Reproducibility Assessment of Enzyme-Linked Immunosorbent Assays to Detect Anti-HPV16 L1-Specific IgG1, IgG3, IgA, and IgM Antibodies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Serum Samples
2.2. Optimization of Reagents
2.3. Setting the Cut-Off Concentration
2.4. ELISAs
2.5. Assessment of Assay Reproducibility and Measurement of Antibody Isotype Levels in CVT Samples
2.6. Statistical Analysis
3. Results
3.1. Optimization of Serum Standards
3.2. Concentrations of HPV16 L1-Specific Total IgG
3.3. Evaluation of Assay Reproducibility for IgG1, IgG3, IgA, and IgM ELISAs
3.4. Pattern of Antibody Responses among Vaccinated Individuals
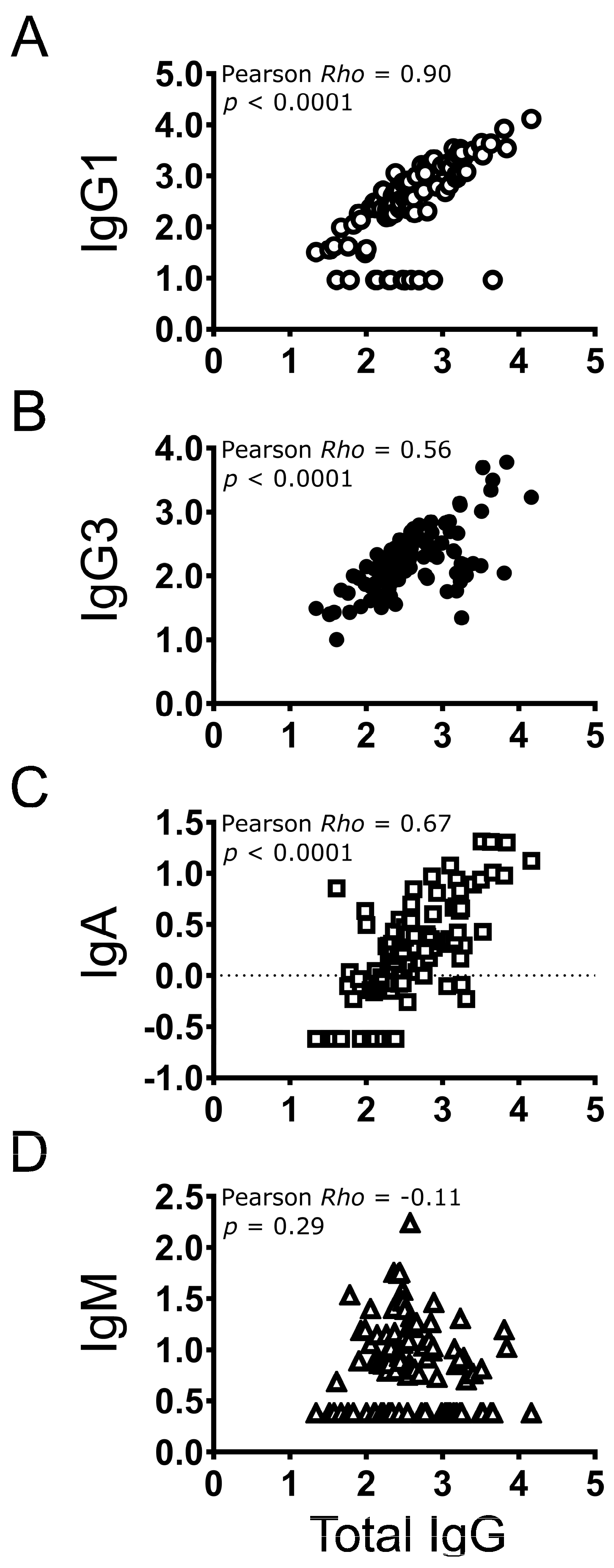
3.5. Correlation between Individual Antibody Types and Total IgG
3.6. Correlation amongst Antibody Isotypes in Vaccinated Individuals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Senkomago, V.; Henley, S.J.; Thomas, C.C.; Mix, J.M.; Markowitz, L.E.; Saraiya, M. Human Papillomavirus-Attributable Cancers—United States, 2012–2016. MMWR Morb. Mortal Wkly. Rep. 2019, 68, 724–728. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cervical Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 8 June 2024).
