Comparative Effectiveness of the Bivalent (Original/Omicron BA.4/BA.5) mRNA COVID-19 Vaccines mRNA-1273.222 and BNT162b2 Bivalent in Adults with Underlying Medical Conditions in the United States
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Study Design and Study Population
2.3. Objectives
2.4. Outcome Measures
2.5. Covariates
2.6. Statistical Analysis
2.7. Sensitivity Analyses
3. Results
3.1. Main Analysis
3.2. Sensitivity Analyses
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lythgoe, K.A.; Golubchik, T.; Hall, M.; House, T.; Cahuantzi, R.; MacIntyre-Cockett, G.; Fryer, H.; Thomson, L.; Nurtay, A.; Ghafani, M.; et al. Lineage Replacement and Evolution Captured by 3 Years of the United Kingdom Coronavirus (COVID-19) Infection Survey. Proc. Biol. Sci. 2023, 290, 20231284. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bhattacharya, M.; Nag, S.; Dhama, K.; Chakraborty, C. A Detailed Overview of SARS-CoV-2 Omicron: Its Sub-Variants, Mutations and Pathophysiology, Clinical Characteristics, Immunological Landscape, Immune Escape, and Therapies. Viruses 2023, 15, 167. [Google Scholar] [CrossRef]
- Tian, D.; Sun, Y.; Xu, H.; Ye, Q. The Emergence and Epidemic Characteristics of the Highly Mutated SARS-CoV-2 Omicron Variant. J. Med. Virol. 2022, 94, 2376–2383. [Google Scholar] [CrossRef]
- Chalkias, S.; Harper, C.; Vrbicky, K.; Walsh, S.R.; Essink, B.; Brosz, A.; McGhee, N.; Tomassini, J.E.; Chen, X.; Chang, Y.; et al. A Bivalent Omicron-Containing Booster Vaccine against COVID-19. N. Engl. J. Med. 2022, 387, 1279–1291. [Google Scholar] [CrossRef]
- Zou, J.; Kurhade, C.; Patel, S.; Kitchin, N.; Tompkins, K.; Cutler, M.; Cooper, D.; Yang, Q.; Cai, H.; Muik, A.; et al. Improved Neutralization of Micron BA.4/5, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 with Bivalent BA.4/5 Vaccine. BioRxiv 2022. [Google Scholar] [CrossRef]
- Rosenblum, H.G. Interim Recommendations from the Advisory Committee on Immunization Practices for the Use of Bivalent Booster Doses of COVID-19 Vaccines—United States, October 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1436–1441. [Google Scholar] [CrossRef]
- Kim, L.; Garg, S.; O’Halloran, A.; Whitaker, M.; Pham, H.; Anderson, E.J.; Armistead, I.; Bennett, N.M.; Billing, L.; Como-Sabetti, K.; et al. Risk Factors for Intensive Care Unit Admission and In-Hospital Mortality among Hospitalized Adults Identified through the US Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin. Infect. Dis. 2021, 72, e206–e214. [Google Scholar] [CrossRef]
- Rosenthal, N.; Cao, Z.; Gundrum, J.; Sianis, J.; Safo, S. Risk Factors Associated with In-Hospital Mortality in a US National Sample of Patients with COVID-19. JAMA Netw. Open 2020, 3, e2029058. [Google Scholar] [CrossRef]
- Boersma, P.; Black, L.I.; Ward, B.W. Prevalence of Multiple Chronic Conditions among US Adults, 2018. Prev. Chronic Dis. 2020, 17, 200130. [Google Scholar] [CrossRef]
- Kopel, H.; Bogdanov, A.; Winer-Jones, J.P.; Adams, C.; Winer, I.H.; Bonafede, M.; Nguyen, V.H.; Mansi, J.A. Comparison of COVID-19 and Influenza-Related Outcomes in the United States during Fall–Winter 2022–2023: A Cross-Sectional Retrospective Study. Diseases 2024, 12, 16. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. COVID-NET: COVID-19-Associated Hospitalization Surveillance Network. Available online: https://www.cdc.gov/coronavirus/2019-ncov/covidnetdashboard/de/powerbi/dashboard.html (accessed on 2 September 2023).
