Enhanced Expression of the L1R Gene of Vaccinia Virus by the tPA Signal Sequence Inserted in a Fowlpox-Based Recombinant Vaccine
Abstract
:1. Introduction
2. Material and Methods
2.1. Cells
2.2. Expression Plasmid
2.3. Recombination Plasmid
2.4. Plasmid Transfection
2.5. Fowlpox Virus Recombinant
2.6. Immunoprecipitation Analysis
2.7. Expression of Viral RNA Transcripts in Vero Cells by pVAXtPA-L1R and FPtPA-L1R
2.8. Over-Time Expression of Viral RNA Transcripts in Vero and MRC-5 Cells
2.9. Immunofluorescence
3. Results
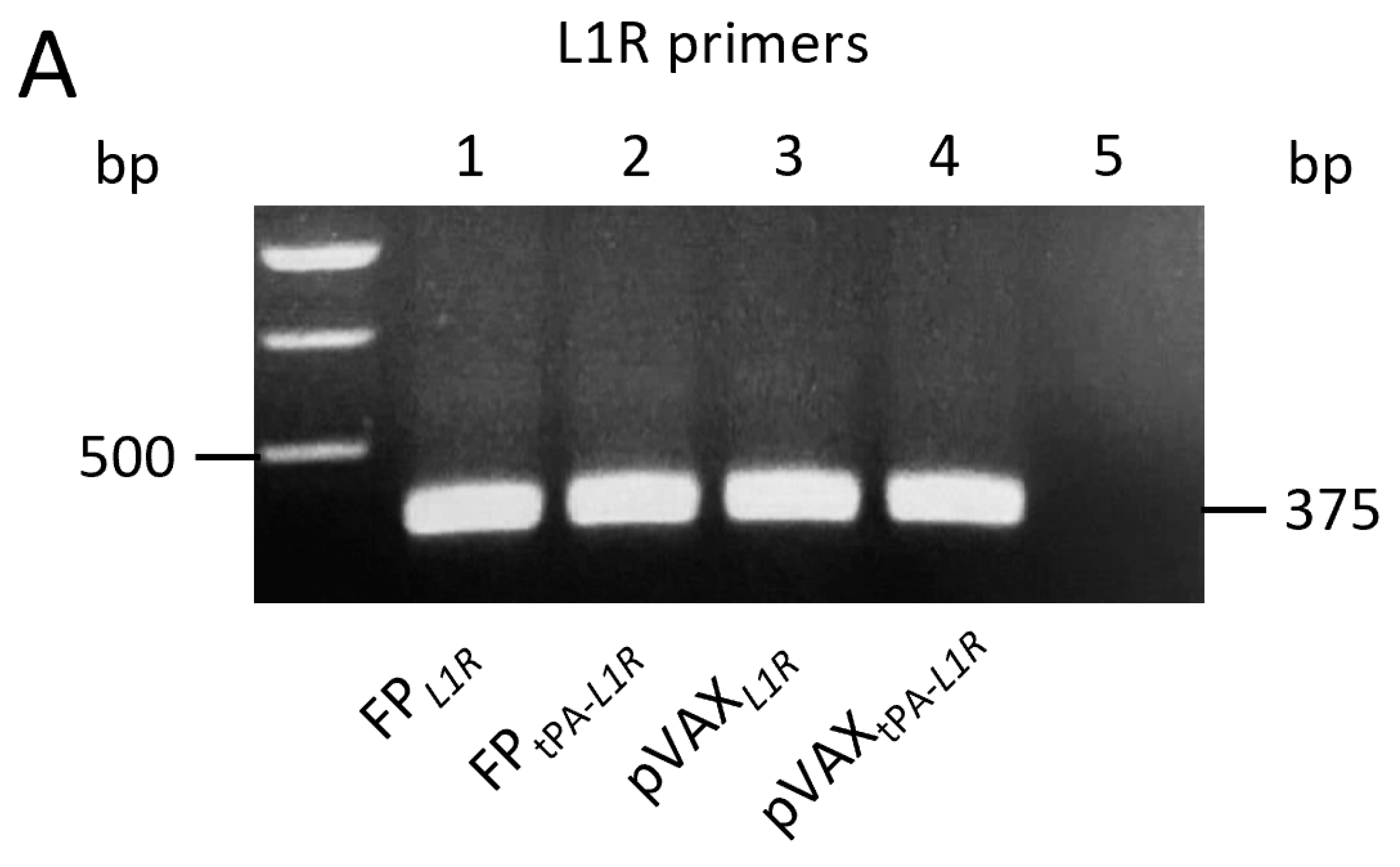
3.1. tPA-L1R Transcript Is Expressed Only by FPtPA-L1R
3.2. FPtPA-L1R Expresses the Transgene in Vero and MRC-5 Cells for More than One Month
3.3. L1 Protein Is Expressed by FPtPA-L1R in Vero, MRC-5, and CEF Cells
3.4. L1 Protein Is Expressed by All the Cell Types in the Cytoplasm and on the Membrane by FPtPA-L1R
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ferrier-Rembert, A.; Drillien, R.; Tournier, J.N.; Garin, D.; Crance, J.M. Short-and long-term immunogenicity and protection induced by non-replicating smallpox vaccine candidates in mice and comparison with the traditional 1st generation vaccine. Vaccine 2008, 26, 1794–1804. [Google Scholar] [CrossRef]
- Baxby, D.; Paoletti, E. Potential use of nonreplicating vectors as recombinant vaccines. Vaccine 1992, 10, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, P.; Frazier, J.; Skinner, M.A. Fowlpox Virus Host Range Restriction: Gene Expression, DNA Replication, and Morphogenesis in Nonpermissive Mammalian Cells. Virology 1993, 197, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.A.; Laidlaw, S.M.; Eldaghayes, I.; Kaiser, P.; Cottingham, M.G. Fowlpox virus as a recombinant vaccine vector for use in mammals and poultry. Expert Rev. Vaccines 2005, 4, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Weinberg, R.; Languet, B.; Desmettre, P.; Paoletti, E. Recombinant fowlpox virus inducing protective immunity in nonavian species. Vaccine 1988, 6, 497–503. [Google Scholar] [CrossRef]
- Zanotto, C.; Pozzi, E.; Pacchioni, S.; Volonté, L.; De Giuli Morghen, C.; Radaelli, A. Canarypox and fowlpox viruses as recombinant vaccine vectors: A biological and immunological comparison. Antivir. Res. 2010, 88, 53–63. [Google Scholar] [CrossRef]
- Bos, R.; Rutten, L.; van der Lubbe, J.E.M.; Bakkers, M.J.G.; Hardenberg, G.; Wegmann, F.; Zuijdgeest, D.; de Wilde, A.H.; Koornneef, A.; Verwilligen, A.; et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines 2020, 5, 91. [Google Scholar] [CrossRef]