- Meites, E.; Szilagyi, P.G.; Chesson, H.W.; Unger, E.R.; Romero, J.R.; Markowitz, L.E. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal Wkly. Rep. 2019, 68, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, E.J.; Einstein, M.H.; Franceschi, S.; Kitchener, H.C. Human papillomavirus and cervical cancer. Lancet 2013, 382, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Sampson, J.N.; Porras, C.; Schiller, J.T.; Kemp, T.; Herrero, R.; Wagner, S.; Boland, J.; Schussler, J.; Lowy, D.R.; et al. Evaluation of Durability of a Single Dose of the Bivalent HPV Vaccine: The CVT Trial. J. Natl. Cancer Inst. 2020, 112, 1038–1046. [Google Scholar] [CrossRef]
- Joshi, S.; Anantharaman, D.; Muwonge, R.; Bhatla, N.; Panicker, G.; Butt, J.; Rani Reddy Poli, U.; Malvi, S.G.; Esmy, P.O.; Lucas, E.; et al. Evaluation of immune response to single dose of quadrivalent HPV vaccine at 10-year post-vaccination. Vaccine 2023, 41, 236–245. [Google Scholar] [CrossRef]
- Watson-Jones, D.; Changalucha, J.; Whitworth, H.; Pinto, L.; Mutani, P.; Indangasi, J.; Kemp, T.; Hashim, R.; Kamala, B.; Wiggins, R.; et al. Immunogenicity and safety of one-dose human papillomavirus vaccine compared with two or three doses in Tanzanian girls (DoRIS): An open-label, randomised, non-inferiority trial. Lancet Glob. Health 2022, 10, e1473–e1484. [Google Scholar] [CrossRef]
- WHO. Human papillomavirus vaccines: WHO position paper (2022 update). Wkly. Epidemiol. Rec. 2022, 50, 645–672. [Google Scholar]
- Baisley, K.; Kemp, T.J.; Mugo, N.R.; Whitworth, H.; Onono, M.A.; Njoroge, B.; Indangasi, J.; Bukusi, E.A.; Prabhu, P.R.; Mutani, P.; et al. Comparing one dose of HPV vaccine in girls aged 9–14 years in Tanzania (DoRIS) with one dose in young women aged 15–20 years in Kenya (KEN SHE): An immunobridging analysis of randomised controlled trials. Lancet Glob. Health 2024, 12, e491–e499. [Google Scholar] [CrossRef]
- Barnabas, R.V.; Brown, E.R.; Onono, M.A.; Bukusi, E.A.; Njoroge, B.; Winer, R.L.; Galloway, D.A.; Pinder, L.F.; Donnell, D.; Wakhungu, I.; et al. Efficacy of single-dose HPV vaccination among young African women. NEJM Evid. 2022, 1, EVIDoa2100056. [Google Scholar] [CrossRef]
- Basu, P.; Malvi, S.G.; Joshi, S.; Bhatla, N.; Muwonge, R.; Lucas, E.; Verma, Y.; Esmy, P.O.; Poli, U.R.R.; Shah, A.; et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: A multicentre, prospective, cohort study. Lancet Oncol. 2021, 22, 1518–1529. [Google Scholar] [CrossRef]
- Cuschieri, K.; Kavanagh, K.; Moore, C.; Bhatia, R.; Love, J.; Pollock, K.G. Impact of partial bivalent HPV vaccination on vaccine-type infection: A population-based analysis. Br. J. Cancer 2016, 114, 1261–1264. [Google Scholar] [CrossRef] [PubMed]