- Kopel, H.; Nguyen, V.H.; Boileau, C.; Bogdanov, A.; Winer, I.; Ducruet, T.; Zeng, N.; Bonafede, M.; Esposito, D.B.; Martin, D.; et al. Comparative Effectiveness of Bivalent (Original/Omicron BA.4/BA.5) COVID-19 Vaccines in Adults. Vaccines 2023, 11, 1711. [Google Scholar] [CrossRef] [PubMed]
- Boikos, C.; McGovern, I.; Molrine, D.; Ortiz, J.R.; Puig-Barberà, J.; Haag, M. Review of Analyses Estimating Relative Vaccine Effectiveness of Cell-Based Quadrivalent Influenza Vaccine in Three Consecutive US Influenza Seasons. Vaccines 2022, 10, 896. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Boileau, C.; Bogdanov, A.; Sredl, M.; Bonafede, M.; Ducruet, T.; Chavers, S.; Rosen, A.; Martin, D.; Buck, P.; et al. Relative Effectiveness of BNT162b2, mRNA-1273, and Ad26.COV2.S Vaccines and Homologous Boosting in Preventing COVID-19 in Adults in the US. Open Forum Infect. Dis. 2023, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals. Available online: https://www.cdc.gov/covid/hcp/clinical-care/underlying-conditions.html (accessed on 5 September 2023).
- Benavidez, G.A.; Zahnd, W.E.; Hung, P.; Eberth, J.M. Chronic Disease Prevalence in the US: Sociodemographic and Geographic Variations by Zip Code Tabulation Area. Prev. Chronic Dis. 2024, 21, E14. [Google Scholar] [CrossRef] [PubMed]
- Hulme, W.J.; Horne, E.M.F.; Parker, E.P.K.; Keogh, R.H.; Williamson, E.J.; Walker, V.; Palmer, T.M.; Curtis, H.J.; Walker, A.J.; Andrews, C.D.; et al. Comparative Effectiveness of BNT162b2 versus mRNA-1273 Covid-19 Vaccine Boosting in England: Matched Cohort Study in OpenSAFELY-TPP. BMJ 2023, 380, e072808. [Google Scholar] [CrossRef]
- Dickerman, B.A.; Gerlovin, H.; Madenci, A.L.; Figueroa Muñiz, M.J.; Wise, J.K.; Adhikari, N.; Ferolito, B.R.; Kurgansky, K.E.; Gagnon, D.R.; Cho, K.; et al. Comparative Effectiveness of Third Doses of mRNA-Based COVID-19 Vaccines in US Veterans. Nat. Microbiol. 2023, 8, 55–63. [Google Scholar] [CrossRef]
- Embi, P.J.; Levy, M.E.; Naleway, A.L.; Patel, P.; Gaglani, M.; Natarajan, K.; Dascomb, K.; Ong, T.C.; Klein, N.P.; Liao, I.-C.; et al. Effectiveness of 2-Dose Vaccination with mRNA COVID-19 Vaccines Against COVID-19-Associated Hospitalizations Among Immunocompromised Adults - Nine States, January-September 2021. MMWR Morb Mortal Wkly Rep. 2021, 70, 1553–1559. [Google Scholar] [CrossRef]
