responses to 10 SARS-CoV-2 Variants in Natural Infection with Wild- Type (B.1) Virus, Kappa (B.1.617.1), and Delta (B.1.617.2) Variants and COVISHIELD Vaccine Immunization in India: Utility of the MSD Platform. Front. Immunol. 2023, 14, 1181991. [Google Scholar] [CrossRef] [PubMed]
- Haritay, S.; Patil, R.; Maldar, A.; Kumar, A.; Reddy, V.; Oswal, D.; Tahashildar, M.A.; Kolakar, A.; Kabbur, S.; Prasad, J.B.; et al. Waning of Antibody Response Among Vaccinees Who Received Two Doses of Covishield Vaccine. J. Glob. Infect. Dis. 2023, 15, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Tegally, H.; Moir, M.; Everatt, J.; Giovanetti, M.; Scheepers, C.; Wilkinson, E.; Subramoney, K.; Makatini, Z.; Moyo, S.; Amoako, D.G.; et al. Emergence of SARS-CoV-2 Omicron Lineages BA.4 and BA.5 in South Africa. Nat. Med. 2022, 28, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Greaney, A.J.; Starr, T.N.; Gilchuk, P.; Zost, S.J.; Binshtein, E.; Loes, A.N.; Hilton, S.K.; Huddleston, J.; Eguia, R.; Crawford, K.H.D.; et al. Complete Mapping of Mutations to the SARS-CoV-2 Spike Receptor-Binding Domain That Escape Antibody Recognition. Cell Host Microbe 2021, 29, 44. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J.; et al. SARS-CoV-2 Variants, Spike Mutations and Immune Escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.S.A.; Karim, Q.A. Omicron SARS-CoV-2 Variant: A New Chapter in the COVID-19 Pandemic. Lancet 2021, 398, 2126–2128. [Google Scholar] [CrossRef] [PubMed]
- Cordelia, F.; Kirsebom, M.; Andrews, N.; Sachdeva, R.; Stowe, J.; Ramsay, M.; Lopez Bernal, J. Effectiveness of ChAdOx1-S COVID-19 Booster Vaccination against the Omicron and Delta Variants in England. Nat. Commun. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Jacobsen, H.; Strengert, M.; Maaß, H.; Ynga Durand, M.A.; Katzmarzyk, M.; Kessel, B.; Harries, M.; Rand, U.; Abassi, L.; Kim, Y.; et al. Diminished Neutralization Responses towards SARS-CoV-2 Omicron VoC after MRNA or Vector-Based COVID-19 Vaccinations. Sci. Rep. 2022, 12, 19858. [Google Scholar] [CrossRef]
- Sudjaritruk, T.; Mueangmo, O.; Saheng, J.; Winichakoon, P.; Salee, P.; Wongjak, W.; Chaito, T.; Praparattanapan, J.; Nuket, K.; Solai, N.; et al. Comparison of Immunogenicity and Reactogenicity of Five Primary Series of COVID-19 Vaccine Regimens against Circulating SARS-CoV-2 Variants of Concern among Healthy Thai Populations. Vaccines 2023, 11, 564. [Google Scholar] [CrossRef] [PubMed]