- Donken, R.; Schurink-Van’t Klooster, T.M.; Schepp, R.M.; van der Klis, F.R.; Knol, M.J.; Meijer, C.J.; de Melker, H.E. Immune Responses After 2 Versus 3 Doses of HPV Vaccination up to 4(1/2) Years After Vaccination: An Observational Study among Dutch Routinely Vaccinated Girls. J. Infect. Dis. 2017, 215, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.; Pollock, K.G.J.; Potts, A.; Love, J.; Cuschieri, K.; Cubie, H.; Robertson, C.; Donaghy, M. Introduction and sustained high coverage of the HPV bivalent vaccine leads to a reduction in prevalence of HPV 16/18 and closely related HPV types. Br. J. Cancer 2014, 110, 2804–2811. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Struyf, F.; Del Rosario-Raymundo, M.R.; Hildeshim, A.; Skinner, S.R.; Wacholder, S.; Garland, S.M.; Herrero, R.; David, M.P.; Mfeeler, C.M.; et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: Combined analysis of data from the Costa Rica Vaccine and PATRICIA trials. Lancet Oncol. 2015, 16, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.; Wallace, L.; Pollock, K.G.; Cuschieri, K.; Robertson, C.; Kavanagh, K.; Cruickshank, M. Prevalence of cervical disease at age 20 after immunisation with bivalent HPV vaccine at age 12-13 in Scotland: Retrospective population study. BMJ 2019, 365, l1161. [Google Scholar] [CrossRef]
- Pasmans, H.; Schurink-Van’t Klooster, T.M.; Bogaard, M.J.M.; van Rooijen, D.M.; de Melker, H.E.; Welters, M.J.P.; van der Burg, S.H.; van der Klis, F.R.M.; Buisman, A.M. Long-term HPV-specific immune response after one versus two and three doses of bivalent HPV vaccination in Dutch girls. Vaccine 2019, 37, 7280–7288. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Joshi, S.; Muwonge, R.; Esmy, P.O.; Basu, P.; Prabhu, P.; Bhatla, N.; Nene, B.M.; Shaw, J.; Poli, U.R.R.; et al. Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine 2018, 36, 4783–4791. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Prabhu, P.R.; Pawlita, M.; Gheit, T.; Bhatla, N.; Muwonge, R.; Nene, B.M.; Esmy, P.O.; Joshi, S.; Poli, U.R.; et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: A multicentre prospective cohort study. Lancet Oncol. 2016, 17, 67–77. [Google Scholar] [CrossRef]
- Zeng, Y.; Moscicki, A.B.; Woo, H.; Hsu, C.H.; Kemp, T.J.; Pinto, L.A.; Szabo, E.; Dimond, E.; Bauman, J.; Sahasrabuddhe, V.V.; et al. HPV16/18 Antibody Responses After a Single Dose of Nonavalent HPV Vaccine. Pediatrics 2023, 152, e2022060301. [Google Scholar] [CrossRef]
- Herrero, R.; Hildesheim, A.; Rodriguez, A.C.; Wacholder, S.; Bratti, C.; Solomon, D.; Gonzalez, P.; Porras, C.; Jimenez, S.; Guillen, D.; et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine 2008, 26, 4795–4808. [Google Scholar] [CrossRef]