- Drawz, P.E.; DeSilva, M.; Bodurtha, P.; Vazquez Benitez, G.; Murray, A.; Chamberlain, A.M.; Dudley, R.A.; Waring, S.; Kharbanda, A.B.; Murphy, D.; et al. Effectiveness of BNT162b2 and mRNA-1273 Second Doses and Boosters for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection and SARS-CoV-2-Related Hospitalizations: A Statewide Report from the Minnesota Electronic Health Record Consortium. Clin. Infect. Dis. 2022, 75, 890–892. [Google Scholar] [CrossRef]
- Molnár, G.A.; Vokó, Z.; Sütő, G.; Rokszin, G.; Nagy, D.; Surján, G.; Surján, O.; Nagy, P.; Kenessey, I.; Wéber, A.; et al. Effectiveness of SARS-CoV-2 Primary Vaccines and Boosters in Patients with Type 2 Diabetes Mellitus in Hungary (HUN-VE 4 Study). BMJ Open Diabetes Res. Care 2024, 12, e003777. [Google Scholar] [CrossRef]
- Kavikondala, S.; Haeussler, K.; Wang, X.; Spellman, A.; Bausch-Jurken, M.T.; Sharma, P.; Amiri, M.; Krivelyova, A.; Vats, S.; Nassim, M.; et al. Immunogenicity of mRNA-1273 and BNT162b2 in Immunocompromised Patients: Systematic Review and Meta-Analysis Using GRADE. Infect. Dis. Ther. 2024, 13, 1419–1438. [Google Scholar] [CrossRef]
- Mavrovouniotis, I.; Fylaktou, A.; Stagou, M.; Ouranos, K.; Lioulios, G.; Evgenikaki, E.; Exindari, M.; Gioula, G. Cellular and Humoral Responses in Dialysis Patients after Vaccination with the BNT162b2 or mRNA-1273 Vaccines. Life 2023, 13, 474. [Google Scholar] [CrossRef]
- Liao, S.-Y.; Gerber, A.N.; Zelarney, P.; Make, B.; Wechsler, M.E. Impaired SARS-CoV-2 mRNA Vaccine Antibody Response in Chronic Medical Conditions: A Real-World Analysis. Chest 2022, 161, 1490–1493. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Dong, F.; Pyle, L.; Michels, A.W.; Yu, L.; Rewers, M. Similar Time Course of Humoral Response to SARS-CoV-2 mRNA Vaccines in People with and without Type 1 Diabetes. Diabetes Technol. Ther. 2023, 25, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Neuhann, J.M.; Stemler, J.; Carcas, A.J.; Frías-Iniesta, J.; Akova, M.; Bethe, U.; Heringer, S.; Salmanton-García, J.; Tischmann, L.; Zarrouk, M.; et al. Immunogenicity and Reactogenicity of a First Booster with BNT162b2 or Full-Dose mRNA-1273: A Randomised VACCELERATE Trial in Adults ≥75 Years (EU-COVAT-1). Vaccine 2023, 41, 7166–7175. [Google Scholar] [CrossRef]
- Clark, A.; Jit, M.; Warren-Gash, C.; Guthrie, B.; Wang, H.H.X.; Mercer, S.W.; Sanderson, C.; McKee, M.; Troeger, C.; Ong, K.L.; et al. Global, Regional, and National Estimates of the Population at Increased Risk of Severe COVID-19 Due to Underlying Health Conditions in 2020: A Modelling Study. Lancet Glob. Health 2020, 8, e1003–e1017. [Google Scholar] [CrossRef] [PubMed]