- Rutten, L.; Lai, Y.T.; Blokland, S.; Truan, D.; Bisschop, I.J.M.; Strokappe, N.M.; Koornneef, A.; van Manen, D.; Chuang, G.Y.; Farney, S.K.; et al. A Universal Approach to Optimize the Folding and Stability of Prefusion-Closed HIV-1 Envelope Trimers. Cell Rep. 2018, 23, 584–595. [Google Scholar] [CrossRef]
- Kou, Y.; Xu, Y.; Zhao, Z.; Liu, J.; Wu, Y.; You, Q.; Wang, L.; Gao, F.; Cai, L.; Jiang, C. Tissue plasminogen activator (tPA) signal sequence enhances immunogenicity of MVA-based vaccine against tuberculosis. Immunol. Lett. 2017, 190, 51–57. [Google Scholar] [CrossRef]
- Ondondo, B.; Abdul-Jawad, S.; Roshorm, Y.; Bridgeman, A.; Hanke, T. Vector delivery-dependant effect of human tissue plasminogen activator signal peptide on vaccine induction of T cells. J. HIV AIDS 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Radaelli, A.; Nacsa, J.; Tsai, W.P.; Edghill-Smith, Y.; Zanotto, C.; Elli, V.; Venzon, D.; Tryniszewska, E.; Markham, P.; Mazzara, G.P.; et al. Prior DNA immunization enhances immune response to dominant and subdominant viral epitopes induced by a fowlpox-based SIVmac vaccine in long-term slow-progressor macaques infected with SIVmac251. Virology 2003, 312, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Pantaleo, G.; Esteban, M.; Jacobs, B.; Tartaglia, J. Poxvirus vector-based HIV vaccines. Curr. Opin. HIV AIDS 2010, 5, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Hayes, P.; Gilmour, J.; von Lieven, A.; Gill, D.; Clark, L.; Kopycinski, J.; Cheeseman, H.; Chung, A.; Alter, G.; Dally, L.; et al. Safety and immunogenicity of DNA prime and modified vaccinia ankara virus-HIV subtype C vaccine boost in healthy adults. Clin. Vaccine Immunol. 2013, 20, 397–408. [Google Scholar] [CrossRef]
- Hooper, J.W.; Custer, D.M.; Schmaljohn, C.S.; Schmaljohn, A.L. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology 2000, 266, 329–339. [Google Scholar] [CrossRef]
- Garcia-Arriaza, J.; Esteban, M. Enhancing poxvirus vectors vacccine immunogenicity. Hum. Vaccines Immunother. 2014, 10, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Ravanello, M.P.; Hruby, D.E. Conditional Lethal Expression of the Vaccinia Virus L1R Myristylated Protein Reveals a Role in Virion Assembly. J. Virol. 1994, 68, 6401–6410. [Google Scholar] [CrossRef]
- Wolffe, E.J.; Vijaya, S.; Moss, B. A Myristylated Membrane Protein Encoded by the Vaccinia Virus L1R Open Reading Frame is the Target of Potent Neutralizing Monoclonal Antibodies. Virology 1995, 211, 53–63. [Google Scholar] [CrossRef]
- Monticelli, G. (Ed.) Sangue e la Linfa. In Fisiologia; Casa Editrice Ambrosiana: Rozzano, Italy, 2011; pp. 429–444. [Google Scholar]