- Costa Clemens, S.A.; Weckx, L.; Clemens, R.; Almeida Mendes, A.V.; Ramos Souza, A.; Silveira, M.B.V.; da Guarda, S.N.F.; de Nobrega, M.M.; de Moraes Pinto, M.I.; Gonzalez, I.G.S.; et al. Heterologous versus Homologous COVID-19 Booster Vaccination in Previous Recipients of Two Doses of CoronaVac COVID-19 Vaccine in Brazil (RHH-001): A Phase 4, Non-Inferiority, Single Blind, Randomised Study. Lancet 2022, 399, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Rose, W.; Raju, R.; Babji, S.; George, A.; Madhavan, R.; Leander Xavier, J.V.; David Chelladurai, J.S.; Nikitha, O.S.; Deborah, A.A.; Vijayakumar, S.; et al. Immunogenicity and Safety of Homologous and Heterologous Booster Vaccination of ChAdOx1 NCoV-19 (COVISHIELDTM) and BBV152 (COVAXIN®): A Non-Inferiority Phase 4, Participant and Observer-Blinded, Randomised Study. Lancet Reg. Health 2023, 12, 100141. [Google Scholar] [CrossRef]
- Ren, S.-Y.; Wang, W.-B.; Gao, R.-D.; Zhou, A.-M. Omicron Variant (B.1.1.529) of SARS-CoV-2: Mutation, Infectivity, Transmission, and Vaccine Resistance. World J. Clin. Cases 2022, 10, 1–11. [Google Scholar] [CrossRef]
- Kupferschmidt, K. Where Did “weird” Omicron Come From? Science 2021, 374, 1179. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.B.; Ma, Y.B.; Lei, Z.H.; Zhang, X.F.; Li, J.; Li, S.S.; Dong, Z.Y.; Liang, Y.; Li, Q.M.; Su, J.G. Identification of Key Mutations Responsible for the Enhancement of Receptor-Binding Affinity and Immune Escape of SARS-CoV-2 Omicron Variant. J. Mol. Graph. Model. 2023, 124, 108540. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yu, Y.; Xu, Y.; Jian, F.; Song, W.; Yisimayi, A.; Wang, P.; Wang, J.; Liu, J.; Yu, L.; et al. Fast Evolution of SARS-CoV-2 BA.2.86 to JN.1 under Heavy Immune Pressure. Lancet Infect. Dis. 2024, 24, e70–e72. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Miyakawa, K.; Ohtake, N.; Yamaoka, Y.; Yajima, S.; Yamazaki, E.; Shimada, T.; Goto, A.; Nakajima, H.; Ryo, A. Vaccine-induced humoral response against SARS-CoV-2 dramatically declined but cellular immunity possibly remained at 6 months post BNT162b2 vaccination. Vaccine 2022, 40, 2652–2655. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, B.; Chabrolles, H.; Archimbaud, C.; Brebion, A.; Cosme, J.; Dutheil, F.; Lambert, C.; Junda, M.; Mirand, A.; Ollier, A.; et al. Decline of Humoral and Cellular Immune Responses Against SARS-CoV-2 6 Months After Full BNT162b2 Vaccination in Hospital Healthcare Workers. Front. Immunol. 2022, 13, 842912. [Google Scholar] [CrossRef] [PubMed]