- Fried, M.W.; Crawford, J.M.; Mospan, A.R.; Watkins, S.E.; Munoz, B.; Zink, R.C.; Elliott, S.; Burleson, K.; Landis, C.; Reddy, K.R.; et al. Patient Characteristics and Outcomes of 11 721 Patients with Coronavirus Disease 2019 (COVID-19) Hospitalized across the United States. Clin. Infect. Dis. 2021, 72, e558–e565. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.L.; Fazio-Eynullayeva, E.; Lane, D.A.; Underhill, P.; Lip, G.Y.H. Comorbidities Associated with Mortality in 31,461 Adults with COVID-19 in the United States: A Federated Electronic Medical Record Analysis. PLoS Med. 2020, 17, e1003321. [Google Scholar] [CrossRef] [PubMed]
- Gallo Marin, B.; Aghagoli, G.; Lavine, K.; Yang, L.; Siff, E.J.; Chiang, S.S.; Salazar-Mather, T.P.; Dumenco, L.; Savaria, M.C.; Aung, S.N.; et al. Predictors of COVID-19 Severity: A Literature Review. Rev. Med. Virol. 2021, 31, 1–10. [Google Scholar] [CrossRef]
- Kompaniyets, L. Underlying Medical Conditions and Severe Illness among 540,667 Adults Hospitalized with COVID-19, March 2020–March 2021. Prev. Chronic Dis. 2021, 18, 210123. [Google Scholar] [CrossRef]
- Shrestha, S.S.; Kompaniyets, L.; Grosse, S.D.; Harris, A.M.; Baggs, J.; Sircar, K.; Gundlapalli, A.V. Estimation of Coronavirus Disease 2019 Hospitalization Costs from a Large Electronic Administrative Discharge Database, March 2020–July 2021. Open Forum Infect. Dis. 2021, 8, ofab561. [Google Scholar] [CrossRef]
- Scott, A.; Ansari, W.; Khan, F.; Chambers, R.; Benigno, M.; Di Fusco, M.; McGrath, L.; Malhotra, D.; Draica, F.; Nguyen, J.; et al. Substantial Health and Economic Burden of COVID-19 during the Year after Acute Illness among US Adults at High Risk of Severe COVID-19. BMC Med. 2024, 22, 46. [Google Scholar] [CrossRef]


| Subgroup | Conditions a |
|---|---|
| Diabetes | Diabetes type 1 or type 2 |
| Cerebro- and cardiovascular | Cerebrovascular disease, heart disease |
| Chronic lung disease | Asthma, other chronic lung diseases (e.g., pulmonary heart diseases, chronic obstructive pulmonary disease, and bronchiectasis) |
| Immunocompromised | Cancer, human immunodeficiency virus, primary immunodeficiencies/other immunocompromising conditions (e.g., hereditary hypogammaglobulinemia, selective deficiency of immunoglobulins, severe combined immunodeficiencies, and common variable immunodeficiencies), solid organ or blood stem cell transplantation, stem cell transplantation, use of immunosuppressive medications |