- Harper, D.M.; Franco, E.L.; Wheeler, C.; Ferris, D.G.; Jenkins, D.; Schuind, A.; Zahaf, T.; Innis, B.; Naud, P.; De Carvalho, N.S.; et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: A randomised controlled trial. Lancet 2004, 364, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.B.; Thompson, C.D. Production of papillomavirus-based gene transfer vectors. Curr. Protoc. Cell Biol. 2007, 37, 26-1. [Google Scholar] [CrossRef] [PubMed]
- Herrin, D.M.; Coates, E.E.; Costner, P.J.; Kemp, T.J.; Nason, M.C.; Saharia, K.K.; Pan, Y.; Sarwar, U.N.; Holman, L.; Yamshchikov, G.; et al. Comparison of adaptive and innate immune responses induced by licensed vaccines for Human Papillomavirus. Hum. Vaccine Immunother. 2014, 10, 3446–3454. [Google Scholar] [CrossRef] [PubMed]
- Leder, C.; Kleinschmidt, J.A.; Wiethe, C.; Müller, M. Enhancement of capsid gene expression: Preparing the human papillomavirus type 16 major structural gene L1 for DNA vaccination purposes. J. Virol. 2001, 75, 9201–9209. [Google Scholar] [CrossRef]
- Matsui, K.; Adelsberger, J.W.; Kemp, T.J.; Baseler, M.W.; Ledgerwood, J.E.; Pinto, L.A. Circulating CXCR5⁺CD4⁺ T Follicular-Like Helper Cell and Memory B Cell Responses to Human Papillomavirus Vaccines. PLoS ONE 2015, 10, e0137195. [Google Scholar] [CrossRef]
- Fraussen, J. IgM responses following SARS-CoV-2 vaccination: Insights into protective and pre-existing immunity. eBioMedicine 2022, 77, 103922. [Google Scholar] [CrossRef]
- Sathe, A.; Cusick, J.K. Biochemistry, Immunoglobulin M. In StatPearls; StatPearls Publishing Copyright © 2024; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Cerutti, A. The regulation of IgA class switching. Nat. Rev. Immunol. 2008, 8, 421–434. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Irani, V.; Guy, A.J.; Andrew, D.; Beeson, J.G.; Ramsland, P.A.; Richards, J.S. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol. Immunol. 2015, 67, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, M.; Bjorkman, P.J. Fc receptors and their interactions with immunoglobulins. Annu. Rev. Cell Dev. Biol. 1996, 12, 181–220. [Google Scholar] [CrossRef]
- Ravetch, J.V.; Bolland, S. IgG Fc receptors. Annu. Rev. Immunol. 2001, 19, 275–290. [Google Scholar] [CrossRef] [PubMed]
- McFall-Boegeman, H.; Huang, X.F. Mechanisms of cellular and humoral immunity through the lens of VLP-based vaccines. Expert Rev. Vaccines 2022, 21, 453–469. [Google Scholar] [CrossRef]
- Damelang, T.; Rogerson, S.J.; Kent, S.J.; Chung, A.W. Role of IgG3 in Infectious Diseases. Trends Immunol. 2019, 40, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Gregorek, H.; Madalinski, K.; Woynarowski, M.; Mikolajewicz, J.; Syczewska, M.; Socha, J. The IgG subclass profile of anti-HBs response in vaccinated children and children seroconverted after natural infection. Vaccine 2000, 18, 1210–1217. [Google Scholar] [CrossRef]