- Gao, F.; He, C.; Liu, M.; Yuan, P.; Tian, S.; Zheng, M.; Zhang, L.; Zhou, X.; Xu, F.; Luo, J.; et al. Cross-reactive immune responses to monkeypox virus induced by MVA vaccination in mice. Virol. J. 2023, 20, 126. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.W.; Golden, J.W.; Ferro, A.M.; King, A.D. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine 2007, 25, 1814–1823. [Google Scholar] [CrossRef]
- Bissa, M.; Quaglino, E.; Zanotto, C.; Illiano, E.; Rolih, V.; Pacchioni, S.; Cavallo, F.; De Giuli Morghen, C.; Radaelli, A. Protection of mice against the highly pathogenic VVIHD-J by DNA and fowlpox recombinant vaccines, administered by electroporation and intranasal routes, correlates with serum neutralizing activity. Antivir. Res. 2016, 134, 182–191. [Google Scholar] [CrossRef]
- Golden, J.W.; Josleyn, M.D.; Hooper, J.W. Targeting the vaccinia virus L1 protein to the cell surface enhances production of neutralizing antibodies. Vaccine 2008, 26, 3507–3515. [Google Scholar] [CrossRef] [PubMed]
- Zanotto, C.; Paolini, F.; Radaelli, A.; De Giuli, M.C. Construction of a recombinant avipoxvirus expressing the env gene of Zika virus as a novel putative preventive vaccine. Virol. J. 2021, 18, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, A.; De Giuli Morghen, C.; Zanotto, C.; Pacchioni, S.; Bissa, M.; Franconi, R.; Massa, S.; Paolini, F.; Muller, A.; Venuti, A. A prime/boost strategy by DNA/fowlpox recombinants expressing a mutant E7 protein for the immunotherapy of HPV-associated cancers. Virus Res. 2012, 170, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Rosel, J.L.; Earl, P.L.; Weir, J.; Moss, B. Conserved TAAATG Sequence at the Transcriptional and Translational Initiation Sites of Vaccinia Virus Late Genes Deduced by Structural and Functional Analysis of the Hindlll H Genome Fragment. J. Virol. 1986, 60, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Zanotto, C.; Pozzi, E.; Pacchioni, S.; Bissa, M.; De Giuli Morghen, C.; Radaelli, A. Construction and characterisation of a recombinant fowlpox virus that expresses the human papilloma virus L1 protein. J. Transl. Med. 2011, 9, 190–200. [Google Scholar] [CrossRef]
- Wiser, I.; Balicer, R.D.; Cohen, D. An update on smallpox vaccine candidates and their role in bioterrorism related vaccination strategies. Vaccine 2007, 25, 976–984. [Google Scholar] [CrossRef]
- Poland, G.A. Smallpox vaccines: From first to second to third generation. Vaccine 2005, 365, 362–363. [Google Scholar] [CrossRef]
- Artenstein, A.W. New generation smallpox vaccines: A review of preclinical and clinical data. Rev. Med. Virol. 2008, 18, 217–231. [Google Scholar] [CrossRef]