| Chronic kidney disease | Chronic kidney disease |
| Pre-Weighting | Post Weighting | ||||||
|---|---|---|---|---|---|---|---|
| mRNA-1273.222 | BNT162b2 Bivalent | SMD | mRNA-1273.222 | BNT162b2 Bivalent | SMD | ||
| Number of patients | 757,572 | 1,204,975 | 758,803 | 1,202,211 | |||
| Age at index in years, mean (SD) | 62 (16) | 60 (16) | 0.096 | 61 (16) | 61 (16) | 0.003 | |
| Sex, N (%) | Female | 431,238 (56.9) | 697,587 (57.9) | 0.020 | 436,546 (57.5) | 691,694 (57.5) | <0.001 |
| Male | 326,334 (43.1) | 507,388 (42.1) | 322,257 (42.5) | 510,518 (42.5) | |||
| Race, N (%) | Black | 47,522 (6.3) | 82,125 (6.8) | 0.031 | 49,881 (6.6) | 79,383 (6.6) | 0.001 |
| Other | 39,457 (5.2) | 60,368 (5.0) | 38,699 (5.1) | 61,213 (5.1) | |||
| White | 379,026 (50.0) | 589,334 (48.9) | 374,392 (49.3) | 592,955 (49.3) | |||
| Unknown | 291,567 (38.5) | 473,148 (39.3) | 295,831 (39.0) | 468,661 (39.0) | |||
| Ethnicity, N (%) | Hispanic | 37,723 (5.0) | 63,334 (5.3) | 0.018 | 38,924 (5.1) | 61,883 (5.1) | <0.001 |
| Non-Hispanic | 631,927 (83.4) | 997,153 (82.8) | 630,037 (83.0) | 997,945 (83.0) | |||
| Unknown | 87,920 (11.6) | 144,483 (12.0) | 89,842 (11.8) | 142,383 (11.8) | |||
| Region, N (%) | Midwest | 153,932 (20.3) | 287,611 (23.9) | 0.089 | 172,470 (22.7) | 272,229 (22.6) | 0.002 |
| Northeast | 194,796 (25.7) | 306,893 (25.5) | 193,511 (25.5) | 307,058 (25.5) | |||
| South | 229,485 (30.3) | 339,329 (28.2) | 218,508 (28.8) | 346,982 (28.9) | |||
| West | 151,598 (20.0) | 229,463 (19.0) | 147,450 (19.4) | 233,374 (19.4) | |||
| Unknown | 27,761 (3.7) | 41,679 (3.5) | 26,865 (3.5) | 42,569 (3.5) | |||
| Month of index, N (%) | August 2022 | 7 (<0.1) | 14 (<0.1) | 0.068 | 9 (<0.1) | 13 (<0.1) | 0.001 |
| September 2022 | 165,746 (21.9) | 295,324 (24.5) | 178,227 (23.5) | 282,665 (23.5) | |||
| October 2022 | 262,769 (34.7) | 417,242 (34.6) | 262,976 (34.7) | 416,425 (34.6) | |||
| November 2022 | 161,844 (21.4) | 241,826 (20.1) | 156,093 (20.6) | 247,126 (20.6) | |||
| December 2022 | 102,636 (13.5) | 152,770 (12.7) | 98,845 (13.0) | 156,509 (13.0) | |||
| January 2023 | 46,739 (6.2) | 70,112 (5.8) | 45,090 (5.9) | 71,559 (6.0) | |||
| February 2023 | 17,831 (2.4) | 27,687 (2.3) | 17,563 (2.3) | 27,915 (2.3) | |||
| Primary-series COVID-19 vaccine, N (%) | Heterologous | 64,763 (8.5) | 133,740 (11.1) | 0.175 | 79,300 (10.5) | 123,200 (10.2) | 0.007 |
| Homologous | 226,626 (29.9) | 272,401 (22.6) | 190,119 (25.1) | 302,057 (25.1) | |||