- Havervall, S.; Marking, U.; Svensson, J.; Greilert-Norin, N.; Bacchus, P.; Nilsson, P.; Hober, S.; Gordon, M.; Blom, K.; Klingstrom, J.; et al. Anti-Spike Mucosal IgA Protection against SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2022, 387, 1333–1336. [Google Scholar] [CrossRef]
- Senger, K.; Hackney, J.; Payandeh, J.; Zarrin, A.A. Antibody Isotype Switching in Vertebrates. Results Probl. Cell Differ. 2015, 57, 295–324. [Google Scholar] [CrossRef] [PubMed]
- Harro, C.D.; Pang, Y.Y.; Roden, R.B.; Hildesheim, A.; Wang, Z.; Reynolds, M.J.; Mast, T.C.; Robinson, R.; Murphy, B.R.; Karron, R.A.; et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J. Natl. Cancer Inst. 2001, 93, 284–292. [Google Scholar] [CrossRef]
- Petter, A.; Heim, K.; Guger, M.; Ciresa-Ko Nig, A.; Christensen, N.; Sarcletti, M.; Wieland, U.; Pfister, H.; Zangerle, R.; Hopfl, R. Specific serum IgG, IgM and IgA antibodies to human papillomavirus types 6, 11, 16, 18 and 31 virus-like particles in human immunodeficiency virus-seropositive women. J. Gen. Virol. 2000, 81, 701–708. [Google Scholar] [CrossRef]
- Ruiz, W.; McClements, W.L.; Jansen, K.U.; Esser, M.T. Kinetics and isotype profile of antibody responses in rhesus macaques induced following vaccination with HPV 6, 11, 16 and 18 L1-virus-like particles formulated with or without Merck aluminum adjuvant. J. Immune Based Ther. Vaccines 2005, 3, 2. [Google Scholar] [CrossRef]
- Scherpenisse, M.; Schepp, R.M.; Mollers, M.; Meijer, C.J.; Berbers, G.A.; van der Klis, F.R. Characteristics of HPV-specific antibody responses induced by infection and vaccination: Cross-reactivity, neutralizing activity, avidity and IgG subclasses. PLoS ONE 2013, 8, e74797. [Google Scholar] [CrossRef]
- Toh, Z.Q.; He, L.; Chen, C.; Huang, A.; Russell, F.M.; Garland, S.M.; Reyburn, R.; Ratu, T.; Tuivaga, E.; Frazer, I.H.; et al. Measurement of Human Papillomavirus-Specific Antibodies Using a Pseudovirion-Based ELISA Method. Front. Immunol. 2020, 11, 585768. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Kjellberg, L.; Abdalla, H.; Wiklund, F.; Eklund, C.; Knekt, P.; Lehtinen, M.; Kallings, I.; Lenner, P.; Hallmans, G.; et al. Type specificity and significance of different isotypes of serum antibodies to human papillomavirus capsids. J. Infect. Dis. 2000, 181, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Weiland, T.; Zgubic, J.; Brcic, L.; Thurnher, D. Detection of antibody subclasses IgA, IgM and IgG against HPV L1 in HPV-positive oropharyngeal squamous cell carcinoma patients: A pilot study. Eur. Arch. Otorhinolaryngol. 2024, 281, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A.; Beard, L.J.; Feldman, R.G. IgG subclass distribution of antibodies to bacterial and viral antigens. Pediatr. Infect. Dis. J. 1990, 9, S16–S24. [Google Scholar] [CrossRef]