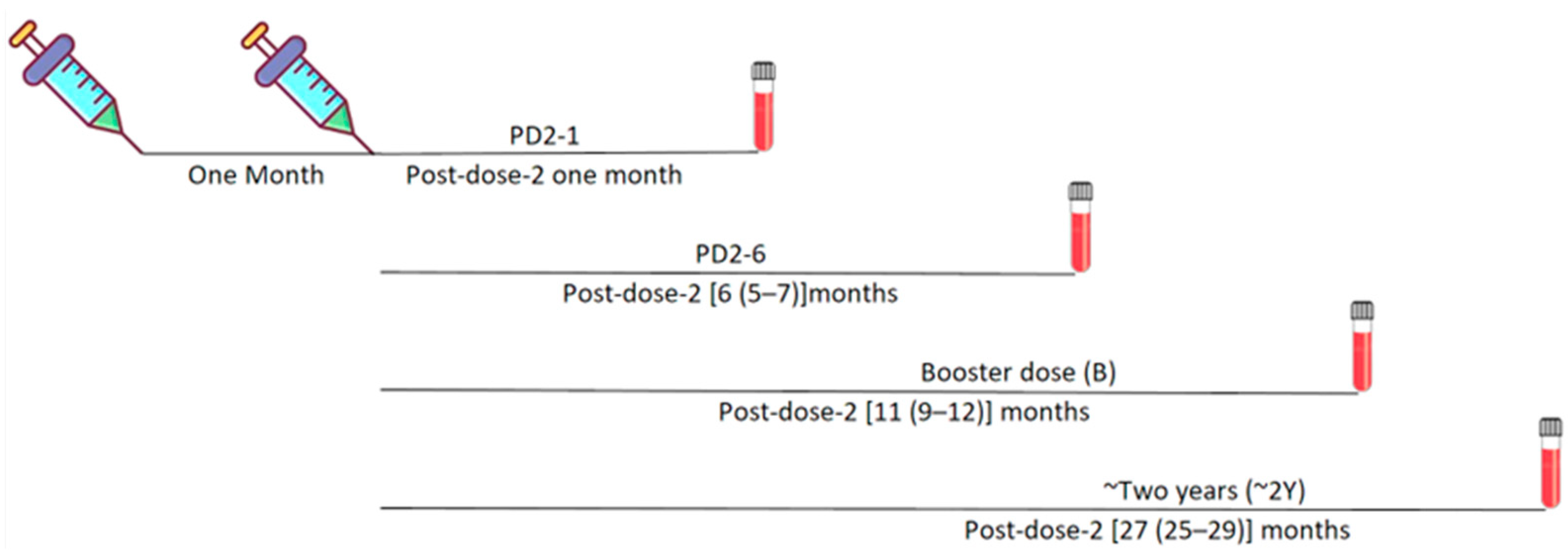





| One Month Post 2nd Dose of Vaccine (PD2-1) | Six Months Post 2nd Dose of Vaccine (PD2-6) | Booster Dose Post-Vaccination (B) | 2 Years Post-Vaccination—PRE-NEGATIVE | 2 Years Post-Vaccination—PRE-POSITIVE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PRE-NEGATIVE | PRE-POSITIVE | PRE-NEGATIVE | PRE-POSITIVE | PRE-NEGATIVE | PRE-POSITIVE | Booster Dose Taken (2Y-B+) | Booster Dose Not Taken (2Y-B-) | Booster Dose Taken (2Y-B+) | Booster Dose Not Taken (2Y-B-) | |
| N | 35 | 31 | 25 | 28 | 16 | 7 | 26 | 1 | 10 | 5 |
| Female (%) | 22 (63) | 15 (48) | 17 (68) | 14 (50) | 11 (68.75) | 4 (57) | 15 (58) | 1 (100) | 5 (50) | 1 (20) |
| Male (%) | 13 (37) | 16 (52) | 8 (32) | 14 (50) | 5 (31.25) | 3 (43) | 11 (42) | 0 (0) | 5 (50) | 4 (80) |
| Age in years | ||||||||||
| Mean | 42 | 35 | 43 | 36 | 43 | 38 | 43 | 26 | 39 | 32 |
| Median | 44 | 33 | 45 | 35 | 41 | 37 | 40 | 26 | 39 | 29 |
| Range | 50 | 31 | 50 | 30 | 43 | 21 | 54 | 0 | 28 | 16 |
| Sample type | Plasma | Plasma | Serum | Serum | Plasma | Plasma | Plasma | Plasma | Plasma | Plasma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, R.; Palkar, S.; Mishra, A.; Arankalle, V. Neutralizing Antibodies against 10 SARS-CoV-2 Variants at Two Years Post-COVISHIELD Vaccination with Special Reference to Omicron Subvariants and Booster Administration. Vaccines 2024, 12, 1039. https://doi.org/10.3390/vaccines12091039
Patil R, Palkar S, Mishra A, Arankalle V. Neutralizing Antibodies against 10 SARS-CoV-2 Variants at Two Years Post-COVISHIELD Vaccination with Special Reference to Omicron Subvariants and Booster Administration. Vaccines. 2024; 12(9):1039. https://doi.org/10.3390/vaccines12091039
Chicago/Turabian StylePatil, Rajashree, Sonali Palkar, Akhileshchandra Mishra, and Vidya Arankalle. 2024. "Neutralizing Antibodies against 10 SARS-CoV-2 Variants at Two Years Post-COVISHIELD Vaccination with Special Reference to Omicron Subvariants and Booster Administration" Vaccines 12, no. 9: 1039. https://doi.org/10.3390/vaccines12091039
APA StylePatil, R., Palkar, S., Mishra, A., & Arankalle, V. (2024). Neutralizing Antibodies against 10 SARS-CoV-2 Variants at Two Years Post-COVISHIELD Vaccination with Special Reference to Omicron Subvariants and Booster Administration. Vaccines, 12(9), 1039. https://doi.org/10.3390/vaccines12091039