| Not reported | 466,183 (61.5) | 798,834 (66.3) | 489,385 (64.5) | 776,955 (64.6) | |||
| Time since last COVID-19 monovalent vaccination, N (%) | ≤90 days | 12,342 (1.6) | 15,007 (1.2) | 0.231 | 10,456 (1.4) | 16,657 (1.4) | 0.002 |
| 91–180 days | 158,876 (21.0) | 161,599 (13.4) | 122,413 (16.1) | 193,483 (16.1) | |||
| >180 days | 443,211 (58.5) | 725,100 (60.2) | 452,371 (59.6) | 717,402 (59.7) | |||
| Not reported | 143,143 (18.9) | 303,269 (25.2) | 173,564 (22.9) | 274,670 (22.8) | |||
| Time since last COVID-19 infection, N (%) | ≤120 days | 32,609 (4.3) | 52,761 (4.4) | 0.030 | 33,104 (4.4) | 52,418 (4.4) | <0.001 |
| 121–180 days | 17,269 (2.3) | 27,424 (2.3) | 17,371 (2.3) | 27,457 (2.3) | |||
| >180 days | 67,135 (8.9) | 117,056 (9.7) | 71,407 (9.4) | 113,132 (9.4) | |||
| Not reported | 640,559 (84.6) | 1,007,734 (83.6) | 636,921 (83.9) | 1,009,204 (83.9) | |||
| Underlying medical conditions, N (%) | Asthma | 107,684 (14.2) | 175,269 (14.5) | 0.009 | 109,615 (14.4) | 173,562 (14.4) | <0.001 |
| Cancer | 99,704 (13.2) | 151,325 (12.6) | 0.018 | 96,642 (12.7) | 153,428 (12.8) | <0.001 | |
| Cerebrovascular disease | 68,237 (9.0) | 104,581 (8.7) | 0.012 | 66,354 (8.7) | 105,537 (8.8) | 0.001 | |
| Chronic kidney disease | 106,577 (14.1) | 164,005 (13.6) | 0.013 | 103,660 (13.7) | 165,086 (13.7) | 0.002 | |
| Chronic liver diseases | 14,318 (1.9) | 22,958 (1.9) | 0.001 | 14,377 (1.9) | 22,830 (1.9) | <0.001 | |
| Chronic lung diseases a | 98,839 (13.0) | 150,479 (12.5) | 0.017 | 95,808 (12.6) | 152,296 (12.7) | 0.001 | |
| Cystic fibrosis | 286 (<0.1) | 422 (<0.1) | 0.001 | 272 (<0.1) | 431 (<0.1) | <0.001 | |
| Diabetes type 1 and 2 | 252,180 (33.3) | 388,331 (32.2) | 0.023 | 246,489 (32.5) | 391,550 (32.6) | 0.002 | |
| Disabilities | 82,976 (11.0) | 141,091 (11.7) | 0.024 | 87,274 (11.5) | 137,767 (11.5) | 0.001 | |
| Heart conditions | 169,131 (22.3) | 256,250 (21.3) | 0.026 | 163,349 (21.5) | 259,743 (21.6) | 0.002 | |
| HIV | 7327 (1.0) | 11,394 (0.9) | 0.002 | 7269 (1.0) | 11,491 (1.0) | <0.001 | |
| Mental health disorders | 201,996 (26.7) | 342,635 (28.4) | 0.040 | 211,585 (27.9) | 334,556 (27.8) | 0.001 | |
| Neurologic conditions | 26,905 (3.6) | 47,214 (3.9) | 0.019 | 28,561 (3.8) | 45,438 (3.8) | <0.001 | |
| Obesity | 255,376 (33.7) | 410,845 (34.1) | 0.008 | 257,461 (33.9) | 408,164 (34.0) | <0.001 | |
| Physical inactivity | 1200 (0.2) | 1941 (0.2) | <0.001 | 1223 (0.2) | 1931 (0.2) | <0.001 | |