- Siber, G.R.; Schur, P.H.; Aisenberg, A.C.; Weitzman, S.A.; Schiffman, G. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N. Engl. J. Med. 1980, 303, 178–182. [Google Scholar] [CrossRef]
- Kemp, T.J.; Hildesheim, A.; Safaeian, M.; Dauner, J.G.; Pan, Y.; Porras, C.; Schiller, J.T.; Lowy, D.R.; Herrero, R.; Pinto, L.A. HPV16/18 L1 VLP vaccine induces cross-neutralizing antibodies that may mediate cross-protection. Vaccine 2011, 29, 2011–2014. [Google Scholar] [CrossRef]



| N | IgG3 | IgG1 | IgA | IgM |
|---|---|---|---|---|
| 1 | 31 | 32 | (0.24) | (2.40) |
| 2 | 25 | 36 | (0.24) | (2.40) |
| 3 | 27 | 42 | (0.24) | (2.40) |
| 4 | 10 | 98 | (0.24) | (2.40) |
| 5 | 60 | 42 | 0.79 | (2.40) |
| 6 | 54 | 112 | 0.59 | (2.40) |
| 7 | 27 | 182 | 0.93 | 7.74 |
| 8 | 100 | 139 | (0.24) | 15.31 |
| 9 | 41 | 238 | 0.72 | 25.07 |
| 10 | 68 | 247 | (0.24) | 11.49 |
| 11 | 129 | 299 | 0.69 | (2.40) |
| 12 | 76 | 236 | 1.11 | 8.01 |
| 13 | 53 | 303 | 0.78 | 7.30 |
| 14 | 217 | 224 | 0.97 | (2.40) |
| 15 | 108 | 516 | 0.87 | 7.16 |
| 16 | 32 | 474 | (0.24) | (2.40) |
| 17 | 50 | 160 | 1.96 | 7.26 |
| 18 | 63 | 174 | 1.56 | (2.40) |
| 19 | 175 | 184 | (0.24) | (2.40) |
| 20 | 48 | 189 | 0.71 | 10.42 |
| 21 | 50 | 262 | 2.06 | (2.40) |
| 22 | 255 | 409 | 1.62 | (2.40) |
| 23 | 114 | 281 | 0.84 | 25.36 |
| 24 | 83 | 183 | 2.69 | 56.53 |
| 25 | 88 | 591 | 1.56 | 55.43 |
| 26 | 194 | 499 | 0.83 | (2.40) |
| 27 | 302 | 233 | 3.54 | 8.56 |
| 28 | 367 | 329 | 0.99 | 56.76 |
| 29 | 139 | 294 | 1.87 | 7.65 |
| 30 | 205 | 737 | 1.58 | 29.75 |
| 31 | 170 | 800 | 1.29 | (2.40) |
| 32 | 227 | 222 | 0.55 | 5.61 |
| 33 | 305 | 735 | 2.52 | 12.28 |
| 34 | 180 | 773 | 1.16 | 7.26 |
| 35 | 135 | 359 | 3.48 | 173.40 |
| 36 | 492 | 364 | 1.26 | 20.91 |
| 37 | 220 | 237 | 3.43 | 6.80 |
| 38 | 272 | 370 | 7.03 | 16.35 |
| 39 | 550 | 187 | 2.45 | 6.39 |
| 40 | 526 | 985 | 1.08 | 18.30 |
| 41 | 198 | 497 | 1.00 | 10.75 |
| 42 | 316 | 1511 | 2.54 | (2.40) |
| 43 | 92 | 205 | 2.41 | 8.28 |
| 44 | 638 | 1146 | 1.49 | 12.34 |
| 45 | 698 | 1850 | 2.29 | 18.39 |
| 46 | 479 | 1227 | 9.34 | 9.66 |
| 47 | 277 | 2126 | 1.87 | 28.74 |
| 48 | 210 | 1524 | 2.17 | (2.40) |
| 49 | 198 | 620 | 6.44 | 5.34 |
| 50 | 667 | 488 | 2.05 | (2.40) |
| 51 | 57 | 1733 | 0.79 | (2.40) |
| 52 | 711 | 659 | 2.29 | (2.40) |
| 53 | 496 | 1421 | 11.96 | (2.40) |
| 54 | 247 | 3464 | 4.63 | (2.40) |
| 55 | 236 | 2261 | 2.19 | 10.10 |
| 56 | 58 | 896 | 4.78 | 7.10 |
| 57 | 111 | 1061 | 8.69 | (2.40) |
| 58 | 467 | 2696 | 2.64 | 7.43 |
| 59 | 1376 | 2526 | 4.31 | 8.15 |
| 60 | 1290 | 3278 | 6.77 | (2.40) |
| 61 | 156 | 1143 | 4.54 | (2.40) |
| 62 | 22 | 2821 | 0.81 | (2.40) |
| 63 | 103 | 1217 | 0.59 | 5.04 |
| 64 | 155 | 3153 | 7.82 | 5.79 |
| 65 | 144 | 3956 | 8.69 | (2.40) |
| 66 | 1026 | 4355 | 20.46 | 6.46 |
| 67 | 5004 | 2564 | 2.68 | (2.40) |
| 68 | 2202 | 4260 | 20.32 | (2.40) |