- Hutin, Y.J.; Williams, R.J.; Malfait, P.; Pebody, R.; Loparev, V.N.; Ropp, S.L.; Rodriguez, M.; Knight, J.C.; Tshioko, F.K.; Khan, A.S.; et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 2001, 7, 434–438. [Google Scholar] [CrossRef]
- Whitley, R.J. Smallpox: A potential agent of bioterrorism. Antivir. Res. 2003, 57, 7–12. [Google Scholar] [CrossRef]
- Vogel, S.; Sardy, M.; Glos, K.K.H.C.; Ruzicka, T.; Wollenberg, A. The Munich outbreak of cutaneous cowpox infection: Transmission by infected pet rats. Acta Derm. Venereol. 2012, 92, 126–131. [Google Scholar] [PubMed]
- Ranasinghe, C.; Eyers, F.; Stambas, J.; Boyle, D.B.; Ramshaw, I.A.; Ramsay, A.J. A comparative analysis of HIV-specific mucosal/systemic T cell immunity and avidity following rDNA/rFPV and poxvirus-poxvirus prime boost immunisations. Vaccine 2011, 29, 3008–3020. [Google Scholar] [CrossRef] [PubMed]
- Golden, J.W.; Gittis, A.G.; Hooper, J.W.; Garboczi, D.N.; Su, H.P. Structural basis for the binding of the neutralizing antibody, 7D11, to the poxvirus L1 protein. Virology 2007, 368, 331–341. [Google Scholar]
- Senkevich, T.G.; White, C.L.; Koonin, E.V.; Moss, B. Complete pathway for protein disulfide bond formation encoded by orthopoxviruses. Proc. Natl. Acad. Sci. USA 2002, 99, 6667–6672. [Google Scholar] [CrossRef]
- Aldaz-Carroll, L.; Whitbeck, J.C.; Ponce de, L.M.; Lou, H.; Lebowitz, J.; Fogg, C.; White, C.L.; Moss, B.; Cohen, G.H.; Eisenberg, R.J. Physical and immunological characterization of a recombinant secreted form of the membrane protein encoded by the vaccinia virus L1R gene. Virology 2005, 341, 59–71. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radaelli, A.; Zanotto, C.; Brambilla, C.; Adami, T.; De Giuli Morghen, C. Enhanced Expression of the L1R Gene of Vaccinia Virus by the tPA Signal Sequence Inserted in a Fowlpox-Based Recombinant Vaccine. Vaccines 2024, 12, 1115. https://doi.org/10.3390/vaccines12101115
Radaelli A, Zanotto C, Brambilla C, Adami T, De Giuli Morghen C. Enhanced Expression of the L1R Gene of Vaccinia Virus by the tPA Signal Sequence Inserted in a Fowlpox-Based Recombinant Vaccine. Vaccines. 2024; 12(10):1115. https://doi.org/10.3390/vaccines12101115
Chicago/Turabian StyleRadaelli, Antonia, Carlo Zanotto, Chiara Brambilla, Tommaso Adami, and Carlo De Giuli Morghen. 2024. "Enhanced Expression of the L1R Gene of Vaccinia Virus by the tPA Signal Sequence Inserted in a Fowlpox-Based Recombinant Vaccine" Vaccines 12, no. 10: 1115. https://doi.org/10.3390/vaccines12101115