| Primary Immunodeficiencies | 15,989 (2.1) | 25,055 (2.1) | 0.002 | 15,724 (2.1) | 25,043 (2.1) | <0.001 | |
| Pregnancy b | 5072 (0.7) | 9587 (0.8) | 0.015 | 5741 (0.8) | 9048 (0.8) | <0.001 | |
| Smoking c | 153,460 (20.3) | 244,998 (20.3) | 0.002 | 153,751 (20.3) | 243,993 (20.3) | <0.001 | |
| Solid organ or hematopoietic cell transplantation | 5078 (0.7) | 8679 (0.7) | 0.006 | 5287 (0.7) | 8429 (0.7) | <0.001 | |
| Tuberculosis | 444 (0.1) | 719 (0.1) | <0.001 | 447 (0.1) | 711 (0.1) | <0.001 | |
| Use of immunosuppressants | 63,796 (8.4) | 98,130 (8.1) | 0.010 | 62,513 (8.2) | 99,075 (8.2) | <0.001 | |
| Pre-Weighting, N (%) | Post Weighting, N (%) | Follow-Up Duration in Days, Median (IQR), Pre-Weighting | ||||
|---|---|---|---|---|---|---|
| mRNA-1273.222 | BNT162b2 Bivalent | mRNA-1273.222 | BNT162b2 Bivalent | mRNA-1273.222 | BNT162b2 Bivalent | |
| N = 757,572 | N = 1,204,975 | N = 758,803 | N = 1,202,211 | N = 757,572 | N = 1,204,975 | |
| Diabetes | 252,180 (33.3) | 388,331 (32.2) | 252,635 (33.3) | 387,233 (32.2) | 195 (140–223) | 197 (142–226) |
| Cerebro- and cardiovasculardisease | 204,933 (27.1) | 311,148 (25.8) | 205,364 (27.1) | 310,143 (25.8) | 198 (144–225) | 201 (145–228) |
| Chronic lung disease | 183,686 (24.2) | 290,887 (24.1) | 183,969 (24.2) | 290,178 (24.1) | 195 (139–224) | 197 (141–226) |
| Immunocompromised | 169,185 (22.3) | 258,467 (21.4) | 169,509 (22.3) | 257,751 (21.4) | 199 (148–226) | 202 (150–229) |
| Chronic kidney disease | 106,577 (14.1) | 164,005 (13.6) | 106,841 (14.1) | 163,426 (13.6) | 196 (140–224) | 199 (141–226) |
| Unadjusted HR a, (95% CI) | Adjusted HR b, (95% CI) | |||
|---|---|---|---|---|
| COVID-19-Related Hospitalization | COVID-19-Related Outpatient Encounter | COVID-19-Related Hospitalization | COVID-19-Related Outpatient Encounter | |
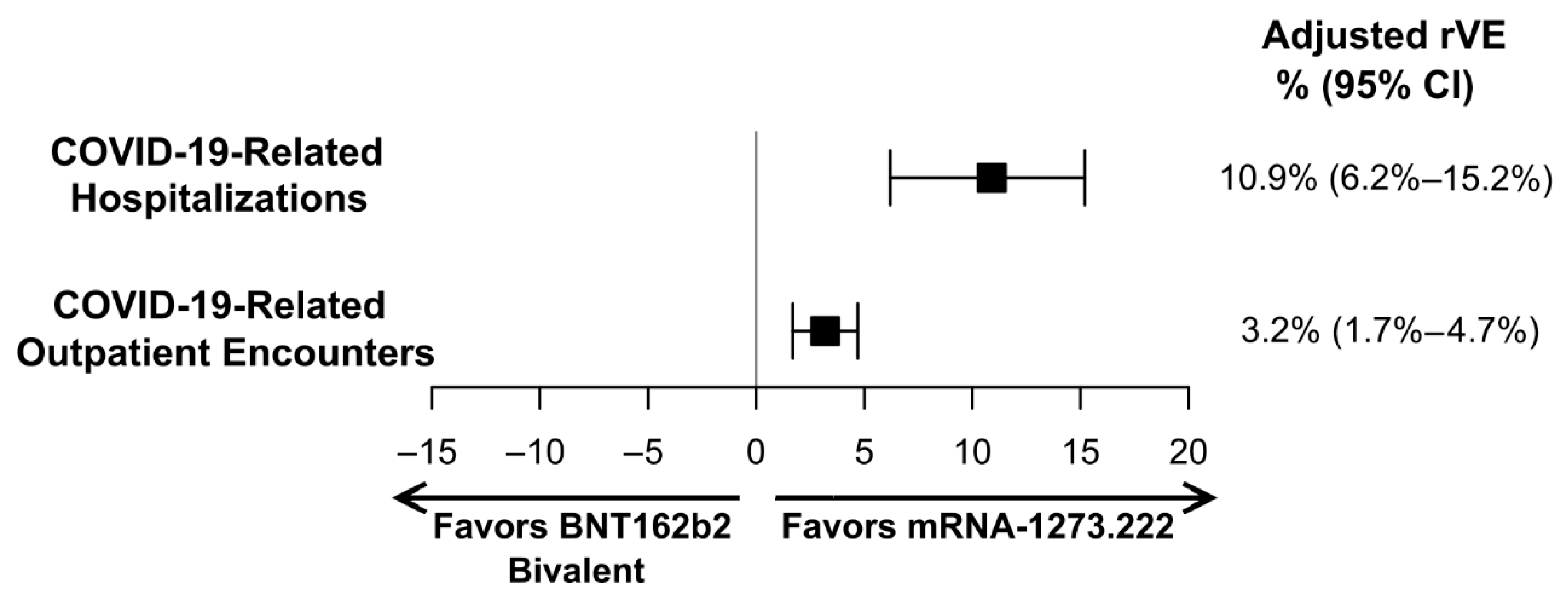
| Overall | 0.873 (0.830–0.919) | 0.971 (0.956–0.986) | 0.891 (0.848–0.938) | 0.968 (0.953–0.983) |
| Subgroup analyses | ||||
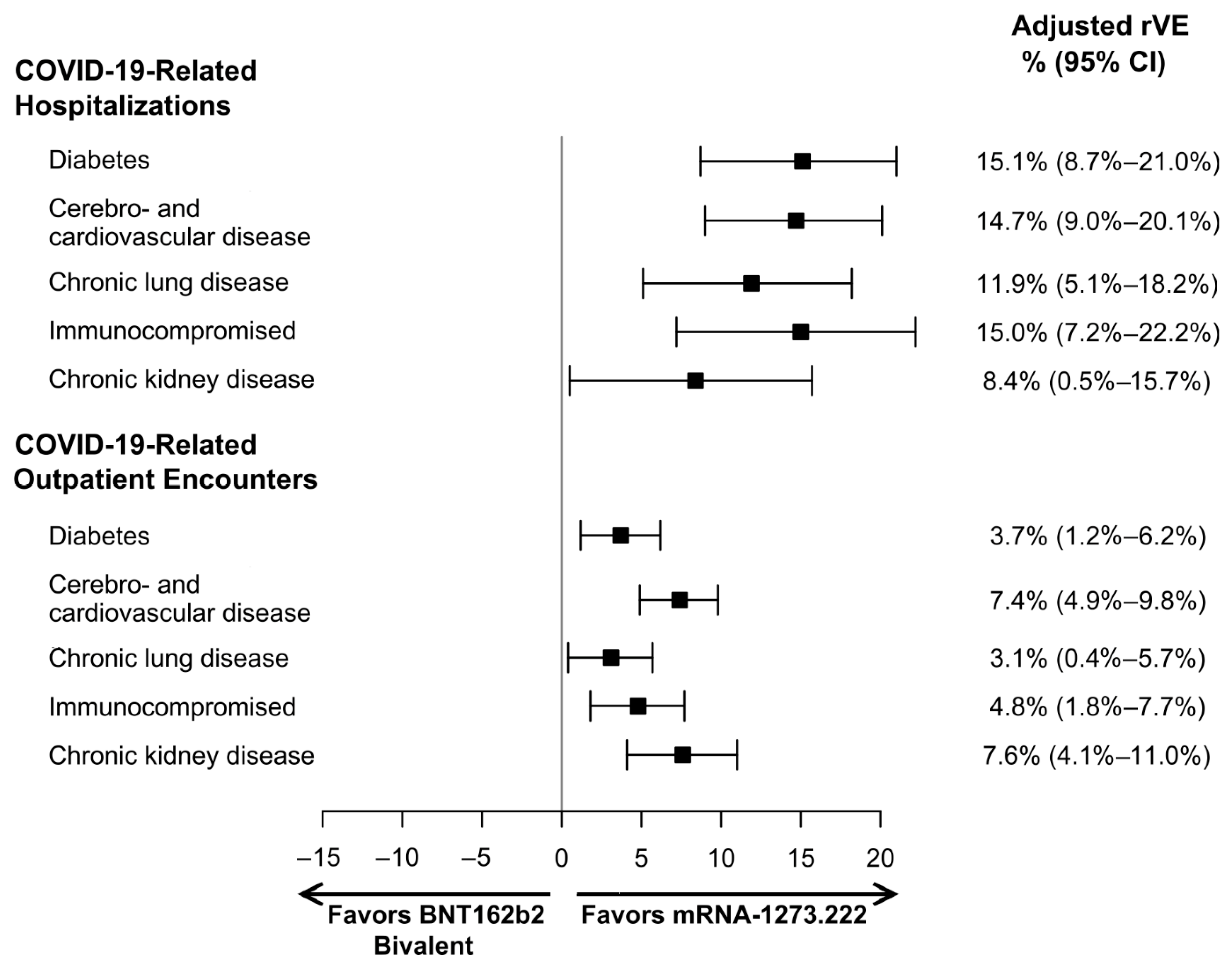
| Diabetes | 0.802 (0.746–0.863) | 0.947 (0.923–0.972) | 0.849 (0.790–0.913) | 0.963 (0.938–0.988) |
| Cerebro- and cardiovascular disease | 0.790 (0.739–0.844) | 0.894 (0.871–0.919) | 0.853 (0.799–0.910) | 0.926 (0.902–0.951) |
| Chronic lung disease | 0.852 (0.791–0.918) | 0.957 (0.932–0.984) | 0.881 (0.818–0.949) | 0.969 (0.943–0.996) |
| Immunocompromised | 0.835 (0.764–0.912) | 0.946 (0.917–0.976) | 0.850 (0.778–0.928) | 0.952 (0.923–0.982) |