| 69 | 3167 | (6.00) | 10.19 | (2.40) |
| 70 | 111 | 8335 | 9.53 | 15.60 |
| 71 | 6055 | 3511 | 20.03 | 10.47 |
| 72 | 1710 | 13,344 | 13.26 | (2.40) |
| ICC | Overall CV | Between-Technician CV | Across-Day CV | |
|---|---|---|---|---|
| IgG1 | ||||
| Overall | 99.8 | 9.0 | 12.8 | 6.3 |
| Low | 99.7 | 5.0 | 5.6 | 4.0 |
| Medium | 99.6 | 6.3 | 8.2 | 4.4 |
| High | 99.8 | 6.1 | 8.0 | 4.1 |
| IgG3 | ||||
| Overall | 99.9 | 7.7 | 22.7 | 6.2 |
| Low | 99.4 | 8.4 | 7.3 | 7.6 |
| Medium | 99.3 | 6.6 | 6.8 | 4.4 |
| High | 99.9 | 5.6 | 16.2 | 4.5 |
| IgA | ||||
| Overall | 99.7 | 10.0 | 21.8 | 9.2 |
| Low | 99.0 | 10.5 | 7.3 | 8.0 |
| Medium | 99.5 | 7.6 | 6.3 | 6.3 |
| High | 99.7 | 6.6 | 18.5 | 6.1 |
| IgM | ||||
| Overall | 98.7 | 31.1 | 15.8 | 30.6 |
| Low | 99.3 | 15.9 | 5.6 | 14.6 |
| Medium | 98.5 | 27.6 | 14.7 | 27.3 |
| High | 99.2 | 9.5 | 12.8 | 8.3 |
| Range (EU/mL) | Mean (EU/mL) | Median (EU/mL) | % Detected | ||
|---|---|---|---|---|---|
| IgG1 | All samples | 31–13344 | 1262 | 498 | 86.3% (82/95) |
| Low | 31–516 | 206 | 184 | 82.9% (29/35) | |
| Medium | 187–2126 | 781 | 620 | 82.9% (29/35) | |
| High | 488–13,344 | 3120 | 2630 | 96.0% (24/25) | |
| IgG3 | All samples | 10–6055 | 411 | 156 | 100% (95/95) |
| Low | 10–270 | 92 | 74 | 100% (35/35) | |
| Medium | 36–698 | 286 | 220 | 100% (35/35) | |
| High | 22–6055 | 1032 | 247 | 100% (25/25) | |
| IgA | All samples | 0.55–20.46 | 3.56 | 1.97 | 88.4% (84/95) |
| Low | 0.59–7.16 | 1.63 | 1.08 | 71.4% (25/35) | |
| Medium | 0.55–9.34 | 2.49 | 1.87 | 97.1% (34/35) | |
| High | 0.59–20.46 | 6.94 | 4.63 | 100% (25/25) | |
| IgM | All samples | 4.85–173.40 | 17.76 | 10.77 | 62.1% (59/95) |
| Low | 4.85–56.53 | 15.56 | 11.49 | 54.3% (19/35) | |
| Medium | 5.34–173.40 | 22.34 | 12.28 | 82.9% (29/35) | |
| High | 5.04–20.05 | 9.50 | 7.79 | 44.0% (11/25) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsui, K.; Hempel, H.A.; Shelton, G.; Ocampo, R.; Kemp, T.J.; Pan, Y.; Pinto, L.A. Reproducibility Assessment of Enzyme-Linked Immunosorbent Assays to Detect Anti-HPV16 L1-Specific IgG1, IgG3, IgA, and IgM Antibodies. Vaccines 2024, 12, 1108. https://doi.org/10.3390/vaccines12101108
Matsui K, Hempel HA, Shelton G, Ocampo R, Kemp TJ, Pan Y, Pinto LA. Reproducibility Assessment of Enzyme-Linked Immunosorbent Assays to Detect Anti-HPV16 L1-Specific IgG1, IgG3, IgA, and IgM Antibodies. Vaccines. 2024; 12(10):1108. https://doi.org/10.3390/vaccines12101108
Chicago/Turabian StyleMatsui, Ken, Heidi Anne Hempel, Gloriana Shelton, Rebecca Ocampo, Troy J. Kemp, Yuanji Pan, and Ligia A. Pinto. 2024. "Reproducibility Assessment of Enzyme-Linked Immunosorbent Assays to Detect Anti-HPV16 L1-Specific IgG1, IgG3, IgA, and IgM Antibodies" Vaccines 12, no. 10: 1108. https://doi.org/10.3390/vaccines12101108