| Chronic kidney disease | 0.843 (0.775–0.916) | 0.894 (0.860–0.928) | 0.916 (0.843–0.995) | 0.924 (0.890–0.959) |
| Sensitivity analyses | ||||
| Open claims | 0.859 (0.833–0.887) | 0.928 (0.919–0.937) | 0.880 (0.853–0.907) | 0.869 (0.818–0.922) |
| Closed claims—cut-off 28 February 2023 | 0.854 (0.804–0.907) | 0.974 (0.957–0.991) | 0.950 (0.941–0.960) | 0.962 (0.946–0.979) |
| Outcome | Adjusted rVE |
|---|---|
| Open claims—cut-off 31 May 2023 | |
| COVID-19-related hospitalization | 12.0% (9.3%–14.7%) |
| COVID-19-related outpatient encounter | 5.0% (4.0%–5.9%) |
| Closed claims—cut-off 28 February 2023 | |
| COVID-19-related hospitalization | 13.1% (7.8%–18.2%) |
| COVID-19-related outpatient encounter | 3.8% (2.1%–5.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopel, H.; Nguyen, V.H.; Bogdanov, A.; Winer, I.; Boileau, C.; Ducruet, T.; Zeng, N.; Winer-Jones, J.P.; Esposito, D.B.; Bausch-Jurken, M.; et al. Comparative Effectiveness of the Bivalent (Original/Omicron BA.4/BA.5) mRNA COVID-19 Vaccines mRNA-1273.222 and BNT162b2 Bivalent in Adults with Underlying Medical Conditions in the United States. Vaccines 2024, 12, 1107. https://doi.org/10.3390/vaccines12101107
Kopel H, Nguyen VH, Bogdanov A, Winer I, Boileau C, Ducruet T, Zeng N, Winer-Jones JP, Esposito DB, Bausch-Jurken M, et al. Comparative Effectiveness of the Bivalent (Original/Omicron BA.4/BA.5) mRNA COVID-19 Vaccines mRNA-1273.222 and BNT162b2 Bivalent in Adults with Underlying Medical Conditions in the United States. Vaccines. 2024; 12(10):1107. https://doi.org/10.3390/vaccines12101107
Chicago/Turabian StyleKopel, Hagit, Van Hung Nguyen, Alina Bogdanov, Isabelle Winer, Catherine Boileau, Thierry Ducruet, Ni Zeng, Jessamine P. Winer-Jones, Daina B. Esposito, Mary Bausch-Jurken, and et al. 2024. "Comparative Effectiveness of the Bivalent (Original/Omicron BA.4/BA.5) mRNA COVID-19 Vaccines mRNA-1273.222 and BNT162b2 Bivalent in Adults with Underlying Medical Conditions in the United States" Vaccines 12, no. 10: 1107. https://doi.org/10.3390/vaccines12101107
APA StyleKopel, H., Nguyen, V. H., Bogdanov, A., Winer, I., Boileau, C., Ducruet, T., Zeng, N., Winer-Jones, J. P., Esposito, D. B., Bausch-Jurken, M., Beck, E., Bonafede, M., & Mansi, J. A. (2024). Comparative Effectiveness of the Bivalent (Original/Omicron BA.4/BA.5) mRNA COVID-19 Vaccines mRNA-1273.222 and BNT162b2 Bivalent in Adults with Underlying Medical Conditions in the United States. Vaccines, 12(10), 1107. https://doi.org/10.3390/vaccines12101107





